Abstract
Protein–protein interactions are typically identified by either biochemical purification coupled to mass spectrometry or genetic approaches exemplified by the yeast two-hybrid assay; however, neither assay works well for the identification of cofactors for poorly soluble proteins. Solubility of a poorly soluble protein is thought to increase upon cofactor binding, possibly by masking otherwise exposed hydrophobic domains. We have exploited this notion to develop a high-throughput genetic screen to identify interacting partners of an insoluble protein fused to chloramphenicol acetyltransferase by monitoring the survival of bacteria in the presence of a drug. In addition to presenting proof-of-principle experiments, we apply this screen to activation-induced cytidine deaminase (AID), a poorly soluble protein that is essential for antibody diversification. We identify a unique cofactor, RING finger protein 126 (RNF126), verify its interaction by traditional techniques, and show that it has functional consequences as RNF126 is able to ubiquitylate AID. Our results underpin the value of this screening technique and suggest a unique form of AID regulation involving RNF126 and ubiquitylation.
Keywords: ubiquitin, immunology, lymphocyte
Most large-scale protein–protein interaction data published to date has been produced with yeast two-hybrid assays (1–3) and in vivo pull-down approaches followed by mass spectrometry to identify proteins that coprecipitate with the protein of interest. Although these purification methods were first developed for small-scale protein identification experiments, they have been successfully adapted for use in genome-wide proteomics studies (4–6). Proteins that are poorly soluble or insoluble when ectopically expressed are least amenable to characterization using these tools. Improper folding or exposure of hydrophobic domains can result in insolubility when proteins are expressed individually; however, soluble complexes can form upon coexpression of a native partner. Based on this idea, we have devised a high-throughput protein–protein interaction screen, which involves the coexpression and cofolding of two proteins: an unknown protein expressed from a cDNA library and a known, insoluble “bait” fused to a protein that confers antibiotic resistance. Thus, solubility is directly linked to a traceable marker. We apply this technique to identify cofactors for activation-induced cytidine deaminase (AID), an enzyme that deaminates deoxycytidines in single-stranded DNA.
AID initiates both somatic hypermutation (SHM) and class-switch recombination (CSR) during antibody diversification (7, 8). A number of cofactors have been identified by a combination of genetic and proteomic approaches, which suggest that AID synchronizes with other broadly important cellular pathways including, but not limited to, RNA splicing and processing (9, 10), cellular trafficking (11–14), and transcription/RNA polymerase stalling (8, 15–17); however, a concise picture of the players involved in the AID reaction and the sequence of events remains unclear. Here, we use our solubility-based screening technique to identify a unique interacting partner for AID, RING finger protein 126 (RNF126). We demonstrate that RNF126 can interact with AID in vitro and in vivo and act as an E3 ubiquitin ligase for AID, modifying AID with the covalent attachment of a single ubiquitin moiety.
Results
Solubility-Based Screen to Identify Interacting Partners.
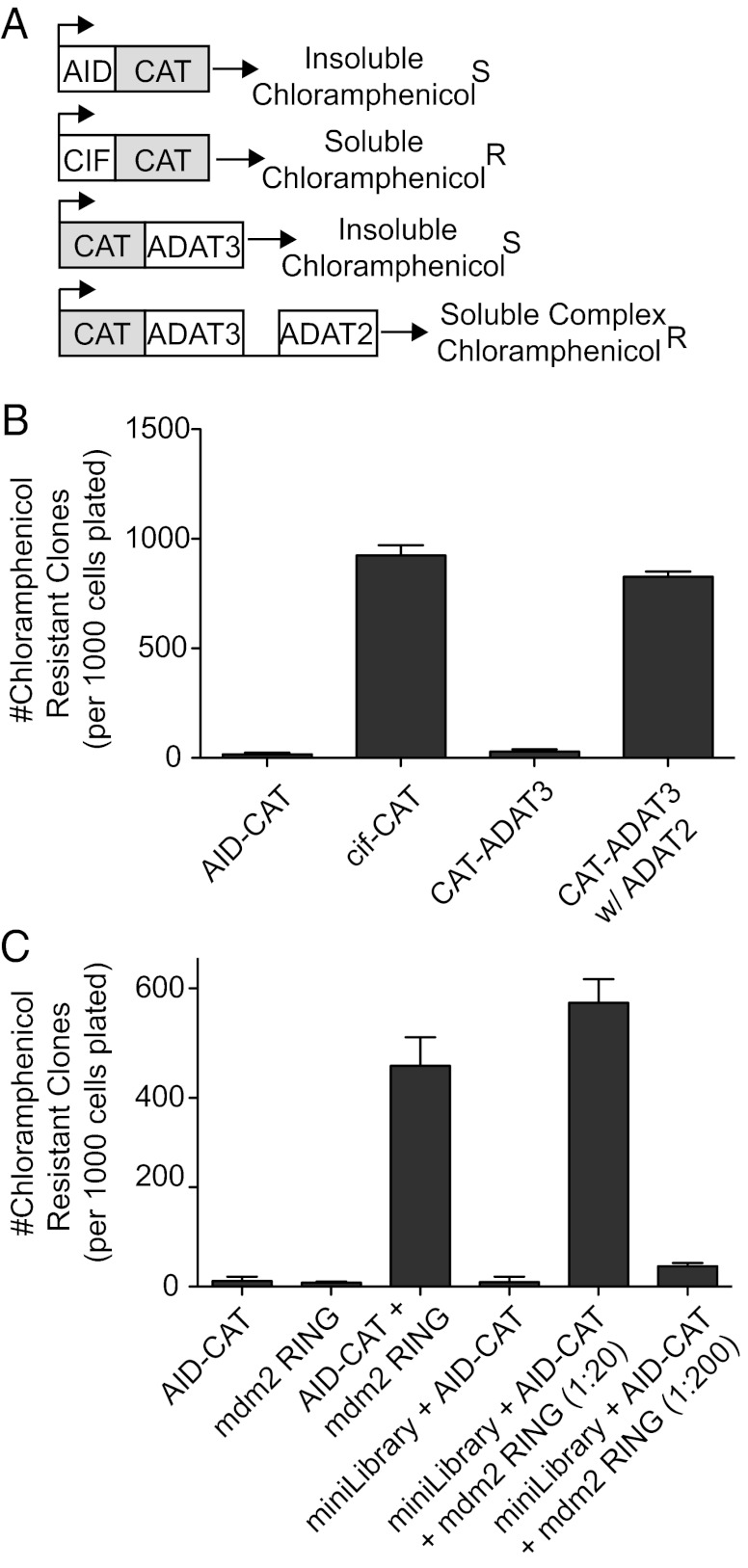
Protein insolubility is often caused by the aberrant exposure of a hydrophobic region that otherwise would be shielded by the binding of a cofactor. Thus, coexpression of an insoluble protein with a library of potential interactors should lead to an increase in solubility upon cofactor binding. This increase in solubility can be monitored if the insoluble protein is fused to a selectable marker [here, chloramphenicol acetyltransferase (CAT)]. We have established that the fusion of CAT with a soluble protein, cell-cycle inhibitory factor (CIF) (18), is soluble and produces chloramphenicol-resistant clones. In addition, fusion of CAT to an insoluble protein, the tRNA editing enzyme, ADAT3, renders cells unable to grow on chloramphenicol (Fig. 1 A and B). Lastly, it has been established that ADAT3, when in complex with its heterodimeric partner, ADAT2, becomes soluble (19). As predicted, solubility of ADAT3-CAT was rescued by coexpression of ADAT2 (Fig. 1 A and B). Together, these results establish that fusions of CAT produce chloramphenicol-resistant clones only when the bait protein is soluble. Furthermore, reconstitution of a soluble complex by coexpression of a necessary binding partner can rescue the ability of bacteria to grow on chloramphenicol plates. However, because of the nature of the selection method, we cannot determine the toxicity of the candidate cDNA because no growth due to toxicity is indistinguishable from no growth due to the inability of the cDNA to solubilize AID. This limitation is a caveat of our technique.
Fig. 1.
A genetic assay that selects for the restoration of solubility to an insoluble protein. (A) A schematic depicts representative CAT fusion proteins. AID and AID-CAT are insoluble and, thus, produce chloramphenicol-sensitive cells; Cif and Cif-CAT are soluble allowing cells to be resistant to chloramphenicol; ADAT3 and ADAT3-CAT are insoluble unless coexpressed with ADAT2. (R, resistant; S, sensitive). (B) Plasmids carrying the indicated genes were transformed into BL21ai E. coli, plated on chloramphenicol containing plates under induction conditions. Resistant colonies were counted. Shown here are colony numbers obtained on LB plates containing 120 μg/mL chloramphenicol. (C) Chloramphenicol-resistant colonies after cotransformation of the indicated expression plasmids were counted. Coexpression of AID-CAT and the Mdm2 RING domain allowed for efficient survival on chloramphenicol plates. These interactions were revealed even when the Mdm2 RING plasmid was diluted 1:20 and 1:200 in a minilibrary of known noninteractors. For B and C, graphs represent the number of chloramphenicol resistant colonies per 1,000 cells plated.
We adapted this system to include AID as the insoluble bait protein. As expected, a C-terminal fusion of AID to CAT left cells unable to grow on chloramphenicol plates (Fig. 1 A and B). We then examined the ability of a published AID cofactor, the C-terminal, RING domain of the E3 ligase, Mdm2, to rescue AID-CAT solubility in this assay (20). Coexpression of this region of Mdm2 rescued the solubility of AID-CAT (Fig. 1C). To determine whether such an interaction can be efficiently isolated from a pool of known negatives, we generated a small library of 20 cDNAs encoding known bacterial virulence factors. Cotransfection of this minilibrary with AID-CAT did not enable survival on chloramphenicol plates. However, dilution of the Mdm2 RING expressing plasmid within the minilibrary in a 20:1 or 200:1 ratio, followed by transformation of the library in the context of AID-CAT, recovered chloramphenicol-resistant clones (Fig. 1C); importantly, all clones coexpressed Mdm2 RING and AID-CAT. Thus, this screening technique is sensitive enough to isolate positive AID interactors even when they constitute a minority of the total population of the library.
Application of the Screen to AID Reveals RING Finger Protein 126 as an Interacting Partner for AID.
To identify potential AID-interacting proteins, we screened in triplicate a full-length, normalized cDNA library from the constitutively mutating RAMOS human B-cell lymphoma line (Fig. 2A and Fig. S1; ref. 21). We generated a list of 127 AID-CAT interactors, of which 36 were cloned in more than one iteration of the screen. The interaction of these 36 proteins with AID was further validated by reciprocal affinity purification experiments in Escherichia coli. Thirty of 36 copurified with AID, suggesting a 16% false positive rate (example, Fig. 3A).
Fig. 2.
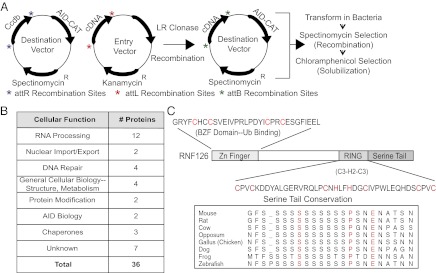
B-cell cDNA library screen for factors that rescue the solubility of the AID-CAT fusion protein. (A) A schematic depicts the cloning and expression of a RAMOS cell-derived cDNA library along with AID-CAT. The full-length cDNA library was cloned into the attL sites of a modified pCDF-duet entry vector by using the Gateway system (Invitrogen). AID-CAT was cloned into a pCDF-duet modified destination vector. Recombination between these vectors is achieved by the addition of the LR Clonase Enzyme Mix, which uses the attR and attL recombination sites. After recombination, the final expression vector contains both a candidate from the cDNA library and AID-CAT. Bacteria are then transformed, selected in spectinomycin to assure that they contain the recombined vector and replica plated on Petri dishes containing IPTG/arabinose to induce expression and chloramphenicol to select for solubility. (B) Pathways representative of putative interactors, which were sequenced from the screen and independently confirmed in bacterial coexpression assays. (C) Cartoon of RNF126 domain structure. The position and sequences of the RING domain and Zinc finger domain are shown. C Inset shows that the amino acid composition of the C-terminal serine-rich domain is evolutionarily conserved.
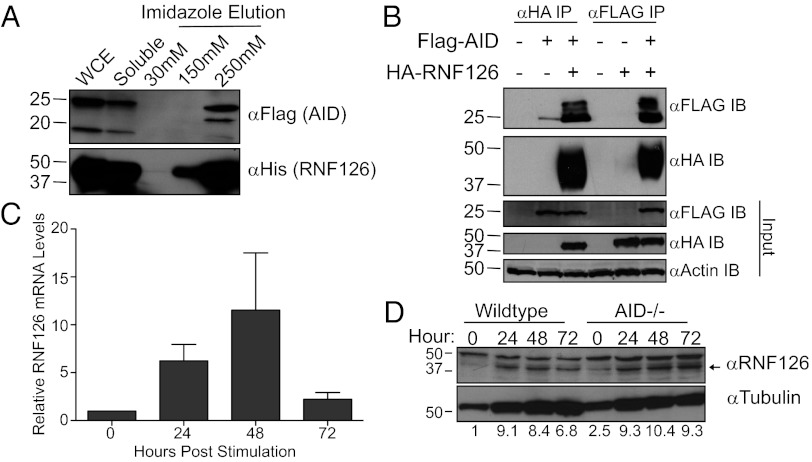
Fig. 3.
RNF126 interacts with AID and its expression is induced in B cells after stimulation. (A) AID copurifies with RNF126 upon coexpression and purification in E. coli. Flag-tagged AID was coexpressed with His-tagged RNF126. Purification of RNF126 on a Talon cobalt column and elution with imidazole reveals that AID coelutes with RNF126. (B) RNF126 interacts with AID in a HEK 293T mammalian system. Flag-tagged AID and HA-tagged RNF126 were coexpressed. Affinity purification of either tag resulted in the purification of the binding partner only when both proteins were expressed. (C) Expression of RNF126 is induced in switching B cells after stimulation for class-switch recombination as assessed by qRT-PCR (normalized to CD19 levels). (D) RNF126 protein levels increase in murine B cells after stimulation to undergo class switch recombination in an AID-independent manner. Arrow denotes RNF126 band (Tubulin: loading control). The values beneath the blot denote a quantification of RNF126 protein levels, normalized to Tubulin.
The majority of the factors identified fall within the category of mRNA transcription, processing, splicing, and export—processes that are thought to be necessary for SHM and CSR. In addition, a significant number of factors fall within the category of DNA repair, a process relevant to CSR. The remaining identified cofactors are either uncharacterized ORFs or factors with known functions but whose involvement in AID-mediated processes is unclear (Fig. 2B and Table S1). Because of the intriguing structural features of one of the unknown genes, RING finger protein 126 (RNF126; Fig. 2C), we chose to further investigate its interaction with AID.
RNF126 Interacts with AID in Bacterial and Mammalian Expression Systems and Is Expressed in Splenic B Cells.
As with the other putative cofactors, we verified that RNF126 could interact with a minimally tagged AID. Indeed, coexpression of a hexa-histidine–tagged RNF126 and a Flag-tagged AID in BL21 E. coli cells, followed by affinity purification on a Talon Cobalt column, demonstrates that AID and RNF126 coelute, suggesting a direct interaction in bacteria (Fig. 3A). Furthermore, size exclusion chromatography of AID expressed alone or with RNF126 reveals that RNF126 coexpression increases AID solubility (Fig. S2). Additional coexpression experiments using truncations of RNF126 and AID/APOBEC2 chimeric proteins suggest specific domain interactions between the two proteins (Fig. S3; ref. 9). Expression of Flag-tagged AID and hemagglutinin (HA)-tagged RNF126 in mammalian 293T cells followed by precipitation on beads conjugated with anti-FLAG or anti-HA antibodies also demonstrates an in-cell interaction. Aside from confirming the bacterial coelution experiments, these co-IP studies in nucleated cells demonstrate that AID and RNF126 coexist in the same cellular compartments (Fig. 3B).
Because AID is primarily expressed in B cells during antibody diversification, it was important to determine whether RNF126 is expressed in a physiological setting alongside AID (7). Using both quantitative RT-PCR and Western blotting, we show that RNF126 is expressed in primary B cells upon stimulation in vitro to undergo CSR (Fig. 3 C and D). Although naïve, unstimulated B cells express RNF126, stimulation increases both RNF126 transcript levels and amounts of protein in a manner that does not depend on the presence of AID or the formation of AID-mediated breaks (Fig. 3D).
RNF126 Ubiquitylates AID.
Although RNF126 is conserved throughout evolution with 62% similarity between human and zebrafish, little is known about its biological role. Three domains exist that are suggestive of function: an N-terminal zinc domain, a C-terminal RING finger domain and an unusual C-terminal stretch of serines (Figs. S3 and S4). The C-terminal serine tail contains a tandem array of ∼12 serines that are conserved from its emergence in zebrafish to human, suggesting an important role in RNF126 biology (Fig. 2C). Upstream of the C-terminal serine stretch lies a C3H2C3-type RING domain, which has been shown to possess E3 ligase activity (22). Interestingly, this domain also interacts with AID residues 88–116 (Fig. S3B). Lastly, the N-terminal Zinc domain shows significant homology (69%) to a ubiquitin binding domain on a related E3 ligase, BCA2 (23).
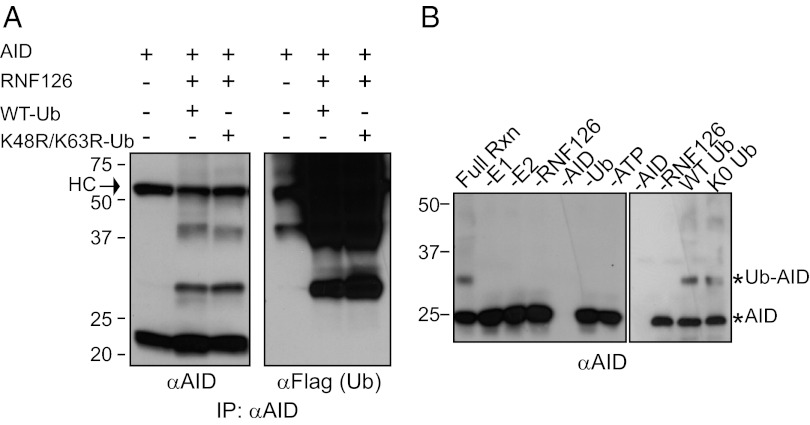
The presence of two ubiquitin-related domains on RNF126 suggests a function in posttranslational modifications. Thus, we asked whether RNF126 not only interacted with, but could also ubiquitylate AID. We used a 293T ubiquitylation assay, in which AID and Flag-ubiquitin are coexpressed in the presence or absence of HA-RNF126. Upon addition of RNF126, AID is ubiquitylated, yielding a reproducible ladder of approximately two to four slower migrating bands that could be representative of either small polyubiquitin chain addition or multiple monoubiquitylation events (Fig. 4A). Polyubiquitin chains can theoretically form at all seven lysines, but linkages at K48 and K63 are most common (24). Expression of the K48R/K63R mutant ubiquitin, which cannot form K48 or K63 polyubiquitin chains, along with AID and RNF126 resulted in a banding pattern similar to that observed with wild-type ubiquitin. This result suggests that the pattern seen is not reflective of polyubiquitylation, but rather multiple monoubiquitylation events (Fig. 4A). This result could also represent the conversion of a single monoubiquitylation event to polyubiquitylation due to E3 ligase overexpression, which has been demonstrated for Mdm2 and p53 (25).
Fig. 4.
RNF126 ubiquitylates AID. (A) AID is ubiquitylated by RNF126 in HEK 293T cells. Coexpression of hAID with HA-tagged RNF126 and Flag-tagged ubiquitin results in the formation of ubiquitylated AID, even in the presence of K48R/K63R mutant ubiquitin. RIPA extracts were prepared and AID immunoprecipitated with an anti-AID antibody. An anti-AID and an anti-FLAG (ubiquitin) blot are shown. The heavy chain band is marked as “HC.” (B) AID is ubiquitylated by RNF126 in vitro. Purified components of the ubiquitin reaction are incubated at 37 °C. (B Left) All components are necessary for ubiquitylation to occur (lane 1). The absence of individual components (lane 2–7) prevents ubiquitylation. (B Right) RNF126 monoubiquitylates AID. Addition of a mutant ubiquitin in which all lysines are mutated to arginine (K0 Ub) shows that the laddering is representative of multiple monoubiquitylation events. An anti-AID immunoblot is shown.
To distinguish between these possibilities, we developed a cell-free ubiquitylation assay and showed that in vitro ubiquitylation of AID also occurred in an RNF126-dependent manner. Further, the reaction depends on all other components of the ubiquitylation cascade, including Ube1 (the ubiquitin activating enzyme or E1), UbcH5b (the ubiquitin conjugating enzyme or E2; Fig. S5), ubiquitin, and energy (ATP) (Fig. 4B). Because the major modified band represents the addition of a single ubiquitin moiety, our data suggests that RNF126 monoubiquitylates AID. We have confirmed this result by using a mutant ubiquitin molecule, which cannot form polyubiquitin chains because all seven lysines are mutated to arginine (Ub K0; Fig. 4B). Finally, E3 ligases are thought to bind their substrate and catalyze the transfer of a Ub moiety from the E2 to the substrate often at a position that is distal from the E3:substrate binding surface. Therefore, our interaction studies cannot a priori suggest the location of the lysine on AID that is modified by RNF126.
RNF126 Selectively Modifies AID.
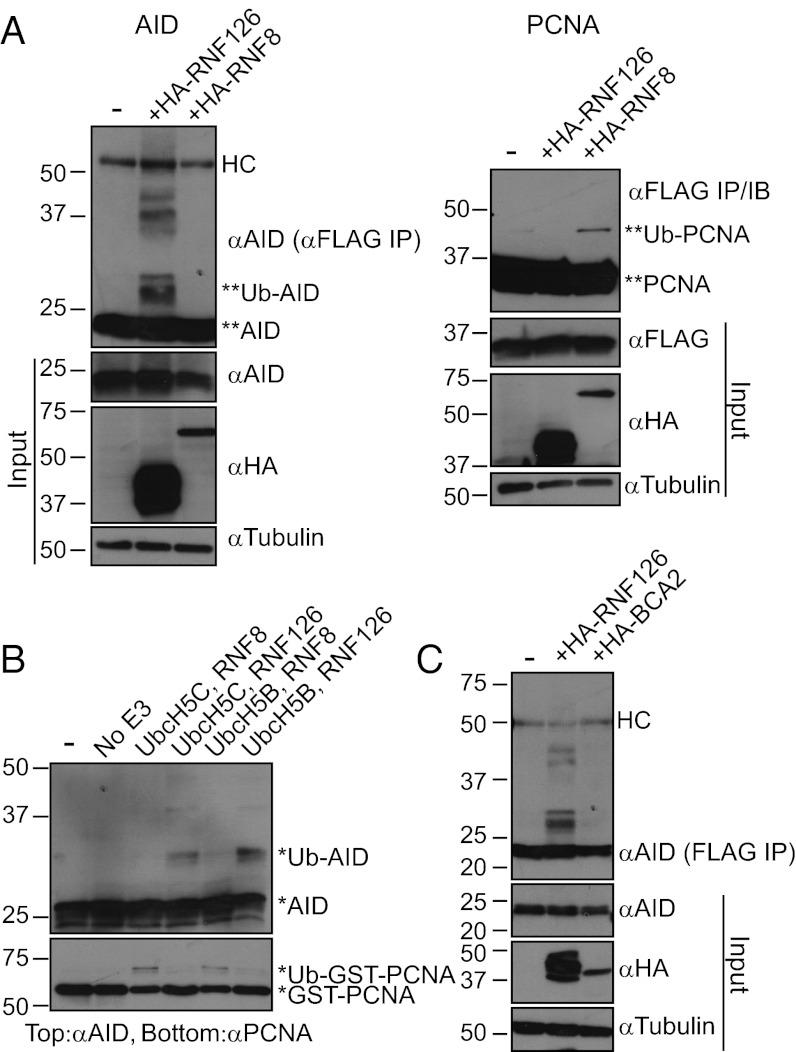
To determine whether RNF126 possesses specificity for AID as a substrate, we have used our ubiquitylation assays to test the ability of other E3 ligases to modify AID. We focused on two ligases: RNF8, which plays distinct roles in CSR (26), and BCA2, a close homolog of RNF126 that is expressed in B cells during CSR. Although coexpression of RNF126 with AID in 293T cells results in the ubiquitylation of AID, coexpression of RNF8 and AID in this setting does not (Fig. 5A, Left). Similarly, RNF126 ubiquitylates AID in vitro when incubated with either UbcH5B, the preferred E2 for RNF126, or UbcH5C, the preferred E2 for RNF8; however, given the same conditions, RNF8 does not (Fig. 5B, Upper). Furthermore, coexpression of BCA2 with AID in HEK 293T cells does not lead to AID ubiquitylation (Fig. 5C).
Fig. 5.
RNF126 selectively modifies AID compared with other B-cell associated E3 ligases. (A) The 293T cells were transfected with Flag-AID, alone or in combination with HA-RNF126 or HA-RNF8 (Left), and Flag-PCNA, alone or in combination with the same ligases (Right). Coexpression of RNF126, but not RNF8, results in AID ubiquitylation. Ubiquitylation of PCNA occurs upon coexpression of RNF8 (lane 3), but not RNF126 (lane 2). Both AID and PCNA were immunoprecipitated with αFlag and blotted with αAID and αFlag, respectively (Top). Western blots of AID (αAID), PCNA (αFlag), RNF126/RNF8 (αHA), and tubulin (αTubulin) of input (whole cell extract) are shown in the bottom three panels. Asterisks mark the unmodified and modified bands of AID and PCNA, and “HC” denotes the heavy chain band. Lane 1 of each image represents the expression of the substrate without exogenous ligase. (B) GST-RNF8 and GST-PCNA were used as an alternate ligase and substrate in the in vitro ubiquitylation assay. Reactions were carried out with either UbcH5b or UbcH5c as the E2 enzyme. In both cases, RNF8 selectively ubiquitylates PCNA and not AID (lanes 3 and 5) and RNF126 selectively ubiquitylates AID and not PCNA (lanes 4 and 6). Lane 1 excludes all components except the substrate and lane 2 only excludes the E3 ligase. Unmodified and modified bands are denoted with an asterisk. (C) BCA2, a homologous E3 ligase, cannot ubiquitylate AID. AID is expressed in 293T cells alone (lane 1) or in the presence of either RNF126 (lane 2) or BCA2 (lane 3). Only coexpression with RNF126, but not BCA2, results in ubiquitylation. The top image represents an αFlag IP and αAID immunoblot. Western blots of AID (αAID), RNF126/BCA2 (αHA), and tubulin (αTubulin) in the input/whole cell extract are shown in the bottom three images. The heavy chain band is marked with “HC.”
Conversely, we wanted to assess the ability of RNF126 to ubiquitylate an alternate substrate involved in the CSR reaction. One such substrate is proliferating cell nuclear antigen (PCNA), which is involved in DNA repair in the context of AID-mediated reactions (27, 28). Monoubiquitylation of PCNA at lysine 164 is thought to recruit the error-prone DNA polymerase, Polη, resulting in the accumulation of mutations at non-C:G base pairs within the variable region and switch regions of the Ig locus (29). Monoubiquitylation of PCNA has been shown to be carried out by at least two different E2/E3 ubiquitin-conjugating enzyme/ligase pairs: Rad6/Rad18 (30, 31) and UbcH5c/RNF8 (32). We compared the ability of RNF8 and RNF126 containing complexes to ubiquitylate PCNA. Whereas we could easily demonstrate that RNF8 had activity toward PCNA both in 293T cells (Fig. 5A, Right) and in vitro (Fig. 5B, Lower), RNF126 appeared inactive in these assays (Fig. 5 A and B).
Although not exhaustive, these experiments establish that RNF126 shows specificity for AID when presented with two substrates known to be present at the Ig locus during CSR and SHM. In addition, two other ligases, one of which has been demonstrated to be present at the Ig locus (RNF8) and one that shows high homology to RNF126 (BCA2), cannot ubiquitylate AID.
Discussion
Popular methods to obtain protein interaction data in a high-throughput fashion include affinity purification followed by mass spectrometric analysis of proteins copurified with the tagged protein of interest and yeast two-hybrid approaches. These approaches are complementary to one another. Affinity-based pull-down methods describe the assembly of multiple proteins that are stably associated with the tagged bait protein, whereas yeast two-hybrid approaches provide information about binary protein–protein interactions. Both of these techniques suffer from a number of limitations, including an inherent inability to detect transient protein–protein interactions.
New protein–protein interaction screens attempt to bypass many of the limitations of these classical approaches. Such screens include, but are not limited to, split molecule complementation (e.g., split GFP; ref. 33), chemical and photo cross-linking followed by specific immunoprecipitation to lock transient interactions in place (34), and the fusion of a bait protein with molecules that can modify and “mark” neighboring proteins (e.g., BirA; ref. 35). None of these methods are particularly reliable for the identification of interactions with a protein that is poorly soluble or insoluble when ectopically expressed. The solubility-based screen we report here specifically targets this latter subset of proteins.
The motivation behind the development of this assay was to identify interacting partners for AID, a potent DNA mutator highly expressed in B lymphocytes (36) yet poorly soluble when expressed in all systems tested. In mature B cells, AID deaminates deoxycytidines at the Ig locus to initiate a cascade of error-prone repair that results in either point mutations (during SHM) or genomic recombination (during CSR). Although AID preferentially targets the Ig locus, loci elsewhere in the genome can be targeted, resulting in point mutations and genomic translocations (37, 38).
Many attempts have been made to identify AID interactors by using either affinity-based purification methods or yeast two-hybrid approaches; as a result, a handful of proteins have been implicated in the AID reaction (reviewed in ref. 8). Using the screen described here, we have successfully identified a number of previously reported AID cofactors: AID, itself, which is thought to be a multimer (39); components of the ssDNA binding complex, RPA, thought to play a role in the recruitment of DNA break repair machinery to the Ig locus (40, 41); and CTNNBL1, a splicing factor (9). Furthermore, the RING domain of Mdm2 can interact with AID (Fig. 1 and ref. 20), but we did not identify full-length Mdm2 in our screen. This result was not surprising because our library does not contain Mdm2 truncations and it is only the RING domain that was shown to interact (20).
The fact that this screening approach has identified known cofactors underscores its validity. More importantly, however, the screen has identified factors not previously known to complex with AID. These include proteins that are likely to play important roles in DNA repair, such as Rad51 and FEN1. In addition, we identified the RRM2 complex, which is responsible for the synthesis of deoxyribonucleotides during DNA repair (42). We have also identified factors with known roles in RNA processing, such as the polyadenylation factor and endonuclease, CPFS73. RNA processing at the Ig locus has increasingly been recognized as a necessary precursor to the completion of CSR and, although the mechanism is still not understood, there likely exists a tight coordination between transcription at the Ig locus, AID-mediated mutation, and double-strand break repair.
We have also identified a number of ubiquitous factors that are potentially very important for the subcellular localization of AID by either mediating cytoplasmic-nuclear trafficking [such as karyopherin (43) and Nup93] or by playing a role in retaining AID in the cytoplasm and excluding it from the nucleus (e.g., TCP1-eta and Bip in analogy to other chaperones; ref. 44). Interestingly, we have also identified another member of the family of ubiquitylation enzymes, UbcH7, although the importance of this enzyme in relation to AID biology has not yet been studied. Finally, we have identified a number of factors of completely unknown function and it will be important to determine their relevance to CSR and SHM in the future.
We have begun to characterize one of these unknown genes, RNF126. We have verified that RNF126 interacts with AID upon expression in bacterial and mammalian systems and that RNF126 is expressed in a physiologically relevant setting for AID biology (Fig. 3). We have also demonstrated that RNF126 can serve as an E3 ligase in a complex that monoubiquitylates AID (Fig. 4) and that other E3 ligases expressed in B cells with specific functions in B-cell biology do not appear to have such activity, arguing for selectivity (Fig. 5). It is tempting to attribute regulatory significance to the interaction between RNF126 and AID given the demonstration by Reynaud et al. (45) that ubiquitylation of AID promotes degradation of nuclear AID; however, functional relevance of the interaction between AID and this E3 ligase must await the generation of RNF126-deficient mice.
A number of roles for RNF126 in the context of AID and antibody diversification can be hypothesized based on sequence similarities of the domains of this E3 ligase with homologous domains of known function. For example, in addition to the RING domain, which interacts with and modifies AID (Figs. S3 and S4), RNF126 contains several intriguing domains. Its N-terminal zinc finger domain is homologous to a recently identified ubiquitin-binding domain (23). The coexistence of the RING and ubiquitin-binding domains within the same protein is reminiscent of the proposed regulation of translesion DNA repair by polη. In this case, ubiquitylation and ubiquitin binding within the same protein results in a closed conformation that prevents an interaction with PCNA (46). Finally, the tandem array of serines at the very C terminus (Fig. 2) is homologous to similar stretches of serines found in transcriptional activators present at RNAPII-dependent promoters. These factors include viral proteins, which bind to general transcription factors to hijack the transcription machinery of the host cell (47), as well as cellular proteins with roles in transcription (48–50). Given the multitude of genetic and proteomic data linking AID to transcription initiation, elucidation of the role of this particular domain of RNF126 in the context of a potential dual interaction with the Ig promoter and AID will be interesting to pursue.
Materials and Methods
Library Screening.
The cDNA library from the Ramos cell line was introduced into a modified pCDF-based destination vector (Fig. 2) by using an LR reaction (Invitrogen) followed by transformation in E. coli BL21ai cells. Transformants were plated on spectinomycin containing LB plates (50 μg/mL) and incubated overnight at 30 °C. These colonies were replica plated on spectinomycin (50 μg/mL), chloramphenicol (60 μg/mL), arabinose (0.02%), and IPTG (0.5 mM) plates that were incubated overnight at 30 °C. Colonies grown under these conditions were picked and grown in liquid culture containing chloramphenicol (120 μg/mL) and spectinomycin (50μg/mL) at 30 °C. Plasmid DNA was prepared and sequenced to determine the identity of the AID-interacting proteins.
HEK 293T Ubiquitylation Assay.
HEK 293T cells were plated in a six-well dish and transfected with 500 ng to 1 μg of pCDNA3.AID/FLAG-AID, 500 ng to 1 μg of pCDNA3.HA-RNF126, and 1 μg of pCDNA3.Flag-Ubiquitin, when indicated, using Lipofectamine 2000 (Invitrogen). Thirty-six to 48 h later, cells were lysed as described (SI Materials and Methods). AID immunoprecipitations were performed by using either 2.5–3.5 μL of anti-AID antibody (Cell Signaling) and protein G-Sepharose beads (Invitrogen), or anti-Flag M2 agarose beads (Sigma). Beads were washed at least three times with lysis buffer and eluted with Laemmli buffer containing 200 mM DTT and boiling. Samples were loaded on a 12.5% (wt/vol) Tris⋅HCl Criterion gel (Bio-Rad), transferred, and blotted with the antibodies indicated.
In Vitro Ubiquitylation Assay.
His-tagged RNF126 was purified as described (SI Materials and Methods). His-tagged Ube1 (E1), His-tagged UbcH5b (E2), and ubiquitin were purchased from BioMol, Lysineless Ubiquitin from Boston Biochem, and hAID from Enzymax. All components were resuspended in ubiquitination buffer (50mM Tris⋅HCl at pH 7.5, 2.5 mM MgCl2, and 1 mM DTT) and incubated at 37 °C for 30 min to 1 h. An equal volume of Laemmli buffer containing 200 mM DTT was added, and the samples boiled before gel loading. Concentrations of components used are as follows: 50 nM Ube1, 500 nM UbcH5b, 300 ng of RNF126, 300 ng of AID, 4 μg of Ubiquitin, 2 mM ATP, in a total volume of 25 μL. Reactions were loaded on a 12.5% (wt/vol) Tris⋅HCl Criterion gel (Bio-Rad), and membranes were blotted with an anti-AID antibody.
Additional experimental methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Reuben Harris (University of Minnesota, Minneapolis, MN) for providing Mdm2 fragment containing expression cassettes. This work was supported by National Institutes of Health Grant AI082329 (National Institute of Allergy and Infectious Diseases) (to F.N.P.) and by graduate fellowships from the National Defense Science and Engineering Foundation and the National Science Foundation (to R.K.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214538110/-/DCSupplemental.
References
- 1.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 2.Ghavidel A, Cagney G, Emili A. A skeleton of the human protein interactome. Cell. 2005;122(6):830–832. doi: 10.1016/j.cell.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Rual J-F, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437(7062):1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- 4.Alber F, et al. Determining the architectures of macromolecular assemblies. Nature. 2007;450(7170):683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- 5.Gavin A-C, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440(7084):631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- 6.Krogan NJ, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440(7084):637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 7.Delker RK, Fugmann SD, Papavasiliou FN. A coming-of-age story: Activation-induced cytidine deaminase turns 10. Nat Immunol. 2009;10(11):1147–1153. doi: 10.1038/ni.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: Induction, targeting and beyond. Nat Rev Immunol. 2012;12(7):517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conticello SG, et al. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol Cell. 2008;31(4):474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Basu U, et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. 2011;144(3):353–363. doi: 10.1016/j.cell.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, et al. 14-3-3 adaptor proteins recruit AID to 5′-AGCT-3′-rich switch regions for class switch recombination. Nat Struct Mol Biol. 2010;17(9):1124–1135. doi: 10.1038/nsmb.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häsler J, Rada C, Neuberger MS. Cytoplasmic activation-induced cytidine deaminase (AID) exists in stoichiometric complex with translation elongation factor 1α (eEF1A) Proc Natl Acad Sci USA. 2011;108(45):18366–18371. doi: 10.1073/pnas.1106729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeevan-Raj BP, et al. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med. 2011;208(8):1649–1660. doi: 10.1084/jem.20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak U, Matthews AJ, Zheng S, Chaudhuri J. The splicing regulator PTBP2 interacts with the cytidine deaminase AID and promotes binding of AID to switch-region DNA. Nat Immunol. 2011;12(2):160–166. doi: 10.1038/ni.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storb U, et al. Targeting of AID to immunoglobulin genes. Adv Exp Med Biol. 2007;596:83–91. doi: 10.1007/0-387-46530-8_8. [DOI] [PubMed] [Google Scholar]
- 16.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302(5653):2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 17.Pavri R, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell. 2010;143(1):122–133. doi: 10.1016/j.cell.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Y, et al. Structure of the cyclomodulin Cif from pathogenic Escherichia coli. J Mol Biol. 2008;384(2):465–477. doi: 10.1016/j.jmb.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio MAT, et al. An adenosine-to-inosine tRNA-editing enzyme that can perform C-to-U deamination of DNA. Proc Natl Acad Sci USA. 2007;104(19):7821–7826. doi: 10.1073/pnas.0702394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDuff DA, Neuberger MS, Harris RS. MDM2 can interact with the C-terminus of AID but it is inessential for antibody diversification in DT40 B cells. Mol Immunol. 2006;43(8):1099–1108. doi: 10.1016/j.molimm.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Sale JE, Neuberger MS. TdT-accessible breaks are scattered over the immunoglobulin V domain in a constitutively hypermutating B cell line. Immunity. 1998;9(6):859–869. doi: 10.1016/s1074-7613(00)80651-2. [DOI] [PubMed] [Google Scholar]
- 22.Burger A, Amemiya Y, Kitching R, Seth AK. Novel RING E3 ubiquitin ligases in breast cancer. Neoplasia. 2006;8(8):689–695. doi: 10.1593/neo.06469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacopulos S, et al. Effects of partner proteins on BCA2 RING ligase activity. BMC Cancer. 2012;12:63. doi: 10.1186/1471-2407-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37(Pt 5):937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 25.Li M, et al. Mono- versus polyubiquitination: Differential control of p53 fate by Mdm2. Science. 2003;302(5652):1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran S, et al. The RNF8/RNF168 ubiquitin ligase cascade facilitates class switch recombination. Proc Natl Acad Sci USA. 2010;107(2):809–814. doi: 10.1073/pnas.0913790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langerak P, Krijger PHL, Heideman MR, van den Berk PCM, Jacobs H. Somatic hypermutation of immunoglobulin genes: Lessons from proliferating cell nuclear antigenK164R mutant mice. Philos Trans R Soc Lond B Biol Sci. 2009;364(1517):621–629. doi: 10.1098/rstb.2008.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roa S, et al. Ubiquitylated PCNA plays a role in somatic hypermutation and class-switch recombination and is required for meiotic progression. Proc Natl Acad Sci USA. 2008;105(42):16248–16253. doi: 10.1073/pnas.0808182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faili A, et al. DNA polymerase eta is involved in hypermutation occurring during immunoglobulin class switch recombination. J Exp Med. 2004;199(2):265–270. doi: 10.1084/jem.20031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419(6903):135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 31.Hibbert RG, Huang A, Boelens R, Sixma TK. E3 ligase Rad18 promotes monoubiquitination rather than ubiquitin chain formation by E2 enzyme Rad6. Proc Natl Acad Sci USA. 2011;108(14):5590–5595. doi: 10.1073/pnas.1017516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, et al. PCNA is ubiquitinated by RNF8. Cell Cycle. 2008;7(21):3399–3404. doi: 10.4161/cc.7.21.6949. [DOI] [PubMed] [Google Scholar]
- 33.Cabantous S, Waldo GS. In vivo and in vitro protein solubility assays using split GFP. Nat Methods. 2006;3(10):845–854. doi: 10.1038/nmeth932. [DOI] [PubMed] [Google Scholar]
- 34.Lowder MA, Appelbaum JS, Hobert EM, Schepartz A. Visualizing protein partnerships in living cells and organisms. Curr Opin Chem Biol. 2011;15(6):781–788. doi: 10.1016/j.cbpa.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196(6):801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102(5):553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 37.Liu M, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451(7180):841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 38.Robbiani DF, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135(6):1028–1038. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conticello SG, Langlois M-A, Neuberger MS. Insights into DNA deaminases. Nat Struct Mol Biol. 2007;14(1):7–9. doi: 10.1038/nsmb0107-7. [DOI] [PubMed] [Google Scholar]
- 40.Vuong BQ, et al. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nat Immunol. 2009;10(4):420–426. doi: 10.1038/ni.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430(7003):992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 42.Niida H, et al. Essential role of Tip60-dependent recruitment of ribonucleotide reductase at DNA damage sites in DNA repair during G1 phase. Genes Dev. 2010;24(4):333–338. doi: 10.1101/gad.1863810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patenaude A-M, Di Noia JM. The mechanisms regulating the subcellular localization of AID. Nucleus. 2010;1(4):325–331. doi: 10.4161/nucl.1.4.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orthwein A, et al. Regulation of activation-induced deaminase stability and antibody gene diversification by Hsp90. J Exp Med. 2010;207(12):2751–2765. doi: 10.1084/jem.20101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoufouchi S, et al. Proteasomal degradation restricts the nuclear lifespan of AID. J Exp Med. 2008;205(6):1357–1368. doi: 10.1084/jem.20070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bienko M, et al. Regulation of translesion synthesis DNA polymerase eta by monoubiquitination. Mol Cell. 2010;37(3):396–407. doi: 10.1016/j.molcel.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 47.Bates PA, DeLuca NA. The polyserine tract of herpes simplex virus ICP4 is required for normal viral gene expression and growth in murine trigeminal ganglia. J Virol. 1998;72(9):7115–7124. doi: 10.1128/jvi.72.9.7115-7124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge H, Zhao Y, Chait BT, Roeder RG. Phosphorylation negatively regulates the function of coactivator PC4. Proc Natl Acad Sci USA. 1994;91(26):12691–12695. doi: 10.1073/pnas.91.26.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge H, Roeder RG. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994;78(3):513–523. doi: 10.1016/0092-8674(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 50.Kretzschmar M, Kaiser K, Lottspeich F, Meisterernst M. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell. 1994;78(3):525–534. doi: 10.1016/0092-8674(94)90429-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.