Abstract
We recently introduced fluorescent false neurotransmitters (FFNs) as optical tracers that enable the visualization of neurotransmitter release at individual presynaptic terminals. Here, we describe a pH-responsive FFN probe, FFN102, which as a polar dopamine transporter substrate selectively labels dopamine cell bodies and dendrites in ventral midbrain and dopaminergic synaptic terminals in dorsal striatum. FFN102 exhibits greater fluorescence emission in neutral than acidic environments, and thus affords a means to optically measure evoked release of synaptic vesicle content into the extracellular space. Simultaneously, FFN102 allows the measurement of individual synaptic terminal activity by following fluorescence loss upon stimulation. Thus, FFN102 enables not only the identification of dopamine cells and their processes in brain tissue, but also the optical measurement of functional parameters including dopamine transporter activity and dopamine release at the level of individual synapses. As such, the development of FFN102 demonstrates that, by bringing together organic chemistry and neuroscience, molecular entities can be generated that match the endogenous transmitters in selectivity and distribution, allowing for the study of both the microanatomy and functional plasticity of the normal and diseased nervous system.
Keywords: dopamine reporter, secretion kinetics, molecular design, multiphoton imaging
Dopamine neurotransmission plays a key role in habit learning, motivation, reward, and motor function (1), and altered dopamine neurotransmission is associated with disorders such as Parkinson’s disease, schizophrenia, and drug addiction (2–4). As a “social” neurotransmitter that overflows relatively long distances beyond its presynaptic terminals, dopamine’s extrasynaptic concentration is principally determined by the combination of exocytotic neurotransmitter release and reuptake by the plasma membrane dopamine transporter (DAT) (5). Psychostimulants, such as cocaine and amphetamine (AMPH), increase extracellular dopamine via interactions with DAT.
Extracellular dopamine concentration, particularly in the striatum where it is present at high levels, has been characterized by microdialysis (6, 7) and rapid electrochemical detection using carbon fiber cyclic voltammetry (8, 9) and amperometry (10). The excellent temporal resolution of the electrochemical methods is well suited for measuring changes in extrasynaptic dopamine concentration associated with neuronal activity. However, these approaches usually measure the release and reuptake of dopamine from large sets of striatal dopamine release sites and lack the spatial resolution required to study synaptic transmission at the level of individual presynaptic terminals.
Optical methods provide vastly improved spatial resolution so that processes by which specific synapses are modulated can be studied. The first group of fluorescent reporters for the study of presynaptic function were the endocytic FM dyes (11), which act as tracers of exocytosis and endocytosis. These labels, however, do not indicate transmitter accumulation or release, but rather the fusion of synaptic vesicle membrane with the plasma membrane. Moreover, the FM dyes label all active presynaptic terminals with recycling synaptic vesicles regardless of the identity of the neurotransmitter. Another approach uses genetically encoded vesicle membrane proteins known as synaptopHluorins (12, 13) that exhibit pH-sensitive fluorescence emission, although these also indicate vesicle membrane fusion rather than neurotransmitter uptake and release.
As a different approach, we recently introduced fluorescent false neurotransmitters (FFNs) as fluorescent tracers of neurotransmitter uptake and release from individual presynaptic terminals (14). In our efforts to develop a second generation of FFNs, we have focused on the design of highly selective, pH-responsive dopamine-mimicking probes (15). Here, we introduce FFN102 as a pH-responsive fluorescent DAT substrate that exhibits high selectivity for dopamine neuronal cell bodies in the midbrain and presynaptic terminals in the dorsal striatum. In addition to the identification of dopamine cells and their processes in acute mouse brain tissue, FFN102 enables the optical measurement of important functional parameters including DAT activity and the modulation of dopamine release within large populations of striatal dopamine terminals.
Results
Photophysical Characterization of FFN102.
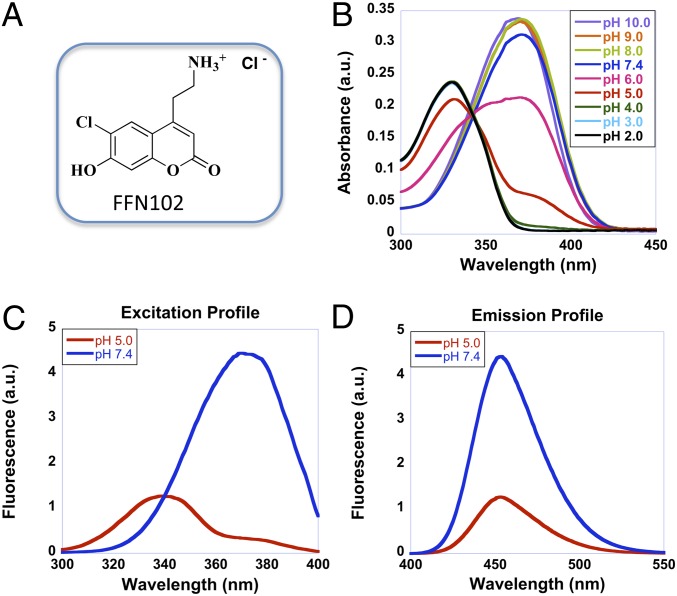
FFN102 (Fig. 1A) was designed as a fluorescent pH reporter responsive at pH values relevant to synaptic vesicle physiology (pH 5–7) (15). Phenols exhibit pH-dependent photophysical properties (light absorption, and in some cases, excitation and emission spectra) due to the equilibrium between the protonated phenol and deprotonated phenolate forms. The phenol group in the 7-position of the coumarin nucleus renders FFN102 ratiometric in the excitation mode with a pKa value of 6.2. The absorption spectra of FFN102 exhibited pH dependence: the absorption maximum shifts toward 331 nm at lower pH values and to 371 nm at higher pH values, corresponding to the protonated and deprotonated forms, respectively (Fig. 1B). As expected, the fluorescence excitation of this probe was also pH dependent, showing a peak at 340 nm at pH 5.0, mimicking the relatively acidic vesicular pH, and a peak at 370 nm at pH 7.4, the approximate pH of the cytoplasm (Fig. 1C). Although the emission wavelength is independent of pH with a maximum at 453 nm, the intensity of emission is highly pH dependent (Fig. 1D). Thus, pH can be measured ratiometrically via optical means using two different excitation wavelengths (15).
Fig. 1.
Structure and photophysical properties of FFN102. (A) Structure of FFN102. (B) Absorption spectra of FFN102 acquired at a range of pH values (2–10). (C) The excitation spectra of FFN102, measured at an emission wavelength of 453 nm, displayed a maximum at 340 nm at pH 5.0 (red), whereas a maximum at 370 nm was observed at pH 7.4 (blue). (D) The emission spectra of FFN102 at both pH 5.0 (red, λex = 340 nm) and pH 7.4 (blue, λex = 370 nm) displayed a maximum at 453 nm.
Pharmacological Characterization of FFN102.
FFN102 was originally identified as a vesicular monoamine transporter 2 (VMAT2) substrate using VMAT2-transfected HEK cells (15). The present imaging studies in mouse brain tissue demonstrate that FFN102 is also a mouse DAT substrate (see below). FFN102 was purposefully designed as a highly polar compound (logD at pH 7.4 is −1.45) to diminish nonselective labeling of tissue and passive membrane diffusion. FFN102 showed no appreciable binding to a broad panel of CNS receptors [38 receptors were screened at 10 μM concentration by the Psychoactive Drug Screening Program (PDSP); Bryan Roth, University of North Carolina, Chapel Hill, NC], including dopamine (D1–5) and serotonin receptors (5HT1–7) (see SI Text for a complete list). FFN102 is therefore unlikely to interfere with synaptic signaling.
FFN102 Labeling of Dopaminergic Axonal Profiles and Presynaptic Terminals in Dorsal Striatum.
We examined the uptake and distribution of the probe in mouse brain striatal slices using two-photon microscopy (Fig. 2). We compared the pattern of FFN102 label with that of green fluorescent protein (GFP) expressed under the control of the tyrosine hydroxylase (TH) promoter (17). A high level of anatomical coincidence between FFN102 and TH-GFP staining would indicate selectivity for dopaminergic axonal profiles.
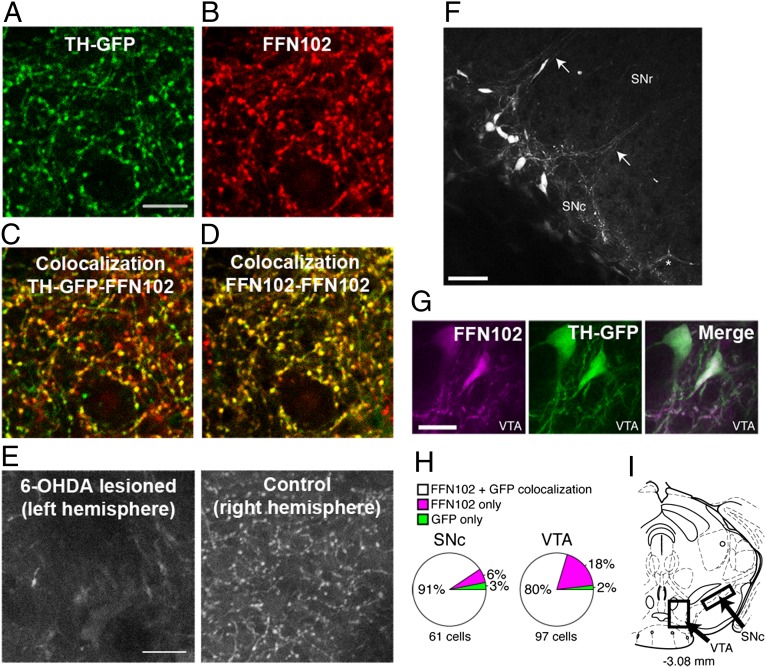
Fig. 2.
FFN102 labels dopaminergic terminals in the dorsal striatum and midbrain neurons of acute mouse brain slices. (A) GFP signal (λex = 910 nm) expressed under the control of the TH promoter. (B) Labeling of FFN102 (λex = 760 nm). (C) Overlap of TH-GFP and FFN102 suggests a high level of colocalization (yellow); 91.1 ± 1.9% (mean ± SEM; n = 3) of puncta labeled with FFN102 were also labeled with TH-GFP. (D) Overlap of two images of FFN102 acquired before and after the TH-GFP image showed 94.8 ± 0.9% (mean ± SEM; n = 3) colocalization. (E) Effect of 6-OHDA lesion on the uptake of FFN102 in the dorsal striatum of acute mouse brain slices. A statistical difference (P < 0.05, t test; n = 3) in the number of labeled terminals was observed in the control hemisphere (Right, 221 ± 10; mean ± SEM) compared with the lesioned hemisphere (Left, 3 ± 2; mean ± SEM). (Scale bar, 10 μm.) (F) FFN102 fluorescent labeling of dopamine neurons in the substantia nigra compacta (SNc) and their dendrites (arrows) projecting into the substantia nigra reticulata (SNr). The asterisk indicates an apparent blood vessel. (Scale bar, 50 μm.) (G) FFN102 accumulation in slices from TH-GFP (λex = 930 nm) mice at the level of the ventral tegmental area (VTA) reveals high degree of FFN102 and GFP colocalization. (Scale bar, 20 μm.) (H) Quantification of FFN102 and GFP colocalization in SNc and VTA illustrates the predominant labeling of dopaminergic neurons. (I) Schematic illustration modified from Franklin and Paxinos (16) showing the approximate regions at which the images were acquired.
Striatal slices from TH-GFP transgenic mice were incubated with FFN102 (10 μM, 30 min) and imaged sequentially at excitation and emission wavelengths corresponding to FFN102 and GFP (FFN102: λexc = 760 nm, λem = 430–470 nm; GFP: λexc = 910 nm, λem = 510–580 nm; the two-photon excitation wavelength used for FFN102 corresponds approximately to the absorption maximum at higher pH values, which is 371 nm for one-photon excitation). The images revealed an extensive overlap (yellow) between GFP (green) (Fig. 2A) and FFN102 labeling (red) (Fig. 2 B and C). We found that 91.1 ± 1.9% (mean ± SEM; n = 3) of the puncta labeled with FFN102 were also labeled with GFP. To account for possible shifts of the field of view in the z plane, the colocalization of two images of FFN102, acquired before and after the GFP channel image, was calculated and determined to be 94.8 ± 0.9% (mean ± SEM; n = 3) (Fig. 2D).
We next investigated FFN102 uptake in slice preparations of mice unilaterally lesioned with the neurotoxin 6-hydroxydopamine (6-OHDA), which selectively destroys catecholaminergic neurons (18) due to the affinity of the neurotoxin for DAT and the norepinephrine plasma membrane transporter (NET) (19). Four weeks after the lesion, striatal slices from the lesioned and nonlesioned hemispheres were incubated with FFN102 as described above. Although 221 ± 10 (mean ± SEM; n = 3) FFN102-positive terminals were present in the nonlesioned hemisphere (right), only 3 ± 2 terminals were identified in the lesioned hemisphere (left) (Fig. 2E). DAT immunolabel confirmed a loss of dopaminergic terminals in the injected hemisphere (Fig. S1). The results thus indicate that FFN102 is selectively accumulated in striatal dopaminergic terminals.
FFN102 Uptake Is Dependent on DAT.
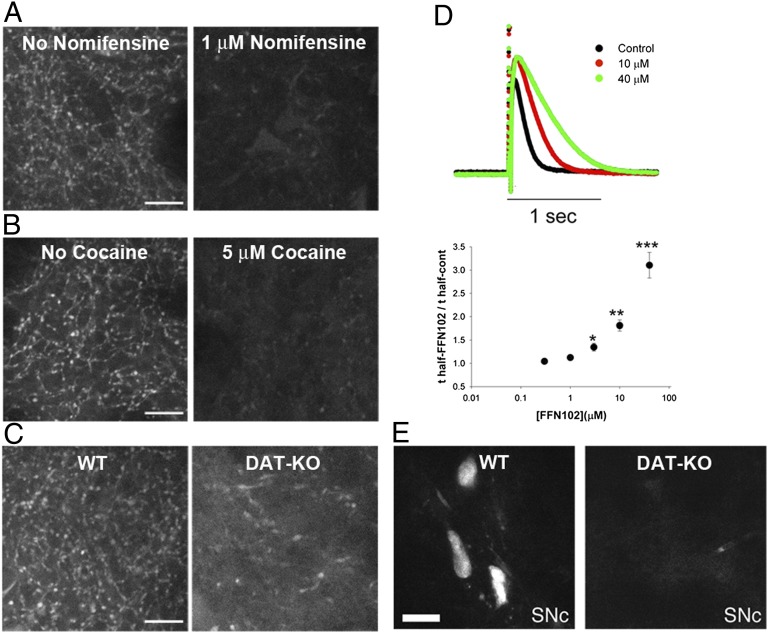
We then examined whether uptake of FFN102 into dopaminergic axonal extensions and terminals was dependent on DAT. Preincubation with the DAT inhibitors (20, 21) nomifensine (1 μM, 10 min) or cocaine (5 μM, 10 min), followed by a 30-min coincubation of FFN102 (10 μM) with the respective uptake blocker resulted in a 15-fold decrease in the number of labeled terminals (Fig. 3 A and B). Slices not exposed to nomifensine displayed 202 ± 9 terminals (mean ± SEM; n = 3) compared with 12 ± 3 terminals observed in slices preincubated with the inhibitor. Similarly, slices not exposed to cocaine displayed 222 ± 15 terminals (mean ± SEM; n = 3), whereas slices pretreated with cocaine showed only 14 ± 2 terminals. Mice with a genetic deletion (“knockout”) of the DAT gene (DAT-KO; kindly provided by Marc Caron, Duke University, Durham, NC) (22) exhibited a striking decrease in uptake of FFN102 compared with wild-type animals (Fig. 3C). Although 217 ± 7 terminals (mean ± SEM; n = 3) were identified in wild-type slices, only 9 ± 3 terminals were present in DAT-KO slices.
Fig. 3.
DAT dependency of FFN102 uptake in the dorsal striatum of acute mouse brain slices. (A) Treatment with 1 μM nomifensine decreased FFN102 uptake (Right, 12 ± 3 puncta; mean ± SEM) compared with untreated slices (Left, 202 ± 9 puncta; mean ± SEM). (B) Treatment with 5 μM cocaine resulted in a similar decreased uptake of FFN102 (Right, 14 ± 2 puncta; mean ± SEM) compared with control slices (Left, 222 ± 15 puncta; mean ± SEM). (C) FFN102 uptake was significantly reduced in the absence of DAT, as seen by the higher number of fluorescent puncta observed in wild-type slices (WT) (Left, 217 ± 7; mean ± SEM), compared with slices from DAT-deficient mice (DAT-KO; Right, 9 ± 3; mean ± SEM). Values obtained for treated and untreated slices (A and B), as well as those obtained for WT and DAT-KO (C), were statistically different (P < 0.05, t test, n = 3). (Scale bar, 10 μm.) (D) FFN102 concentrations of 4–40 µM inhibited the reuptake of dopamine released by electrical stimulation, as measured by cyclic voltammetry in the dorsal striatum (P < 0.05, two-tailed Student t test). The height of the signal mostly represents dopamine release, whereas the decay is mainly dependent on DAT-mediated reuptake. (E) FFN102-positive cells were found in the midbrain (SNc shown) of WT (Left) but not DAT-deficient (Right) mice (n = 3). (Scale bar, 20 µm.)
As an independent means to test whether FFN102 is a DAT substrate, we examined the effect of FFN102 on dopamine reuptake during activity-dependent dopamine release using cyclic voltammetry. As also seen with established DAT blockers (23–25), FFN102 prolonged the decay of the evoked dopamine signal at concentrations of 4 µM and above (Fig. 3D) (P < 0.05, two-tailed Student t test). The affinity of FFN102 for DAT can be estimated by making an assumption that it interacts competitively with endogenous DA. We previously determined, using a random walk simulation of amperometric and cyclic voltammetry signals, that the DA Km-apparent in the striatal slice is ∼0.8 µM and is shifted to larger values by the competitive inhibitors AMPH and nomifensine (25). Similarly, we found that increasing concentrations of FFN102 progressively shifted the Km-apparent for DA clearance. Ten micromolar FFN102 yielded a shift of Km-apparent to ∼3 µM, consistent with an FFN102 affinity toward DAT of ∼4.2 µM: FFN102 thus is a weaker DAT blocker than AMPH or nomifensine (25). In uptake assays using cloned human DAT expressed in HEK293 cells (PDSP), FFN102 inhibited uptake, although its affinity was lower than 10 µM (n = 4) (SI Text). Differences in the affinity estimates may be due to differences between human and mouse DAT or between the assay systems. The ability of FFN102 to function as a substrate for NET was not investigated in the slice preparation (hNET binding assay was negative) (SI Text).
In conclusion, FFN102 is a mouse DAT substrate, as shown by its DAT-dependent uptake and its ability to inhibit DA uptake.
FFN102 Labeling of Dopaminergic Cell Bodies and Dendrites.
We analyzed FFN102 uptake in acute midbrain slices comprising the substantia nigra (SN) pars compacta and reticulata (Fig. 2 F, H, and I) and the ventral tegmental area (VTA) (Fig. 2 G–I). FFN102 (10 μM; 30–45 min) was accumulated into SN pars compacta and VTA dopamine cell bodies as well as both proximal and distal dendrites (Fig. 2 F and G). We occasionally observed FFN102 labeling of structures that are likely to be blood vessels, based on their anatomical characteristics (Fig. 2F). The vast majority of the cells in the SN pars compacta and VTA labeled with FFN102 were positive for GFP in the TH-GFP mouse line (Fig. 2 G and H), confirming that FFN102 was selectively accumulated into dopaminergic neurons in these brain regions. Somatodendritic FFN102 accumulation in the midbrain was due to uptake via DAT, as DAT-deficient mice had no FFN102-labeled cells (Fig. 3E).
Thus, FFN102 shows high selectivity for nigrostriatal and mesocorticolimbic dopamine neurons, which is primarily ascribed to its ability to function as a DAT substrate. Moreover, the mostly homogeneous distribution of FFN102 throughout the cell bodies, dendrites, and projection field terminals of midbrain dopamine neurons suggests that the localization of this probe is not restricted to synaptic vesicles and is also present in the cell cytoplasm.
Release of FFN102 by AMPH.
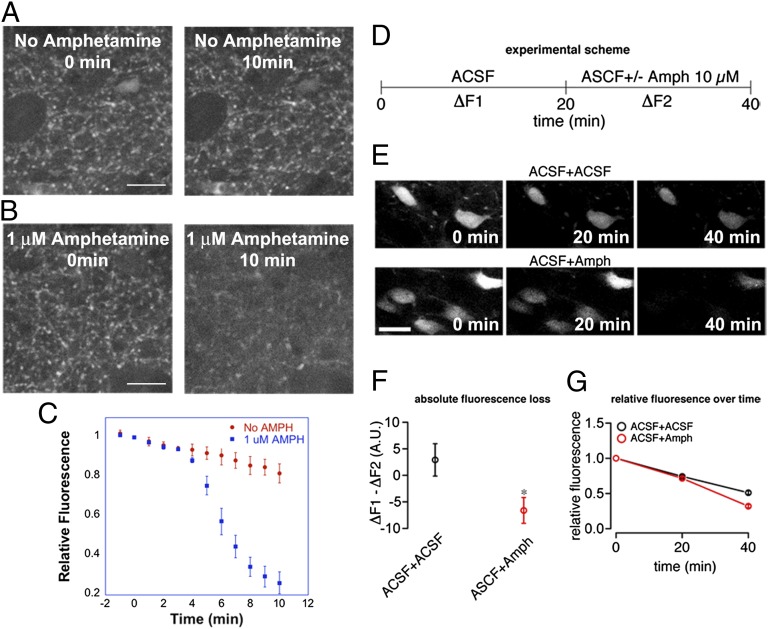
AMPH releases dopamine from both cytoplasmic and synaptic vesicle pools (23, 26, 27) due to its role as a substrate for both DAT and VMAT2, as well as a weak base collapsing the interior acidic pH gradients across synaptic vesicle membranes. We observed a substantial loss of striatal axonal FFN102 fluorescence after perfusion with 1 μM AMPH, whereas in the absence of AMPH, fluorescence of FFN102 decreased at a much slower rate over the same time period (Fig. 4 A–C). We similarly observed relatively slow but appreciable destaining of somatodendritic label in control slices (Fig. 4 D–G), with a time-dependent decrease in the rate of fluorescence loss, yielding a positive value for the subtraction between ΔF1 and ΔF2, the first and second 20-min imaging periods (see detailed definition in legend of Fig. 4 D–F). In AMPH-treated slices, the rate of fluorescence loss was increased upon AMPH application during ΔF2, yielding a negative value for ΔF1 − ΔF2 (Fig. 4F) that differed significantly from control slices (P < 0.05, two-tailed t test; Fig. 4 F and G). These data suggest that FFN102 is spontaneously released from dopaminergic neurons and that AMPH increases the rate of release. Thus, FFN102 could provide a means to optically examine the mechanisms involved in AMPH-induced dopamine release.
Fig. 4.
AMPH induces release of FFN102 from dorsal striatum and midbrain. (A and B) Images of an acute striatal slice loaded with FFN102 taken 0 and 10 min after perfusion with ACSF containing no AMPH (A) or 1 µM AMPH (B). (C) Quantification of FFN102 puncta fluorescence loss in the absence and presence of 1 μM AMPH, normalized to the intensity at time 0 (expressed as mean ± SEM; n = 3). (D) Schematic illustration of midbrain AMPH-induced FFN102 release experiments. FFN102-loaded midbrain slices were perfused with ACSF for 15 min in the imaging chamber and images were acquired 0, 20, and 40 min thereafter. One-half of the slices were perfused with ACSF containing 10 µM AMPH during the 20- to 40-min period, whereas the other half was perfused with regular ACSF throughout the entire experiment. (E) Representative images of FFN102-filled SNc DA neurons acquired after 0, 20, and 40 min of ACSF (Upper) or ACSF plus 10 μM AMPH (Lower) perfusion. (F) Quantification of fluorescence intensity shows a decreased rate of fluorescence loss between the 20- and 40-min time points (ΔF2) compared with the 0- to 20-min period (ΔF1) in control slices, but increased rate in AMPH-treated slices. (G) Fluorescence over time after normalization. Note the more rapid decrease in fluorescence in the presence of AMPH. *P < 0.05 compared with control slices (t test; n = 28–29 cells from 6 slices per treatment). For F and G, results are displayed as mean ± SEM. (Scale bar: A and B, 10 µm; E, 20 µm.)
Activity-Dependent Release of FFN102.
Exocytotic release of dopamine from synaptic vesicles can be evoked by neuronal depolarization with a high concentration of potassium or by electrical stimulation. Perfusion of FFN102-labeled slices with artificial cerebrospinal fluid (ACSF) containing 40 mM KCl for 5 min resulted in the loss of fluorescent signal (760-nm excitation) from presynaptic terminals and an overall increase in background fluorescence (Fig. S2B). The increased background is expected from the effect of pH on FFN102’s signal, as upon exocytosis, FFN102 would be redistributed from the acidic environment of the synaptic vesicle lumen to the neutral extracellular space, resulting in increased fluorescence at 760 nm. The release of FFN102 in response to K+ depolarization was blocked by cadmium chloride (CdCl2) (200 μM), a calcium channel blocker (Fig. S2C), whereas the loss of FFN102 in the presence of KCl and CdCl2 was not different from that observed under continuous ACSF perfusion in the same time period (Fig. S2 A and C). These results demonstrate that FFN102 is released in response to depolarization in a Ca2+-dependent manner.
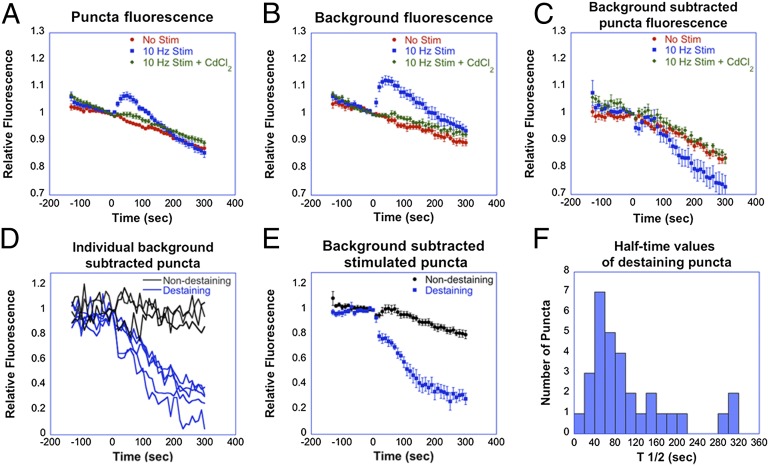
We then investigated the exocytotic release of FFN102 using local electrical stimulation to control the frequency and number of pulses applied. Slices were imaged for 140 s without stimulation, and for an additional 300 s period during which a 10-Hz stimulus train was locally applied with a bipolar electrode. Within 10–40 s of the onset of stimulation, an increase in fluorescence intensity measured at the puncta was observed (Fig. 5A). An even greater relative increase was observed in areas that excluded the puncta (“background”), indicating that this fluorescence increase was due to diffusion of the probe from presynaptic release sites (Fig. 5B). No significant increase in puncta or background fluorescence was observed when voltage-gated calcium channels were blocked by 200 μM CdCl2 or in the absence of stimulation. Because the fluorescence emitted by the probe present in the extracellular space is likely to contribute to the overall fluorescence intensity measured at the puncta, due to both light scattering and insufficient z resolution of two-photon microscopy, we subtracted average background fluorescence values from the total fluorescence signals measured at each punctum (Fig. 5C). The background subtraction removed the increase in fluorescence measured at fluorescent puncta in response to stimulation (Fig. 5C). These results indicate that the increased fluorescence upon stimulation is mainly due to an increase in background fluorescence values, reflecting the release of reporter from acidic synaptic vesicles into the neutral extracellular space where the fluorescence intensity of FFN102 at 760-nm excitation is enhanced.
Fig. 5.
Calcium-dependent release of FFN102 in the dorsal striatum of acute mouse brain slices in response to electrical stimulation. (A–C) Normalized mean fluorescence intensity of fluorescent puncta (A), regions that do not include the puncta, i.e., “background” (B), and fluorescent puncta after background subtraction (C). Mean values are shown for unstimulated slices (red) and slices stimulated at 10 Hz in the absence (blue) or presence of 200 µM CdCl2 (green). Fluorescence intensities for each curve were normalized to the corresponding intensity values at the time point before the onset of stimulation (t = 0). Each point represents a mean ± SEM (n = 5). (D) Relative fluorescence over time of representative individual destaining (blue) and nondestaining terminals (black). For calculation of half-time (t1/2) values, each curve was fit with a one-phase exponential decay function. For the exponential curves shown, t1/2 values were calculated to be 59.5, 76.5, 100.4, 146.3, and 310.3, with R2 values of 0.89, 0.97, 0.92, 0.93, and 0.95, respectively. (E) Relative signal over time of destaining (blue) and nondestaining puncta (black), after background subtraction, in slices stimulated at 10 Hz. Each curve represents the mean ± SEM (n = 5). For A–E, stimulation was initiated at time 0 s. (F) Histogram of t1/2 values of individual FFN102-labeled terminals that destain under 10-Hz stimulation. (Bin size, 20 s.)
Background subtraction also uncovered a difference (P < 0.001, ANOVA) in the rate of puncta fluorescence loss between stimulated and nonstimulated slices, indicating that stimulation-induced destaining of fluorescent puncta had occurred (Fig. 5C). Upon examination of background-subtracted curves from individual fluorescent puncta, we found that stimulated slices exhibited both destaining and nondestaining puncta within the same field of view. Representative examples of destaining and nondestaining curves are shown in Fig. 5D. This finding implies that averaging the fluorescence intensities of all puncta, as shown in Fig. 5C, underestimates the fluorescence loss of destaining puncta elicited by electrical stimulation. In an effort to better represent the different responses of FFN102 puncta, we grouped the destaining and nondestaining puncta into separate cohorts and produced average intensity plots depicted in Fig. 5E (see also Fig. S3). Destaining puncta responded to stimulation with an exponential decay of fluorescence (Fig. 5 D and E) with a mean destaining half-time (t1/2) of 88.0 ± 9.4 s (mean ± SEM). The distribution of t1/2 values obtained for all individual destaining puncta is shown as a histogram in Fig. 5F. A small fraction of destaining puncta in stimulated slices appeared to show either a transient increase in fluorescence intensity followed by destaining or a lag period between the onset of stimulation and destaining. These kinetics, which may reflect for example incomplete background subtraction (SI Text), did not follow a clear exponential decay upon stimulation, and, therefore, these puncta were not included in the analysis or the average data presented in Fig. 5E. All puncta considered as destaining presented R2 exponential fitting values of 0.6 or higher (SI Text). Puncta considered as nondestaining displayed the same slow loss of signal as puncta from nonstimulated controls or from slices exposed to 10-Hz stimulation in the presence of CdCl2. The time course observed for the transient increase in background fluorescence mentioned above approximately matched the kinetics of fluorescence loss of the destaining puncta, which is consistent with an activity-dependent exocytotic release of FFN102. Our results demonstrate that FFN102 enables optical detection of the overall release of synaptic content into the extracellular space as well as examination of release kinetics of individual presynaptic terminals in striatal slices.
Discussion
We recently introduced FFNs as optical probes to visualize neurotransmission at the level of individual presynaptic terminals, as well as pH-dependent FFNs that can be used to determine pH of VMAT1-containing vesicles in PC12 cells (14, 15). FFN102 is to date the most selective probe for dopamine presynaptic terminals in mouse acute brain tissue and can be used to visualize DAT activity. FFN102 can in addition be used for functional studies in somatodendritic regions of dopamine neurons as well as to address synaptic activity of individual dopaminergic terminals. Furthermore, in contrast to the first generation of FFNs that only permitted measuring the levels of FFN that remain in presynaptic terminals, the reporter FFN102 provides an approach to observe both the FFN that is released into the extracellular milieu and the label remaining within individual terminals.
The high selectivity of this probe in the brain slice can be attributed to its ability to function as a DAT substrate and to its high polarity. Moreover, the lack of interaction of FFN102 with a broad panel of CNS receptors bodes well for the use of FFN102 to study presynaptic activity and plasticity, diminishing potential concerns in data interpretation due to modulation of neurotransmitter receptors. Although FFN102 accumulation in striatal slices was clearly DAT dependent, we were unable to observe FFN102 uptake in dopamine cell culture, possibly due to lower DAT or VMAT2 levels in cultured postnatal neurons.
Other fluorescent monoamine transporter substrates have been developed to visualize multiple aspects of monoamine neuron function. For example, the fluorescent serotonin (5HT) analog 5,7-dihydroxytryptamine (5,7-dHT) can identify 5HT and dopamine neurons in culture due to its uptake by serotonin transporter (SERT) and DAT, respectively. 5,7-dHT has recently been applied to study somatic release of 5HT from serotonergic neurons in brain slice preparations (28). However, this probe is weakly fluorescent, prone to oxidation and toxic. Furthermore, 5,7-dHT vesicular uptake requires long incubation times (3 h) in the presence of high concentration of monoamine oxidase inhibitors, which renders this probe less suited for physiological studies of synaptic plasticity in brain tissue. In contrast, FFN102 exhibits rapid uptake (within 30 min) and is chemically and photochemically stable, highly fluorescent, and produced no apparent toxicity in in vitro and in situ studies. These properties render this agent suitable for time-lapse two-photon microscopy imaging of DAT function and neurotransmitter release. Similarly, NET activity at murine sympathetic terminals has been measured optically using a fluorescent NET/DAT/SERT substrate present in the commercially available NTUA systems (neurotransmitter transporter uptake assay) (29). However, it is currently unknown whether this system can be used for studies of synaptic activity in brain tissue.
The loss of striatal terminal FFN102 elicited by AMPH is reminiscent of the effect of this psychostimulant in the redistribution of endogenous dopamine. However, AMPH-induced release of dopamine and FFN102 proceeds with different time courses. Cyclic voltammetry recordings indicate that AMPH-induced dopamine release reaches a plateau after 30 min (23). In contrast, we observed an almost complete release of FFN102 in response to AMPH within 10 min. Interestingly, AMPH-induced dopamine efflux becomes faster upon VMAT2 inhibition (23), which likely reflects the time course of reverse transport of cytoplasmic dopamine. The slower release measured in the absence of VMAT2 inhibitors is probably due to the redistribution of vesicular dopamine to the cytosol that precedes efflux via reverse transport through DAT. We thus hypothesize that a significant fraction of FFN102 is localized in the cytosol. This is further supported by the high correlation of FFN102 and TH-GFP labeling (Fig. 2) including both apparent presynaptic terminals and axonal regions that do not include clear puncta (Fig. S4), and also by the apparently homogeneous distribution of FFN102 within cell bodies and dendrites (Fig. 2).
Ca2+-dependent release of FFN102 elicited by high-K+ or electrical stimulation was detected in striatal terminals as (i) an overall increase in background signal, reflecting the release of FFN102 during vesicle fusion from acidic synaptic vesicles to the neutral extracellular space and (ii) a decrease in fluorescence of individual puncta. Analysis of the response of individual fluorescent puncta to a 10-Hz stimulation train revealed the presence of destaining and nondestaining puncta. The background-subtracted fluorescence intensity of destaining puncta exhibited an exponential decay upon the onset of stimulation with a mean t1/2 of 88.0 ± 9.4 s. Nondestaining puncta may either represent axonal areas that do not contain synaptic vesicles but contain FFN102 in the cytosol or dopaminergic terminals that do not undergo detectable synaptic vesicle exocytosis in response to stimulation. Further studies combining the use of FFNs and probes that measure vesicle fusion are underway to address this issue.
In conclusion, FFN102 is a bright fluorescent probe compatible with a range of red and green fluorescent markers (e.g., GFP, FM dyes) that exhibits high selectivity toward dopamine neurons. For this reason, FFN102 is a promising tool to visualize neuronal degeneration in the 6-OHDA and other Parkinson’s disease models and to study important functional parameters such as DAT activity, somatodendritic dopamine dynamics, dopamine exocytosis at individual presynaptic terminals, and mechanisms underlying presynaptic plasticity and the action of drugs of abuse.
Methods
Slice Preparation.
Unless otherwise noted, all animals used for slice preparation were 2- to 4-month-old male C57BL/6 mice obtained from The Jackson Laboratory. All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University.
For striatal slice preparation, mice were decapitated and acute 300-μm-thick coronal slices were cut on a vibratome and allowed to recover for 1 h before use at room temperature in oxygenated [95% O2, 5% CO2 (vol/vol)] ACSF containing the following (in mM): 125 NaCl, 2.5 KCl, 26 NaHCO3, 0.3 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 0.8 NaH2PO4, 10 glucose (pH 7.2–7.4, 292–296 mOsm/L).
For coronal midbrain slice preparation, brains were dissected and immersed in 4 °C cutting solution containing the following (in mM): 180 sucrose, 10 NaCl, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 10 glucose (pH 7.3, 300–305 mOsm/L). Coronal sections (250 µm) were cut through the midbrain and equilibrated for 30 min in 32 °C ACSF. Slices were then transferred to room temperature (22–25 °C) for 30 min before use.
Loading and Imaging of FFN102.
FFN102 (10 μM) (available from Abcam Biochemical) was loaded into presynaptic terminals by a 30- to 45-min incubation at room temperature in oxygenated ACSF. Slices were then transferred to an imaging chamber (QE-1; Warner Instruments), held in place with a platinum wire and nylon custom-made holder (30), and superfused (1–3 mL/min) with oxygenated ACSF. Slices were allowed to wash in the perfusing chamber for 5–10 min before imaging.
Unless otherwise noted, dopaminergic terminals and dopamine cell bodies were visualized at >30-µm depth in the slice using either a Prairie Ultima Multiphoton Microscopy System (Prairie Technologies) or a Leica DM6000 with titanium–sapphire MaiTai lasers (Spectra-Physics) equipped with either 60×, 0.9 N.A., or 40×, 0.8 N.A. water-immersion objectives. FFN102 was excited at 760 nm and visualized using an emission range of either 440–500 nm (Prairie) or 430–470 nm (Leica).
Please refer to SI Text for methodology regarding FFN102 photophysical characterization, 6-OHDA injections, cyclic voltammetry recordings, FFN102-GFP colocalization experiments, effects of drugs, KCl, and electrical stimulation on FFN102 loading and/or destaining, and image processing and analysis.
Supplementary Material
Acknowledgments
We thank Paolomi Merchant and Matthew Dunn for experimental support, the National Institute of Mental Health (NIMH) Psychoactive Drug Screening Program (University of North Carolina, Chapel Hill) for pharmacological characterization, Marc Caron (Duke University) for providing the DAT knockout mice, and Eugene Mosharov (Columbia University) for ImageJ macros. This work was supported by The G. Harold and Leila Y. Mathers Charitable Foundation, NIMH Grants R01MH086545 and R21 MH090356, the JPB Foundation, the McKnight Foundation, the Parkinson’s Disease Foundation, National Institute on Drug Abuse Grant R01DA07418, and National Institute of Neurological Disorders and Stroke Udall Center of Excellence for Parkinson’s Disease Research. P.C.R. was supported by the National Science Foundation predoctoral fellowship. A.B. was supported by fellowships from the Swedish Research Council and the Sweden–America Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213569110/-/DCSupplemental.
References
- 1.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 2.Verhoeff NP. Radiotracer imaging of dopaminergic transmission in neuropsychiatric disorders. Psychopharmacology (Berl) 1999;147(3):217–249. doi: 10.1007/s002130051163. [DOI] [PubMed] [Google Scholar]
- 3.Nikolaus S, Antke C, Müller H-W. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav Brain Res. 2009;204(1):1–31. doi: 10.1016/j.bbr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Nikolaus S, Antke C, Müller H-W. In vivo imaging of synaptic function in the central nervous system: II. Mental and affective disorders. Behav Brain Res. 2009;204(1):32–66. doi: 10.1016/j.bbr.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Sotnikova TD, Beaulieu J-M, Gainetdinov RR, Caron MG. Molecular biology, pharmacology and functional role of the plasma membrane dopamine transporter. CNS Neurol Disord Drug Targets. 2006;5(1):45–56. doi: 10.2174/187152706784111579. [DOI] [PubMed] [Google Scholar]
- 6.Khan SH, Shuaib A. The technique of intracerebral microdialysis. Methods. 2001;23(1):3–9. doi: 10.1006/meth.2000.1101. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M-Y, Beyer CE. Measurement of neurotransmitters from extracellular fluid in brain by in vivo microdialysis and chromatography-mass spectrometry. J Pharm Biomed Anal. 2006;40(3):492–499. doi: 10.1016/j.jpba.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Stamford JA. In vivo voltammetry—prospects for the next decade. Trends Neurosci. 1989;12(10):407–412. doi: 10.1016/0166-2236(89)90081-7. [DOI] [PubMed] [Google Scholar]
- 9.Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49(10):1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 10.Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat Methods. 2005;2(9):651–658. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- 11.Gaffield MA, Betz WJ. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat Protoc. 2006;1(6):2916–2921. doi: 10.1038/nprot.2006.476. [DOI] [PubMed] [Google Scholar]
- 12.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394(6689):192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, et al. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc Natl Acad Sci USA. 2005;102(17):6131–6136. doi: 10.1073/pnas.0501145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubernator NG, et al. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science. 2009;324(5933):1441–1444. doi: 10.1126/science.1172278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee M, Gubernator NG, Sulzer D, Sames D. Development of pH-responsive fluorescent false neurotransmitters. J Am Chem Soc. 2010;132(26):8828–8830. doi: 10.1021/ja101740k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 17.Sawamoto K, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci USA. 2001;98(11):6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson G, Sachs C. Uptake and accumulation of 3H-6-hydroxydopamine in adrenergic nerves. Eur J Pharmacol. 1971;16(1):55–62. doi: 10.1016/0014-2999(71)90056-2. [DOI] [PubMed] [Google Scholar]
- 20.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: Structure, regulation and function. Nat Rev Neurosci. 2003;4(1):13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 21.Brogden RN, Heel RC, Speight TM, Avery GS. Nomifensine: A review of its pharmacological properties and therapeutic efficacy in depressive illness. Drugs. 1979;18(1):1–24. doi: 10.2165/00003495-197918010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 23.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87(2):273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: Effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21(16):5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Sulzer D, et al. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15(5 Pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colgan LA, Putzier I, Levitan ES. Activity-dependent vesicular monoamine transporter-mediated depletion of the nucleus supports somatic release by serotonin neurons. J Neurosci. 2009;29(50):15878–15887. doi: 10.1523/JNEUROSCI.4210-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker LK, Shanks JA, Kennard JA, Brain KL. Dynamic monitoring of NET activity in mature murine sympathetic terminals using a fluorescent substrate. Br J Pharmacol. 2010;159(4):797–807. doi: 10.1111/j.1476-5381.2009.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong MY, Sulzer D, Bamford NS. Imaging presynaptic exocytosis in corticostriatal slices. Methods Mol Biol. 2011;793:363–376. doi: 10.1007/978-1-61779-328-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.