Abstract
Background
Although risk factors for periprosthetic joint infection (PJI) and mortality after total hip arthroplasty (THA) have been identified, interactions between specific patient risk factors are poorly understood. Therefore, it is difficult for surgeons to counsel patients on their individual risk of PJI or mortality after THA.
Questions/purposes
We evaluated the interaction between patient clinical and demographic factors on the risk of PJI and mortality after THA and developed an electronic risk calculator for estimating the patient-specific risk of PJI and mortality in Medicare patients with THA.
Methods
We used the Medicare 5% sample claims database to calculate the risk of PJI within 2 years and mortality within 90 days after THA in 53,252 Medicare patients with primary THAs between 1998 and 2009. Logistic regression using 29 comorbid conditions, age, sex, race, and socioeconomic status were used as inputs to develop an electronic risk calculator to estimate patient-specific risk of PJI and mortality after THA.
Results
The overall 2-year risk of PJI and 90-day risk of mortality after primary THA were 2.07% and 1.30%, respectively. White women aged 70 to 74 years with alcohol abuse, depression, electrolyte disorder, peptic ulcer disease, urinary tract infection, rheumatologic disease, preoperative anemia, cardiopulmonary (cardiac arrhythmia, congestive heart failure, ischemic heart disease, chronic pulmonary disease) comorbidities, and peripheral vascular disease were at highest risk for PJI. White women aged 65 to 69 years with electrolyte disorder, hemiplegia/paraplegia, hypertension, hypothyroidism, metastatic tumor, preoperative anemia, coagulopathy, cardiopulmonary (congestive heart failure, chronic pulmonary disease) and psychiatric (psychoses, depression) comorbidities, malignancies, and peripheral vascular disease were at highest risk for mortality. An electronic risk calculator was developed to estimate the risk of PJI and mortality in Medicare patients with THA.
Conclusions
This electronic risk calculator can be used to counsel Medicare patients regarding their patient-specific risks of PJI and mortality after THA.
Level of Evidence
Level II, prognostic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Despite the widely reported success of THA in improving quality of life and function in patients with disabling hip disease [7, 10, 14], devastating complications such as periprosthetic joint infection (PJI) and death can and do occur. Although individual patient risk factors for PJI and mortality after THA have been identified, including advanced age, congestive heart failure, and diabetes mellitus [8, 13], the interactions between and synergistic effect of those risk factors have not been previously studied. Therefore, the patient-specific risk of PJI or mortality for individual patients who are considering THA is poorly understood.
When discussing risks of surgery with patients who are considering THA, surgeons often cite average rates for risk of PJI (0.59%–2%) [1, 12] and mortality (0.3%–1.0%) [3, 9, 11]. However, these rates reflect the average risk for a diverse population of patients with THAs and may not be accurate for individual patients, depending on their specific demographic (eg, age, sex, socioeconomic status [SES]) or clinical (eg, comorbid conditions) characteristics. Having a more accurate assessment of a patient’s own unique risk of PJI or mortality after THA would help inform shared medical decisions between patients and their surgeons regarding elective surgery, particularly for high-risk patients.
The increasing use of applications (apps), smartphones, and other mobile technology by orthopaedic surgeons has created opportunities for surgeons to use these tools in their clinical practice [4]. We therefore developed an easily accessible electronic risk calculator that can be used to counsel patients regarding their individual risks of PJI and mortality after THA.
Patients and Methods
We used the Medicare 5% sample claims database to calculate the risk of PJI within 2 years and the risk of mortality within 90 days after primary THA as a function of clinical and demographic characteristics in 53,252 Medicare patients who underwent primary THAs between 1998 and 2009. The Medicare 5% sample includes administrative claims from inpatient, outpatient (including physician office-based claims), carrier, skilled nursing facility, hospice care, home health, and durable medical equipment analytic data files. During the duration that a patient is enrolled in Medicare, all the claims associated with the patient are collected in the database. Because each enrollee is assigned a unique, encrypted Medicare beneficiary identifier, this was used to follow each patient longitudinally.
Patients’ Medicare enrollment status and mortality were tracked using a linked denominator file provided by the Centers for Medicare & Medicaid Services that accompanied the analytic data sets. In addition to diagnosis and procedure codes, variables in the Medicare dataset include patient factors (age, race, sex, Medicare buy-in status [public assistance]), US census region, and hospital characteristics (urban versus rural location, teaching status, bed size, ownership).
Logistic regression using 29 comorbid conditions, age, sex, race, and SES were used as inputs to develop an electronic risk calculator to estimate patient-specific risk of PJI and mortality after THA. Logistic regression was evaluated using the 53,252 patients who had primary THAs identified between 1998 and 2009. Separate logistic regression models were developed for PJI and mortality. These models included the patient demographics and comorbidities as independent covariates, and two-way interaction terms.
Based on the 2-year PJI and 90-day mortality rates determined from the logistic regression analysis, an electronic risk calculator was developed to provide real-time accessible, patient-specific evaluation of the estimated risk of PJI and mortality after primary THA. The risk calculator was created as an Apple® iPad (Apple Inc, Cupertino, CA, USA) app. The user interface for the app allows entry of patient data on the first screen (Fig. 1), comorbid conditions on the second screen (Fig. 2), and the calculated risks on the third screen (Fig. 3).
Fig. 1.
A screenshot shows the first screen of the app for entering patient demographic information (age, sex, race, and Medicaid as a secondary payer).
Fig. 2.
Screenshots show the second screen of the app for selecting specific patient comorbid conditions.
Fig. 3A–B.
The third screen of the app shows the calculated probability of PJI within 2 years and mortality within 90 days for the entered patient data and comorbidities. The examples include scenarios where (A) all comorbid conditions and (B) only a subset of conditions were found. The corresponding rate for patients of the same demographic with no comorbidities and the average rate for the overall Medicare THA population are included for comparative purposes. PJI = periprosthetic joint infection.
The entries for the patient data on the first screen (Fig. 1) were designed to match the corresponding inputs to the logistic regression model for risk of PJI and mortality. Specifically, age, sex, race, public assistance for Medicare premiums (a proxy for low SES), height, and weight are entered into the risk calculator. For inputs with only two available options, ie, sex (male/female) and public assistance for Medicare (yes/no), the selection buttons were designed in the form of switches. The remaining inputs are selected via a scrolling menu at the top of the screen. The choices for age range and race were defined to match the choices in the logistic regression model. The user then proceeds to the second screen to enter the patient’s comorbid conditions by tapping on the Select Comorbid Conditions button near the bottom of the first screen.
A patient’s specific comorbid conditions then are selected from the list of 29 comorbid conditions on the second screen (Fig. 2). The conditions are arranged in alphabetical order to allow easy identification and selection of comorbidities. Once these have been selected, the user can calculate the risk of PJI and mortality by tapping the “Calculate Probability of Periprosthetic Joint Infection/Mortality” button at the bottom of the screen. The patient-specific estimated probability of PJI and mortality then are calculated and reported on the third screen (along with the 95% CIs) based on the combination of patient demographic data and comorbid conditions (Fig. 3A). For comparative purposes and to provide baseline rates, the corresponding PJI and mortality rates for patients of the same demographics (eg, age, sex, race, SES) with no comorbidities (Same Demographic with No Comorbidity) and the average rate for the overall Medicare THA population (Medicare Population Average) also are reported. These rates are presented in numerical form and in graphic form for visual comparison. The rate for patients of the same demographics with no comorbidities will change depending on the selected patient data (inputs from the first screen), whereas the average rate for the overall Medicare THA population will remain unchanged regardless of the user input. The previously entered patient demographic data from the first screen and comorbid conditions from the second screen also are listed on this final screen so the user has the patient demographics, comorbid conditions, and risks of PJI and mortality on a single screen for summary purposes.
Because there were 29 possible comorbid conditions for each patient demographic group and 60 possible combinations of demographic groups, this corresponds to 229 × 60 = 3.22 × 1010 possible combinations of patient and comorbidity combinations in the risk calculator application (229 = 5.37 × 108; five age × two sex × three race × 2 SES = 60 combinations; 5.37 × 108 × 60 = 3.22 × 1010). However, the Medicare dataset did not include patients corresponding to every possible combination of demographic and clinical characteristics. Therefore, in situations when no perfect match of comorbidities can be found for a specific demographic, the app will indicate the reported rate was based on available combinations of conditions by stating “*Highest Rate for [Mortality/Periprosthetic Joint Infection] based on available combinations of conditions” (Fig. 3B). For example, if a patient presents with a history of cardiac arrhythmia, chronic liver disease, congestive heart failure, diabetes, hypertension, and ischemic heart disease and that specific combination of comorbid conditions cannot be found, the app will report the highest mortality rate from any remaining combination of these conditions, which in this case is the rate for patients with cardiac arrhythmia, diabetes, and ischemic heart disease only.
Results
Of the 29 comorbid conditions, hypertension was the most prevalent (66%) in the Medicare THA population, followed by ischemic heart disease (28%), hypercholesterolemia (23%), malignancy (22%), and cardiac arrhythmia (20%) (Table 1). The overall 2-year rate of PJI and 90-day rate of mortality were 2.07% (95% CI, 1.66%–2.56%) and 1.30% (95% CI, 1.11%–1.53%), respectively.
Table 1.
Comorbid conditions included in the analysis of risk factors
| Comorbid conditions | Disease prevalence (%) | Absolute number of patients |
|---|---|---|
| Hypertension | 66 | 27,153 |
| Ischemic heart disease | 28 | 11,298 |
| Hypercholesterolemia | 23 | 9528 |
| Malignancy | 22 | 9011 |
| Cardiac arrhythmia | 20 | 8279 |
| Chronic pulmonary disease | 20 | 8097 |
| Urinary tract infection | 18 | 7518 |
| Diabetes | 18 | 7367 |
| Hypothyroidism | 17 | 6935 |
| Peripheral vascular disease | 14 | 5716 |
| Valvular disease | 13 | 5472 |
| Cerebrovascular disease | 12 | 5017 |
| Congestive heart failure | 11 | 4577 |
| Anemia | 11 | 4327 |
| Rheumatologic disease | 9 | 3523 |
| Depression | 7 | 2954 |
| Coagulopathy | 5 | 1880 |
| Psychoses | 4 | 1692 |
| Obesity | 4 | 1587 |
| Renal disease | 3 | 1333 |
| Chronic liver disease | 2 | 979 |
| Dementia | 2 | 756 |
| Pulmonary circulation | 2 | 645 |
| Metastatic tumor | 1 | 606 |
| Peptic ulcer disease | 1 | 416 |
| Lymphoma | 1 | 402 |
| Alcohol abuse | 1 | 267 |
| Hemiplegia/paraplegia | 0.5 | 203 |
| Drug abuse | 0.3 | 104 |
Prevalence based on 53,252 patients with primary THAs; denominator prevalence is 40,919 patients with primary THAs.
Using the risk calculator, the combinations of demographic characteristics and comorbidities that are associated with the highest rate of 2-year PJI are shown (Fig. 4). White women aged 70 to 74 years with alcohol abuse, depression, electrolyte disorder, peptic ulcer disease, urinary tract infection, rheumatologic disease, preoperative anemia, cardiopulmonary (cardiac arrhythmia, congestive heart failure, ischemic heart disease, chronic pulmonary disease) comorbidities, and peripheral vascular disease were at highest risk for PJI (27.18% [95% CI, 14.66%–44.78%]).
Fig. 4.
A screenshot shows the combination of demographic characteristics and comorbidities associated with the highest rate of PJI within 2 years postoperatively. White women aged 70 to 74 years with alcohol abuse, depression, electrolyte disorder, peptic ulcer disease, urinary tract infection, rheumatologic disease, preoperative anemia, cardiopulmonary (cardiac arrhythmia, congestive heart failure, ischemic heart disease, chronic pulmonary disease) comorbidities, and peripheral vascular disease were at highest risk for PJI (27.18% [95% CI, 14.66%–44.78%]). PJI = periprosthetic joint infection.
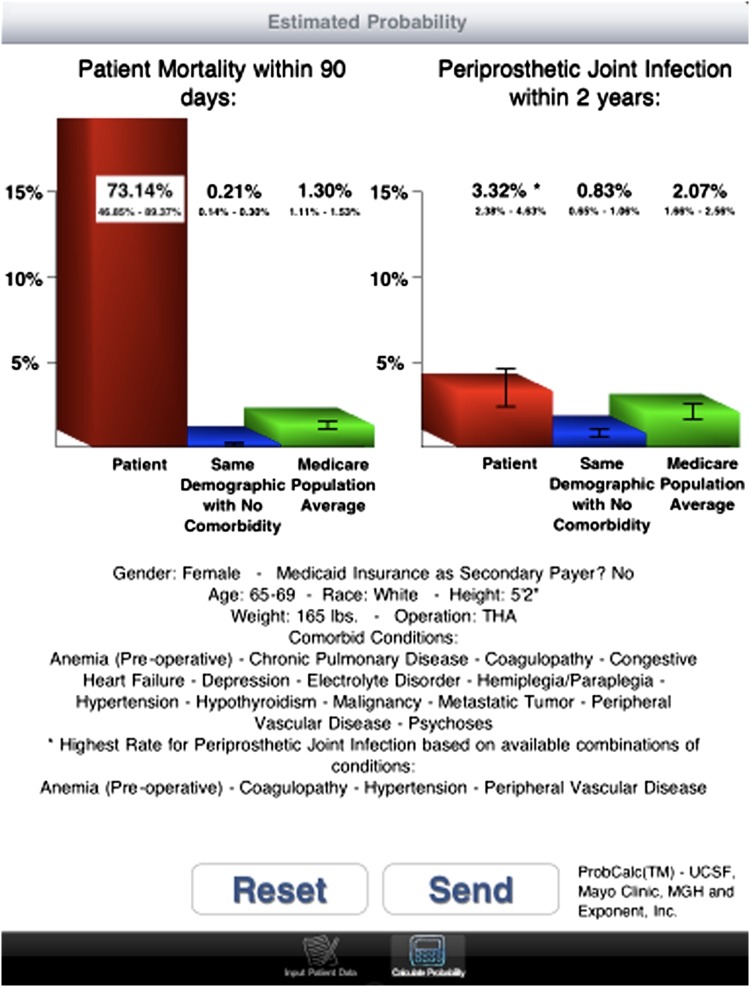
Similarly, the combinations of demographic characteristics and comorbidities associated with the highest rate of 90-day mortality are shown (Fig. 5). White women aged 65 to 69 years with electrolyte disorder, hemiplegia/paraplegia, hypertension, hypothyroidism, metastatic tumor, preoperative anemia, coagulopathy, cardiopulmonary (congestive heart failure, chronic pulmonary disease) and psychiatric (psychoses, depression) comorbidities, malignancies, and peripheral vascular disease were at highest risk for mortality (73.14% [95% CI, 46.86%–89.37%]). Low SES was associated with increased risk of PJI and mortality.
Fig. 5.
A screenshot shows the combination of demographic characteristics and comorbidities associated with the highest rate of 90-day postoperative mortality. White women aged 65 to 69 years with electrolyte disorder, hemiplegia/paraplegia, hypertension, hypothyroidism, metastatic tumor, preoperative anemia, coagulopathy, cardiopulmonary (congestive heart failure, chronic pulmonary disease) and psychiatric (psychoses, depression) comorbidities, malignancies, and peripheral vascular disease were at highest risk for mortality (73.14% [95% CI, 46.86%–89.37%]).
Finally, the rates of PJI (1.08% [95% CI, 0.84–1.38%]) and mortality (0.10% [95% CI, 0.06–0.15%]) for a more typical, low-risk patient with THA, a white male age 65 to 69 years with hypertension and hypercholesterolemia are shown (Fig. 6).
Fig. 6.
A screenshot shows the rate of PJI (1.08% [95% CI, 0.84–1.38%]) and mortality (0.10% [95% CI, 0.06–0.15%]) for a low-risk patient having THA, a white male age 65 to 69 years with hypertension and hypercholesterolemia. PJI = periprosthetic joint infection.
Discussion
Previous studies using Medicare administrative claims data have identified rheumatologic disease, obesity, coagulopathy, preoperative anemia, longer procedure duration, low SES, and male sex as risk factors associated with increased risk of PJI after THA [2, 12]. In other studies with smaller sample sizes, diabetes mellitus, total number of medical comorbidities, absence of prophylactic antibiotics, complications after operation, discharging wounds, previous operations, and remote infection were associated with an increased risk of PJI after THA [8, 16]. Using Medicare claims data, Bozic et al. [2] reported congestive heart failure, metastatic cancer, psychoses, renal disease, dementia, hemiplegia/paraplegia, cerebrovascular disease, and chronic pulmonary disease are associated with an increased adjusted risk of 90-day postoperative mortality in Medicare patients with THAs. Other investigators have found preexisting cardiovascular disease, male sex, and advanced age as risk factors for postoperative mortality [6, 13]. Although each of these studies provides important insight into the patients’ clinical and demographic characteristics associated with an increased risk of PJI and mortality after THA, little is known about the interaction between or synergistic effect of specific patient risk factors, especially in high-risk patients who have multiple comorbidities. We therefore evaluated the interaction between patients’ clinical and demographic characteristics on the risk of PJI and mortality after THA and developed an electronic risk calculator for estimating the patient-specific risk of PJI and mortality after THA in Medicare patients.
Our study is subject to limitations. First, we assumed the risks did not change during the years of the study and did not include year as a factor in the analysis. Therefore, the risk estimates provided by the app reflect average risks in the period of data, in this study 1998 to 2009. Advances in medical care, treatment, and diagnosis for PJI hopefully should reduce such risk with time. The risk of PJI or mortality after THA in the 1990s may not be the same as in 2012. Since our app is not static, regular updates and new releases of the app will be able to incorporate more recent and enhanced Medicare claims data. Second, the data used in the app are from Medicare claims and therefore may not be generalizable to the non-Medicare population. Also, certain comorbid conditions, such as obesity, are almost certainly undercoded in administrative claims, which could lead to an underestimate of their impact on the risk of PJI and/or mortality. Furthermore, the followup period was limited to only 24 months, and therefore late PJI would not be detected. Finally, since we used administrative claims data to estimate the risks of PJI and mortality, future studies will be needed to validate the risk calculator using comorbidities and clinical outcomes extracted from the clinical record.
There is growing interest in the development of healthcare-related electronic apps. Franko [4] reviewed 61 iPhone and 13 Android™ orthopaedic surgery-specific related apps and found, although there is high use of smartphones and interest in orthopaedic-related apps, there are few apps that have positive reviews among users and are desirable among orthopaedic surgeons. iOrtho/Clinical (examination), SpineDecide/Education (patient), and Orthopedics Hyperguide/Educational (reference) were among the most popular orthopaedic-related apps from the Apple® app store. That study also found, in a survey of 476 orthopaedic surgeons, the most desired types of apps include textbook or reference apps, and one of the advantages of having an app on a smartphone is having the data easily accessible and portable [4]. Franko and Tirrell [5] conducted a survey among Accreditation Council for Graduate Medical Education training programs and found physicians, not limited to orthopaedic surgeons, do currently use apps in their clinical practice. The most commonly used app types were drug guides, textbook/reference materials, classification/treatment algorithms, and apps with general medical knowledge [5].
Concerns also have been raised regarding the validity and accuracy of the content included in healthcare apps. Rosser and Eccleston [15] reviewed currently available pain-related apps and found 86% did not have a healthcare professional involved in the development or evaluation of the content in the app and only 12% had a physician as an app creator. Additionally, the source of the app content and data was not reported for the majority of apps reviewed.
We developed an electronic risk calculator that can be used to estimate the patient-specific risk of PJI and mortality after THA in Medicare patients. Using this app, surgeons have the ability to provide their patients with individualized estimates of their probabilities of PJI and mortality based on patient-specific data such as age, sex, race, SES, and comorbid conditions. This easily accessible, easy-to-use risk calculator was developed specifically for orthopaedic surgeons for use in clinical practice when counseling elderly patients with hip disease who are considering THA. A better understanding of individual patient risks will facilitate shared medical decision making between surgeons and their patients who are considering elective THA. This will be especially valuable for elderly patients with multiple comorbid conditions who may be at higher risk for PJI and/or mortality after THA. The app will be made easily accessible and available in the future at no fee through the Apple® app store pending FDA approval. Additional apps will be developed to estimate the impact of patient-specific demographic and clinical characteristics on the risk of other complications (eg, venous thromboembolism) and outcomes (eg, readmission to the hospital and revision surgery).
Acknowledgments
We thank Doruk Baykal BS, Drexel University, Philadelphia, PA, USA, for assistance in programming the app and Vanessa Chan MPH, for help in preparing this manuscript.
Footnotes
The institution of one or more of the authors (KJB) has received, during the study period, funding from the Orthopaedic Research and Education Foundation (Rosemont, IL, USA). One of the authors (SMK) certifies that he has received or may receive payments or benefits, during the study period, an amount in excess of $100,000, from Exponent, Inc (Philadelphia, PA, USA). One of the authors (KO) certifies that he has received or may receive payments or benefits, during the study period, an amount in excess of $100,000, from Exponent, Inc. One of the authors (EL) certifies that he has received or may receive payments or benefits, during the study period, an amount in excess of $100,000, from Exponent, Inc. One of the authors (DJB) certifies that he has received or may receive payments or benefits, during the study period, an amount in excess of $100,000, from DePuy Orthopaedics, Inc (Warsaw, IN, USA). One of the authors (TPV) certifies that he has received or may receive payments or benefits, during the study period, an amount in excess of $10,000, from DePuy Orthopaedics, Inc. One of the authors (HER) certifies that he has received or may receive payments or benefits, during the study period, an amount in excess of $100,000, from Zimmer, Inc (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that the institutions where the work was performed approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at University of California, San Francisco (San Francisco, CA, USA) and Exponent, Inc (Philadelphia, PA, USA; Menlo Park, CA, USA).
References
- 1.American Academy of Orthopaedic Surgeons. Total hip replacement. Available at: http://orthoinfo.aaos.org/topic.cfm?topic=a00377. Accessed November 22, 2011.
- 2.Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in Medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470:130–137. doi: 10.1007/s11999-011-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dearborn JT, Harris WH. Postoperative mortality after total hip arthroplasty: an analysis of deaths after two thousand seven hundred and thirty-six procedures. J Bone Joint Surg Am. 1998;80:1291–1294. doi: 10.2106/00004623-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Franko OI. Smartphone apps for orthopaedic surgeons. Clin Orthop Relat Res. 2011;469:2042–2048. doi: 10.1007/s11999-011-1904-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franko OL, Tirrell TF. Smartphone app use among medical providers in ACGME training programs. J Med Syst. 2012;36:3135–3139. doi: 10.1007/s10916-011-9798-7. [DOI] [PubMed] [Google Scholar]
- 6.Gaston MS, Amin AK, Clayton RA, Brenkel IJ. Does a history of cardiac disease or hypertension increase mortality following primary elective total hip arthroplasty? Surgeon. 2007;5:260–265. doi: 10.1016/S1479-666X(07)80021-7. [DOI] [PubMed] [Google Scholar]
- 7.Hamel MB, Toth M, Legedza A, Rosen MP. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430–1440. doi: 10.1001/archinte.168.13.1430. [DOI] [PubMed] [Google Scholar]
- 8.Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22:651–656. doi: 10.1016/j.arth.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96:1140–1146. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222. doi: 10.1186/1471-2474-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller KA, Callaghan JJ, Goetz DD, Johnston RC. Early postoperative mortality following total hip arthroplasty in a community setting: a single surgeon experience. Iowa Orthop J. 2003;23:36–42. [PMC free article] [PubMed] [Google Scholar]
- 12.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24(6 suppl):105–109. doi: 10.1016/j.arth.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83:1524–1528. doi: 10.2106/00004623-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Rat AC, Guillemin F, Osnowycz G, Delagoutte JP, Cuny C, Mainard D, Baumann C. Total hip or knee replacement for osteoarthritis: mid- and long-term quality of life. Arthritis Care Res (Hoboken). 2010;62:54–62. doi: 10.1002/acr.20014. [DOI] [PubMed] [Google Scholar]
- 15.Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare. 2011;17:308–312. doi: 10.1258/jtt.2011.101102. [DOI] [PubMed] [Google Scholar]
- 16.Surin VV, Sundholm K, Backman L. Infection after total hip replacement: with special reference to a discharge from the wound. J Bone Joint Surg Br. 1983;65:412–418. doi: 10.1302/0301-620X.65B4.6874711. [DOI] [PubMed] [Google Scholar]