Abstract
Background
Periprosthetic infection after total hip arthroplasty (THA) is a devastating complication. Reported rates of infection control range from 80% to 95% but mortality rates associated with treatment of infected THA are also substantial and we suspect underreported.
Questions/Purposes
For patients selected for two-stage treatment of infected THA we therefore determined (1) mortality; (2) rate of reimplantation; and (3) rate of reinfection.
Methods
We identified 202 patients (205 hips) with infected primary or revision THA treated with a two-stage protocol between 1996 and 2009 in our prospectively collected practice registry. Patients underwent two-stage treatment for infection, including removal of all implants and foreign material with implantation of an antibiotic-laden cement spacer in the first stage followed by intravenous culture-specific antibiotics for a minimum of 6 weeks. Second-stage reimplantation was performed if erythrocyte sedimentation rate and C-reactive protein were trending toward normal and the wound was well healed. Thirteen patients (13 hips) were lost to followup before 24 months. The minimum followup in surviving patients was 24 months or failure (average, 53 months; range, 24–180 months).
Results
Fourteen patients (7%; 14 hips) died before reimplantation and two were not candidates because of medical comorbidities. The 90-day mortality rate after the first-stage débridement was 4% (eight patients). Of the 186 patients (189 hips) who underwent reimplantation, 157 (83%) achieved control of the infection. Including all patients who underwent the first stage, survival and infection control after two-stage reimplantation was 76%.
Conclusion
Two-stage treatment of deep infection in primary and revision THA is associated with substantial mortality and a substantial failure rate from both reinfection and inability to perform the second stage.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA achieves durable pain relief and restoration of function in most patients with various degenerative conditions of the hip. Data from Medicare, the Nationwide Inpatient Sample as well as a single-center joint arthroplasty database estimate the incidence of infection after primary THA to be less than 1% [11, 14, 24]. Although not common, it remains one of the most devastating complications of THA, requiring revision surgery, which poses increased risk to the patient [17, 30, 37] as well increased resource use and institutional cost [12, 14]. Despite the increased cost and resource use, rates of successful control of infection may still range as low as 79% [17] (Table 1).
Table 1.
Organism incidence and rate of eradication
| Organism | Incidence | Eradication rate |
|---|---|---|
| Pan-sensitive staphylococcus | 63 (31%) | 86% |
| Resistant staphylococcus | 37 (18%) | 62% |
| Streptococcus species | 17 (8%) | 82% |
| Polymicrobial | 19 (9%) | 84% |
| Pseudomonas | 9 (4%) | 100% |
| Culture-negative | 32 (16%) | 91% |
Although various treatment options exist for infected THA, a two-stage protocol with insertion of some type of antibiotic spacer is widely reported [2, 4–6, 9, 10, 13, 16, 17, 19, 23, 25, 27, 28, 30–33, 35, 36]. Synthetic models have favored a direct exchange approach [34]; however, pooled clinical data have demonstrated the superiority of the two-stage protocol over a period of decades [7, 15]. A staged protocol has been applied with control of infection in the setting of massive bone loss [16], resistant organisms [17], and when used with oral [3] and abbreviated courses of intravenous antibiotics [33]. The use of high-dose antibiotic-laden cement spacers for the first-stage operation has yielded infection control rates superior to those with resection arthroplasty alone [2]; although this has not been a uniformly consistent finding [26, 30], it is the most commonly reported approach. Varying types of high-dose antibiotic cement spacers have been studied, including beads [5], hydroxyapatite blocks [29], and, most commonly, some form of articulating spacer [4, 6, 9, 10, 16, 17, 25, 27, 33, 35]. Comparison of static versus articulating spacers for treatment of infected THA has not been reported.
Reports of infection control and clinical success overshadow less emphasized, but also important, reports of treatment failure and mortality. This highlights the inconsistency of data reporting. For example, Toulson et al. [30] report a 95% rate of infection control, but 5.7% of THAs never underwent the second-stage operation or reimplantation, 25.8% of the patients died before minimum 2-year followup, and 6% of patients were lost. Additionally, Leung et al. [17] note a 79% rate of infection control and a 24% mortality rate associated with the treatment of resistant organisms, including three deaths (6%) before undergoing the second stage and nine (18%) at an average of 4 years after the second-stage operation. To report control of infection in 95% [30] or 79% [17] of patients undergoing treatment when 25.8% and 24%, respectively, die within the study period does not provide the basis for counseling patients regarding treatment outcomes when an infection is discovered nor does it accurately inform surgeon expectation. Death before completing the minimum followup interval as a means of exclusion from the final analysis in reporting two-stage revision outcomes should be reconsidered. Based on the data reported in the current study, the authors believe mortality rates after two-stage revision are underreported, or at least deemphasized, and that understanding the risk of mortality after staged revision for infection will shape both patient and surgeon behavior and expectations.
In patients for whom two-stage treatment of infected THA was selected, we therefore determined (1) the mortality rate; (2) rate of reimplantation; and (3) rate of reinfection. We then determined (4) differences in infection control between resistant and susceptible organisms; and (5) between static and articulating spacers.
Patients and Methods
From our practice registry of 8725 hip arthroplasty procedures performed between 1996 and 2009, we identified 202 patients with 205 infected primary and revision hip arthroplasties treated with a two-stage protocol. The indications for two-stage exchange were: (1) elevated erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) with a culture-positive hip aspiration; (2) elevated ESR and CRP with hip aspirate synovial fluid analysis demonstrating > 3000 WBC/mL with > 60% polymorphonuclear cells (PMNs); (3) late wound drainage (> 6 weeks postoperatively) with elevated ESR and CRP; (4) purulence noted at the time of THA revision surgery; and (5) intraoperative synovial fluid analysis demonstrating > 3000 WBC/mL with > 60% PMNs at the time of THA revision surgery. The contraindication was medical comorbidities precluding a single or multiple surgeries as determined by an independent group of general medical consultants. During that same time, we treated no other patients for infected THA using alternative protocols. Fifty-three percent of patients were male and 47% female. The average age at the time of the first stage was 65 years (range, 32–90 years). The mean weight was 195 pounds (range, 94–400 pounds). The mean height was 67 inches (range, 57–80 inches) and the mean body mass index was 30.1 kg/m2 (range, 17–57 kg/m2). Thirteen patients (13 hips) were lost to followup before minimum 2-year inclusion criteria. Minimum followup was 24 months (average, 54 months; range, 24–180 months). Patients who had not been evaluated within the past 2 years or had not returned for 2-year followup were contacted by phone and data were obtained from medical records and radiographs. All patients signed an institutional review board-approved general research consent allowing for retrospective review.
The infected arthroplasty treated with the two-stage protocol and an antibiotic spacer was a primary THA in 94 cases (46%), hemiarthroplasty in seven (3%), and revision THA in 90 hips (44%). In 11 hips, the immediately preceding surgery was two-stage treatment of infected THA (5%). There were two cases of infected resurfacing and one unknown previous status (primary versus revision).
After diagnosis of THA infection (Table 1) and medical clearance, patients underwent the first of two stages. The débridement stage involved removal of all implants, hardware, cement, or other foreign bodies from the hip. We performed a complete sharp synovectomy and excision of all nonviable tissue (including bone and soft tissue). The acetabulum and femur were then prepared for implant insertion using standard instrumentation. Copious irrigation with an average of 9 L of fluid was performed.
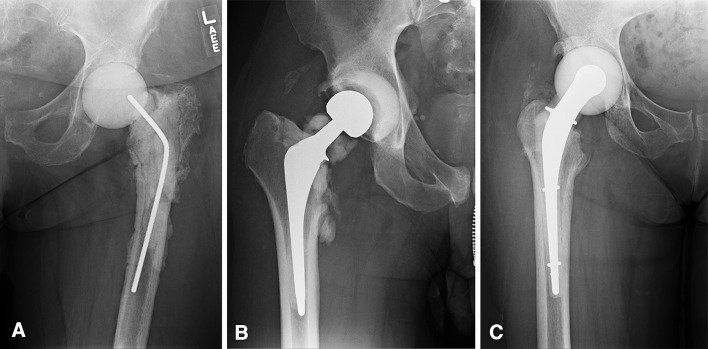
In 62 hips (30%), we used a nonarticulating spacer (Fig. 1). In these cases, after débridement, one to three units of high-dose antibiotic-laden cement were placed into the acetabulum and proximal femur. In 143 hips (70%), an articulating spacer was created and inserted after débridement. Over the timeframe of the study, the articulating spacers differed from hand-molded hemiarthroplasty, to pseudoimplant spacers, to intraoperatively created spacers using prefabricated molds with endoskeleton implants (Fig. 2A–C). Throughout the study period, the high-dose antibiotic concentration remained constant with 3.0 g vancomycin and 3.6 g tobramycin or gentamicin per unit of cement. Depending on the bone loss and deformity, we used between two and four units of cement in each case.
Fig. 1.
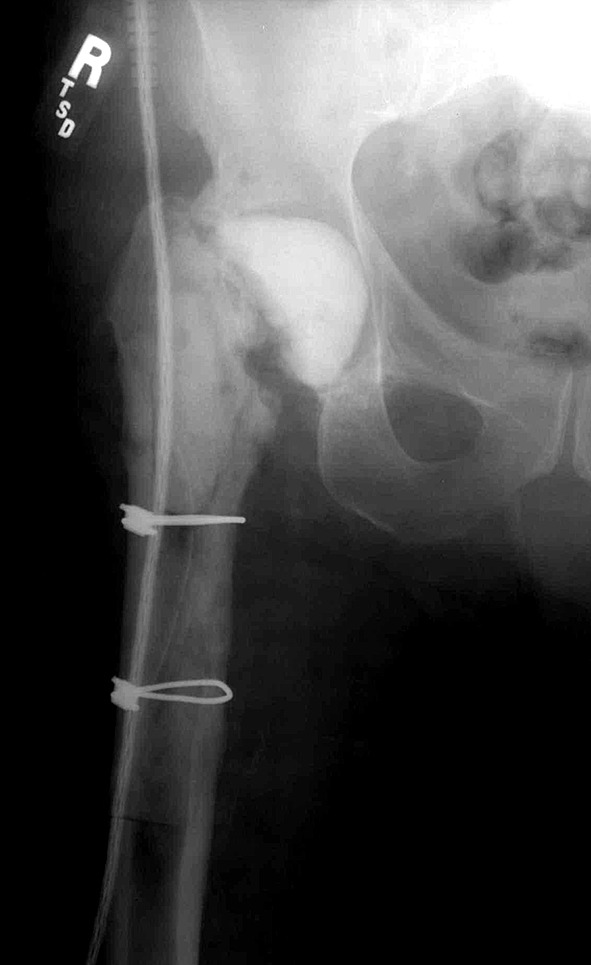
In this patient, a nonarticulating spacer of antibiotic-laden cement was used.
Fig. 2A–C.
(A) The earliest type of articulating spacer used was a hand-molded hemiarthroplasty of antibiotic-laden cement, as shown. (B) Articulating spacers evolved to devices incorporating actual implants with antibiotic-laden cement such as the construct shown. (C) Current technology for articulating spacers is shown, which involves intraoperatively created antibiotic-laden spacers using prefabricated molds with endoskeleton implants.
All patients were maintained on intravenous culture-specific antibiotics for a minimum of 6 weeks under the direction of an infectious disease specialist. Routine weekly surveillance of ESR and CRP was performed. Patients were asked to be toe-touch weightbearing with the use of an assistive device during the intervening treatment.
Patients underwent second-stage reimplantation if their ESR and CRP were trending downward; normal values were not required; if the wound was well healed; and the minimum 6 weeks of antibiotic therapy was completed. No specific laboratory values or antibiotic holiday were used for determining the timing or performance of the second stage. On reimplantation, we removed all spacer or spacer material and the entire wound was redébrided. Routine revision-type reconstruction was performed. All patients received noncemented components at the time of reimplantation.
Patients were seen in followup by the treating physician or a Physician Assistant 3 weeks postoperatively for evaluation of the wound and for staple removal. They were evaluated again 6 weeks postoperatively at which point the wound was again evaluated for the presence of drainage and radiographs were obtained, including an AP pelvis, frog lateral, and femoral views to include the entire stem. If patients were doing well, followup was performed on an as-needed basis and yearly. If there was concern, followup at more appropriate frequent intervals was performed. After the second stage, the infectious disease consultant remained involved following intraoperative cultures, which included five tissue samples and one synovial fluid sample to final status. If cultures were positive, organism-specific antibiotics were reinitiated. Perioperative antibiotics were administered per routine for revision THA, including a first-generation cephalosporin and gentamicin before incision and postoperatively for 48 hours. Harris hip scores (HHS) [8] were obtained on all patients, prestage 1, poststage 2 at 6 weeks, and yearly. At the time of this review, the Social Security Death Index was queried for all patients not seen within the prior 6 months to determine mortality if the patient was not known to have died. Control of infection, for this study, was defined as no further surgery on the index hip for infection. Continuation of antibiotics for chronic suppression was not known. All medications, treatments, and devices used have been approved by the US Food and Drug Administration.
The mortality rate, rate of reimplantation, and rate of reinfection were calculated as a percentage of the total available hips. Differences in infection control between resistant and susceptible organisms and between static and articulating spacers were analyzed using Fisher’s exact test. Post hoc power analysis was performed and a power of 80% to detect a difference of 5% in rate of infection control at p = 0.05 was noted with the numbers analyzed.
Results
Fourteen patients (7%; 14 hips) died before the second-stage reimplantation procedure. The 90-day mortality rate after the first-stage débridement was 4% (eight patients) with seven patients (3%) who died before reimplantation and one patient (0.5%) who died after the second stage. Overall, 91 patients (48%; 93 hips) died during the study period. Of the initial 202 patients, 186 (189 hips; 92%) underwent the second stage of treatment with reimplantation. Fourteen patients died and two patients were not considered candidates for a second stage based on medical comorbidities.
Of the 186 patients (189 hips) who underwent the second-stage reimplantation, 157 were free from infection at an average of 53 months followup. Thus, there was an 83% success rate for control of infection. If mortality is included in the failure rate, the success of two-stage reimplantation to provide control of infection is 76%.
When examining the virulence of the infecting organism, 37 hips (18%) were infected with antibiotic-resistant strains. The ability to control infection (Table 1) was lowest in the resistant organism infections (62%) when compared with sensitive staphylococcal (86%), streptococcal (82%), polymicrobial (84%), pseudomonas (100%), and culture-negative infections (91%). Infections with resistant organisms were significantly less likely to be controlled (p = 0.0012).
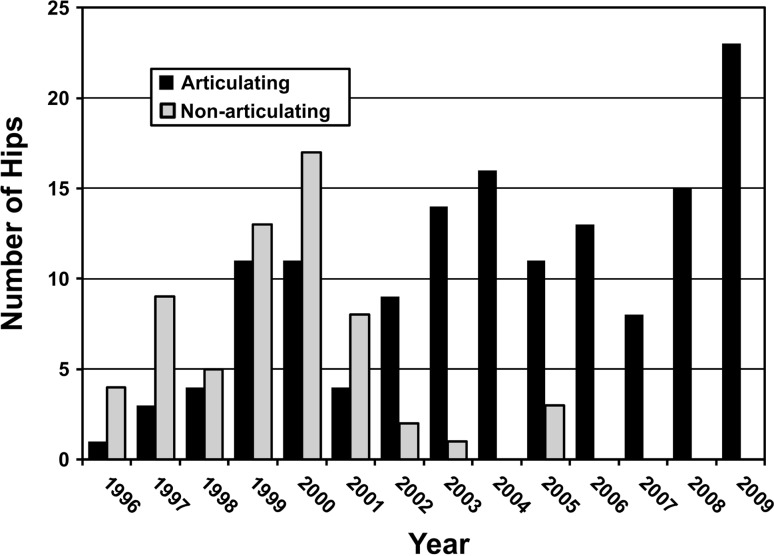
With the numbers available, there were no differences in any variable examined between hips treated with static (62 hips) or articulating (143 hips) spacers. The use of articulating spacers increased during the study period (Fig. 3). The 90-day mortality rate was similar (p = 0.27): 4% and 5%, respectively. Static spacers and articulating spacers were also associated with similar rates of patients not reimplanted as a result of death or morbidity: seven hips (11%) versus nine hips (6%), respectively. In hips that underwent a second stage, we found similar rates (p = 0.16) of recurrence of infection: 17% of articulating and 16% of static spacers had recurrence of infection. The clinical outcome as measured by HHS was similar (p = 0.054) but low in both groups with articulating spacers averaging 65 and static spacers averaging 63.
Fig. 3.
Spacer type use over the time of the study has evolved from static to articulating spacers.
Discussion
THA achieves durable pain relief and restoration of function in most patients with various degenerative conditions of the hip, but infection does occur in a small percentage of these patients and remains one of the most devastating complications of THA. Treatment of infection poses increased risk to the patient [17, 30, 37] as well as increased resource use and institutional cost [12, 14], but rates of infection control may still range below 80% [17]. Two-stage treatment of infected THA with high-dose antibiotic-laden cement spacers before reimplantation is commonly performed with infection control rates consistently better than 80% (Table 2), but the morbidity associated with this treatment is deemphasized in the reporting of these excellent clinical outcomes [17, 30]. The purpose of this study therefore was to report the rate of infection control for two-stage treatment of infected TKA in terms of (1) mortality; (2) successful reimplantation; and (3) rate of reinfection. Differences in success between resistant and susceptible organisms and static and articulating spacers are also reported.
Table 2.
Two-stage treatment of infected THA
| Study | Year | Device | Number of hips undergoing first stage | Number of hips undergoing reimplantation | Interval to reimplantation (months) | Mean followup (months) | 90-day mortality rate | Overall mortality rate | Number of infections controlled after reimplantation/number undergoing first stage | Number of infections controlled after reimplantation/number reimplanted |
|---|---|---|---|---|---|---|---|---|---|---|
| McDonald et al. [21] | 1989 | No spacer | 82 | 82 | 18 | 65 | NR | 13/82 (16%) | 71/82 (87%) | 71/82 (87%) |
| Nestor et al. [22] | 1994 | No spacer | 34 | 34 | 8 | 47 | NR | NR | 28/34 (82%) | 28/34 (82%) |
| Lieberman et al. [18] | 1994 | No spacer | 49 | 34 | 2 | 40 | 1/49 (2%) | 5/49 (8%) | 29/46 (63%) | 29/32 (91%) |
| Tsukayama et al. [31] | 1996 | Beads | 34 | 34 | 3.7 | 46 | 0/34 (0%) | 0/34 (0%) | 29/34 (85%) | 29/34 (85%) |
| Younger et al. [36] | 1997 | Articulating | 61 | 56 | 3 | 43 | NR | 4/61 (6.6%) | 52/61 (89%) | 52/56 (93%) |
| Wang and Chen [32] | 1997 | Beads (13), no spacer (9) | 22 | 22 | 6.6 | 48 | NR | NR | 20/22 (87%) | 20/22 (87%) |
| Fehring et al. [5] | 1999 | Beads (19), no spacer (6) | 25 | 25 | 4.8 | 41 | NR | NR | 23/25 (88%) | 23/25 (88%) |
| Magnan et al. [20] | 2001 | Articulating | 10 | 8 | 5 | 35 | NR | NR | 8/10 (80%) | 8/8 (80%) |
| Koo et al. [13] | 2001 | Articulating | 24 | 22 | 1.5 | 41 | NR | 2/24 (8%) | 21/22 (95%) | 21/22 (95%) |
| Yamamoto et al. [35] | 2003 | Articulating | 17 | 15 | 4.3 | 38 | 0/17 (0%) | 0/17 (0%) | 15/17 (88%) | 15/17 (88%) |
| Evans [4] | 2004 | Articulating | 23 | 23 | 2.8 | > 24 | NR | NR | 22/23 (96%) | 22/23 (96%) |
| Buttaro et al. [1] | 2005 | No spacers | 30 | 30 | 3.5 | 32 | 0/30 (0%) | 0/30 (0%) | 29/30 (97%) | 29/30 (97%) |
| Hofmann et al. [9] | 2005 | Articulating | 42 | 35 | 3 | 76 | NR | 8/42 (19%) | 26/42 (62%) | 26/27 (96%) |
| Nusem and Morgan [23] | 2006 | Articulating (17), beads (1) | 18 | 18 | 5 | 108 | 0/18 (0%) | 5.6% (1/18) | 17/18 (94%) | 17/18 (94%) |
| Scharfenberger et al. [27] | 2007 | Articulating (28) | 28 | 8 | NR | > 24 | NR | 1/28 (3.6%) | 8/28 (29%) | 8/8 (100%) |
| Cabrita et al. [2] | 2007 | Articulating (38), no spacer (30) | 68 | 56 | NR | 48 | NR | 5/68 (7%) | 51/68 (75%) | 51/56 (91%) |
| Stockley et al. [28] | 2008 | Beads | 114 | 114 | 6.4 | 74 | NR | Not stated | 100/114 (88%) | 100/114 (88%) |
| Whittaker et al. [33] | 2009 | Articulating | 44 | 44 | 5 | 49 | NR | 3/44 (7%) | 41/44 (93%) | 41/44 (93%) |
| Lim et al. [19] | 2009 | Articulating (31), beads (6) | 45 | 42 | 4.5 | 54 | NR | 1/45 (2.2%) | 38/45 (84%) | 35/42 (83%) |
| Incavo et al. [10] | 2009 | Articulating | 11 | 11 | > 1.5 | NR | NR | NR | 9/11 (82%) | 9/11 (82%) |
| Sanchez-Sotelo et al. [26] | 2009 | Spacer (31), no spacer (138) | 169 | 169 | 9.4 | 84 | NR | 4/169 (2.4%) | 157/169 (93%) | 157/169 (93%) |
| Cordero-Ampuero et al. [3] | 2009 | No spacer | 36 | 20 | 9.1 | 53 | NR | 4/36 (11%) | 20/36 (55%) | 20/20 (100%) |
| Toulson et al. [30] | 2009 | Spacer (56), no spacer (28) | 130 | 110 | 3.4 | 64.8 | NR | 34/130 (26%) | 80/130 (68%) | 80/84 (95%) |
| Fink et al. [6] | 2009 | Articulating | 40 | 40 | NR | 35 | NR | 1/40 (2.5%) | 40/40 (100%) | 40/40 (100%) |
| Takigami et al. [29] | 2010 | HA blocks | 8 | 8 | 4 | 49 | 0/8 (0%) | NR | 8/8 (100%) | 8/8 (100%) |
| Romano et al. [25] | 2010 | Articulating | 102 | 102 | ~2.7 | 48 | NR | 3/102 (2.0%) | 98/102 (96%) | 98/102 (96%) |
| Lee et al. [16] | 2011 | Articulating | 27 | 27 | 5.5 | 98 | 0% | 2/27 (7.5%) | 26/27 (96%) | 26/27 (96%) |
| Leung et al. [17] | 2011 | Articulating | 50 | 47 | 6 | 58 | NR | 12/50 (24%) | 30/50 (60%) | 30/38 (79%) |
| Current study | 2012 | Static spacer (62); articulating (143) | 205 | 189 | ? | 53 | 8/202 (3.9%) | 91/202 (45%) | 157/202 (77%) | 157/189 (83%) |
HA = hyaluronate; NR = not reported.
We caution readers of the limitations of our study. First patients were not recalled for examination, only phone survey for the purposes of study followup, and the data were collected from our prospectively collected database; therefore, the current status of all patients in terms of implant fixation and function cannot be definitively stated. However, we do report 90-day mortality separately from overall mortality rate, which is informative regarding death occurring in the early perioperative period that is more likely to be associated with the morbidity of the procedures. Second, there is not a comparison group; therefore, our study does not provide information regarding mortality rates of the two-stage procedure relative to an alternative treatment such as single-stage revision, and mortality rates should be interpreted as a result of treating this patient population and not necessarily as a result of the treatment. Third, during the timeframe of the study, the authors did not have exact criteria for proceeding with the second stage or determining control of the infection. We describe our protocol and the resultant outcomes only. Lastly, we do attempt to stratify mortality risk or control of infection according to patient comorbidities and there may be important variables not examined that are related to both. These shortcomings aside, the current report represents the largest in the literature regarding two-stage treatment of infection.
We noted high perioperative mortality. In patients who underwent the second-stage procedure, there was an 83% rate of infection control at an average of 53 months followup. This rate of infection control is on par with similar series reported in the literature (Table 2). Mortality associated with the two-stage treatment of periprosthetic hip infection appears to be high both in the perioperative period but also within the followup interval. Toulson et al. [30] reported a 25.8% rate of death before 2-year followup in their series of two-stage treatment. In the treatment of resistant organisms, a 24% mortality rate has also been reported [17]. In the current series, 45% of patients had died at an average of 4.7 years after treatment. A slightly higher mortality rate of 50% was noted in those patients who had recurrent infection. A total of 14 patients (15 hips; 7%) died within 90 days of the first stage with seven patients (4%) dying after the first stage of treatment and before undergoing reimplantation.
We believe success of a two-stage treatment should include not only control of infection, but also consider those patients who did not undergo successful second-stage reimplantation as failures. Control of the infection is not achieved if death occurs before the second-stage operation and therefore death should not be considered a success in the rate of infection control. Many times authors only include those patients in whom the second stage was completed in their analysis of success. Thus, undergoing the second stage may be an important outcome measure in reporting of results. Lim et al. [19] reported that 92% of their cases underwent reimplantation with 8% requiring permanent resection as a result of continued sepsis. Eighty-two of 87 hips treated in another series were reimplanted for a rate of 94.3% [30]. Similarly, 92% of hips in the current series underwent a second stage with the aforementioned 14 patients (15 hips) dying and two additional patients unfit medically for reimplantation.
Once death and the rate of successful second stage are considered, only then can a true rate of infection control for a treatment strategy be calculated. Toulson et al. [30] report an infection control rate of 95%, yet only 94.5% of hips underwent a second-stage reimplantation. Perhaps a more appropriate success rate in their series would be 78 of 82 patients, or a 90% true control rate with a two-stage protocol. Sanchez-Sotelo et al. [26], in a midterm to long-term series, appear to only report on their patients who underwent successful second-stage reimplantation but offer little information about patients who might not have undergone the second stage. In their series, the rate of infection control was 87.5% but mechanical failure of the second-stage reconstruction dropped their implant survivorship to 75.2%. We observed an 83% rate of infection control in patients successfully reconstructed, but this survival rate drops to 76% if mortality during the perioperative period is included.
With few exceptions, the ability to control highly virulent organisms or resistant strains is compromised compared with sensitive strains [17]. We noted the highest failure rate in resistant strains with an infection control rate of 62%. This is compared with 86% and 82% success for treatment of sensitive staphylococcal and streptococcal strains, respectively. Lim et al. [19] noted this higher failure with all of their failed procedures falling into the resistant group (33% failure). Although most series have supported the notion that resistant strains have a higher failure rate, all 21 resistant infections in the series of Toulson et al. [30] were controlled at 2 years.
Although our belief was that the use of an articulating spacer would result in better outcomes, no differences in the use of these devices were observed. Neither control of infection nor final hip score was different. We noted average HHS of 65 and 63 with articulating and static, respectively. This is remarkably similar to the results of Scharfenberger et al. [27] and their report of the use of a PROSTALAC implant (DePuy Orthopaedics, Inc, Warsaw, IN, USA) during the first stage in which their average HHS was 62. Clearly, the final results of the two-stage treatment of periprosthetic hip infection are severely compromised compared with primary THA and revision THA regardless of the use of an articulating spacer. Whether the second stage is made simpler with the use of an articulating spacer deserves further study.
Periprosthetic infection is a devastating and complicated problem after THA. We highlight the substantial mortality also associated with this treatment strategy despite reimplantation rates and control of infection rates similar to those reported in the literature. Infection with highly virulent resistant organisms was controlled less frequently than sensitive strains and no particular advantage was noted with use of articulating versus static spacers. Along with the technical aspects of débridement and spacer creation, surgeons need to be familiar with these high rates of death. Perhaps better perioperative optimization of nutrition, smoking, and overall health status can lead to fewer deaths and better infection control rates.
Footnotes
The institution of one or more of the authors (KRB, AVL, MJM) has funding from Biomet, Inc (Warsaw, IN, USA) and Stryker (Mahwah, NJ, USA). One of the authors (KRB) certifies that he has or may receive payments or benefits, in any one year, an amount in excess of $100,000 from Biomet, Inc. One of the authors (AVL) certifies that he has or may receive payments or benefits, in any one year, an amount in excess of $1,000,000 from Biomet, Inc and Innomed, Inc (Savannah, GA, USA). One of the authors (MJM) certifies that he has or may receive payments or benefits, in any one year, an amount in excess of $10,000 from Biomet, Inc.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Joint Implant Surgeons, Inc, New Albany, OH, USA.
References
- 1.Buttaro MA, Pusso R, Piccaluga F. Vancomycin-supplemented impacted bone allografts in infected hip arthroplasty. J Bone Joint Surg Br. 2005;87:314–319. doi: 10.1302/0301-620X.87B3.14788. [DOI] [PubMed] [Google Scholar]
- 2.Cabrita HB, Croci AT, de Camargo OP, de Lima ALLM. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clinics (Sao Paulo). 2007;62:99–108. doi: 10.1590/S1807-59322007000200002. [DOI] [PubMed] [Google Scholar]
- 3.Cordero-Ampuero J, Esteban J, Garcia-Cimbrelo E. Oral antibiotics are effective for highly resistant hip arthroplasty infections. Clin Orthop Relat Res. 2009;467:2335–2342. doi: 10.1007/s11999-009-0808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 5.Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty. 1999;14:175–181. doi: 10.1016/S0883-5403(99)90122-5. [DOI] [PubMed] [Google Scholar]
- 6.Fink B, Grossmann A, Fuerst M, Schafer P, Fromelt L. Two-stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res. 2009;467:1848–1858. doi: 10.1007/s11999-008-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvin KL, Hanssen AD. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am. 1995;77:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 9.Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874–879. doi: 10.1016/j.arth.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 10.Incavo SJ, Russell RD, Mathis KB, Adams H. Initial results of managing severe bone loss in infected total joint arthroplasty using customized articulating spacers. J Arthroplasty. 2009;24:607–613. doi: 10.1016/j.arth.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83:1622–1629. doi: 10.1302/0301-620X.83B3.10487. [DOI] [PubMed] [Google Scholar]
- 12.Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res. 2010;96:124–132. doi: 10.1016/j.otsr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Koo K-H, Yang J-W, Cho S-H, Song H-R, Park H-B, Ha Y-C, Chang J-D, Kim S-Y, Kim Y-H. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–892. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Lange J, Troelsen A, Thomsen RW, Soballe K. Chronic infections in hip arthroplasties: comparing risk of reinfection following one-stage and two-stage revision: a systematic review and meta-analysis. Clin Epidemiol. 2012;4:57–73. doi: 10.2147/CLEP.S29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee PTH, Clayton RA, Safir OA, Backstein DJ, Gross AE. Structural allograft as an option for treating infected hip arthroplasty with massive bone loss. Clin Orthop Relat Res. 2011;469:1016–1023. doi: 10.1007/s11999-010-1673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung F, Richards CJ, Garbuz DS, Masri BA, Duncan CP. Two-stage total hip arthroplasty: how often does it control methicillin-resistant infection? Clin Orthop Relat Res. 2011;469:1009–1015. doi: 10.1007/s11999-010-1725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;301:205–212. [PubMed] [Google Scholar]
- 19.Lim S-J, Park J-C, Moon Y-W, Park Y-S. Treatment of periprosthetic hip infection caused by resistant microorganisms using 2-stage reimplantation protocol. J Arthroplasty. 2009;24:1264–1269. doi: 10.1016/j.arth.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Magnan B, Regis D, Biscaglia R, Barolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics. Acta Orthop Scand. 2001;72:591–594. doi: 10.1080/000164701317269003. [DOI] [PubMed] [Google Scholar]
- 21.McDonald DJ, Fitzgerald RH, Ilstrup DM. Two-stage reconstruction of a total hip arthroplasty because of infection. J Bone Joint Surg Am. 1989;71:828–834. [PubMed] [Google Scholar]
- 22.Nestor BJ, Hanssen AD, Ferrer-Gonzalez R, Fitzgerald RH. The use of porous prosthesis in delayed reconstruction of total hip replacements that have failed because of infection. J Bone Joint Surg Am. 1994;76:349–359. doi: 10.2106/00004623-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Nusem J, Morgan DAF. Structural allografts for bone stock reconstruction in two-stage revision for infected total hip arthroplasty. Acta Orthop. 2006;77:92–97. doi: 10.1080/17453670610045740. [DOI] [PubMed] [Google Scholar]
- 24.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romano CL, Romano D, Logoluso N, Meani E. Long-stem versus short-stem preformed antibiotic-loaded cement spacers for two stage revision of infected total hip arthroplasty. Hip Int. 2010;20:26–33. doi: 10.1177/112070001002000104. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Sotelo J, Berry DJ, Hanssen AD, Cabanela ME. Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res. 2009;467:219–224. doi: 10.1007/s11999-008-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharfenberger A, Clark M, Lavoie G, O’Connor G, Masson E, Beaupre LA. Treatment of an infected total hip replacement with the PROSTALAC system. Part 1: infection resolution. Can J Surg. 2007;50:24–28. [PMC free article] [PubMed] [Google Scholar]
- 28.Stockley I, Mockford BJ, Hoad-Reddick A, Norman P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J Bone Joint Surg Br. 2008;90:145–148. doi: 10.1302/0301-620X.90B2.19855. [DOI] [PubMed] [Google Scholar]
- 29.Takigami I, Yoshiki I, Ishimaru D, Ogawa H, Mori N, Shimizu T, Terabayashi N, Shimizu K. Two-stage revision surgery for hip prosthesis infection using antibiotic-loaded porous hydroxyapatite blocks. Arch Orthop Trauma Surg. 2010;130:1221–1226. doi: 10.1007/s00402-009-0991-9. [DOI] [PubMed] [Google Scholar]
- 30.Toulson C, Walcott-Sapp S, Hur J, Salvati EA, Bostrum M, Brause BD, Westrich GH. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol. J Arthroplasty. 2009;24:1051–1060. doi: 10.1016/j.arth.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Wang J-W, Chen C-E. Reimplantation of infected hip arthroplasties using bone allografts. Clin Orthop Relat Res. 1997;335:202–210. doi: 10.1097/00003086-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Whittaker JP, Warren RE, Jones RS, Gregson PA. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br. 2009;91:44–51. doi: 10.1302/0301-620X.91B1.20930. [DOI] [PubMed] [Google Scholar]
- 34.Wolf CF, Gu NY, Doctor JN, Manner PA, Leopold SS. Comparison of one and two-stage revision of total hip arthroplasty complicated by infection. J Bone Joint Surg Am. 2011;93:631–639. doi: 10.2106/JBJS.I.01256. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Miyagawa N, Masaoka T, Katori Y, Shishido T, Imakiire A. Clinical effectiveness of antibiotic-impregnated cement spacers for the treatment of infected implants of the hip joint. J Orthop Sci. 2003;8:823–828. doi: 10.1007/s00776-003-0722-y. [DOI] [PubMed] [Google Scholar]
- 36.Younger ASE, Duncan CP, Masri BA, McGraw RW. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplasty. 1997;12:615–623. doi: 10.1016/S0883-5403(97)90133-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89:526–533. doi: 10.2106/JBJS.F.00952. [DOI] [PubMed] [Google Scholar]