Abstract
Background
The Articular Surface Replacement™ (ASR™) metal-on-metal hip arthroplasty system (DePuy Orthopaedics, Inc, Warsaw, IN, USA) reportedly has a higher than anticipated early failure rate leading to a voluntary recall. This prompted us to evaluate all ASR™ components implanted at our center.
Questions/Purposes
In all ASR™ components, we reported (1) revision rate, (2) blood metal ion levels, and (3) intraoperative findings for revisions related to adverse reaction to metal debris (ARMD).
Methods
We retrospectively reviewed all 172 patients (190 hips) who underwent THA (149 hips) or hip resurfacing (41 hips) with the ASR™ system. We determined failure rates. We obtained blood metal ion concentrations from 93 patients at last followup. We evaluated MRI studies and intraoperative histopathology. Minimum followup was 12 months (mean, 40 months; range, 12–74 months).
Results
At latest followup, we had revised 24 of 190 hips (13%): in 18 patients with THA and five patients with resurfacing. Mean time to revision was 45 months (range, 12–75 months). Mean blood concentrations were 13 μg/L (range, 0–150 μg/L) for cobalt and 6 μg/L (range, 0–87 μg/L) for chromium. Mean prerevision blood metal ion levels were higher in the revised group (cobalt: 48 μg/L; chromium: 18 μg/L) than in the nonrevised group (cobalt: 5 μg/L; chromium: 2 μg/L). ARMD was present in 14 of the 24 hips revised in this study.
Conclusions
Surgeons must have a low threshold for concern for ARMD in patients with ASR™ systems. Blood metal ion levels and MRI can be used to evaluate patients with underperforming implants. Intraoperative histopathologic analysis and joint fluid cytology can help diagnose ARMD at the time of revision.
Level of Evidence
Level III, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Modern metal-on-metal (MOM) bearing surfaces have recently been widely utilized in THA and hip resurfacing procedures in the United States [3]. Since the popularization of the first-generation MOM designs such as the McKee-Farrar prosthesis [23] in the 1960s, improvements in implant design and fixation have provided modern MOM implants with two major theoretical advantages over their metal-on-polyethylene counterparts. MOM articulations using cobalt-chromium-molybdenum alloys reportedly produce considerably less volumetric wear debris than standard metal-on-polyethylene components [32, 42]. Additionally, for a given external diameter, an all-metal acetabular component can be made thinner, allowing the use of a larger-diameter femoral head. These large-head MOM articulations are thought to provide increased stability and ROM compared to implants with small head diameters [5, 20, 29, 33].
However, a major drawback to MOM articulations is the generation of metal debris from mechanical and corrosive wear, which has been associated with increased blood metal ion levels in patients [4, 21, 28]. The systemic long-term effects of elevated circulating metal ion levels in the body remain unclear [9, 15, 36, 39]. In the local pelvic soft tissue environment, MOM articulations have been well described by recent literature to contribute to implant failure through metallosis, macroscopic necrosis, large sterile hip effusions, and corrosive osteolysis. The umbrella term adverse reaction to metal debris (ARMD) has been used to categorize this spectrum of findings [16, 18]. Willert et al. [41] performed a histologic examination of periprosthetic tissue from failed implants suspected of ARMD and described a delayed-type hypersensitivity reaction known as an aseptic lymphocyte-dominant vasculitis-associated lesion (ALVAL). Pandit et al. [26] described a separate phenomenon known as a pseudotumor, which can be readily identified on pelvic MRI as a formation of periprosthetic solid and cystic masses. It is unclear exactly how these reactions to metal debris in the local soft tissue environment contribute to overall MOM implant survivorship.
Many hip prosthesis manufacturers have produced THA and hip resurfacing implants that utilize a MOM bearing. The Articular Surface Replacement™ (ASR™) monobloc acetabular component (DePuy Orthopaedics, Inc, Warsaw, IN, USA) was released outside of the United States in 2003. It was initially designed as part of the ASR™ Hip Resurfacing System (DePuy Orthopaedics). It was subsequently approved by the FDA for use in THA in 2006 as part of the ASR™ XL Acetabular System and represents a modern large-diameter MOM hip bearing connected to a stem by a Morse taper. This taper can be a potential source of fretting wear and corrosion that is not present in the hip resurfacing system. Major concerns have been raised relating to the early failure of the ASR™ acetabular component and MOM articulations as a class. On August 24, 2010, DePuy Orthopaedics issued a voluntary recall of the ASR™ acetabular component used in both THA and hip resurfacing procedures, citing a higher than expected revision rate of 12% in the ASR™ Hip Resurfacing System and 13% in the ASR™ Acetabular System at 5 years [7]. On May 6, 2011, the FDA issued orders for postmarket surveillance studies to 21 manufacturers of MOM hip systems [37].
We therefore reported (1) revision rate, (2) blood metal ion levels, and (3) intraoperative findings for revisions related to ARMD in all ASR™ components used in THA and hip resurfacing procedures by two surgeons at a single institution.
Patients and Methods
Between October 2004 and June 2010, 192 patients (214 hips) underwent primary THA using the ASR™ XL MOM articulation or the ASR™ Hip Resurfacing System. During that same time, we treated approximately 1000 patients with THA. All monoblock acetabular components and modular femoral heads were part of the standard cobalt-chrome DePuy ASR™ system. Tapered titanium Summit® femoral stems (DePuy Orthopaedics) were used for all THA procedures. Sizing of the components was determined at the time of each procedure at the discretion of the attending surgeon. The indications for the ASR™ THA system were (1) young candidates for THA with long life expectancy hoping to return to high levels of postoperative activity and (2) ability to implant a large-diameter femoral head. The indications for the resurfacing system were (1) 65 years old or younger, (2) active preoperative lifestyle, and (3) good proximal femoral bone quality and morphology. The contraindications for these systems were (1) known metal sensitivity, (2) inflammatory arthritis, (3) severely altered acetabular morphology, (4) renal insufficiency, and (5) women of child-bearing age. Two patients had died of unrelated causes before the study. Eighteen patients (22 hips) were lost to followup before the minimum 1-year evaluation required for inclusion in the analysis (mean followup, 5 months; range, 2–12 months). Thus, of the 192 patients (214 hips), 172 (190 hips) (149 THA, 41 resurfacing) met the criteria for inclusion in this study with complete clinical and radiographic followup of at least 12 months or a revision procedure within the first year (Table 1). There were 126 hips in males and 64 hips in females, with a mean age at the time of surgery of 50 years (range, 17–78 years). The minimum followup for the included patients was 12 months (mean, 40 months; range, 12–74 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. We obtained prior institutional review board approval for this review.
Table 1.
Patient demographics, failure rates, component details, and metal ion concentrations
| Variable | Overall | THA | Resurfacing |
|---|---|---|---|
| Number of hips | 190 | 149 | 41 |
| Primary | 143 | 122 | 41 |
| Revision | 27 | 27 | |
| Number of patients | 172 | 131 | 41 |
| Followup (months)* | 40 (12–74) | 36 (12–61) | 54 (12–74) |
| Male:female (number of hips) | 126:64 | 93:56 | 33:08 |
| Age (years)* | 50 (17–78) | 50 (17–78) | 50 (33–65) |
| Harris hip score (points)* | 92 (24–100) | 91 (24–100) | 93 (56–100) |
| VAS pain score (points)* | 1.9 (0–8) | 2.0 (0–8) | 1.7 (0–8) |
| Cup inclination (°)* | 46 (35–61) | 46 (34–61) | 47 (39–59) |
| Femoral head diameter (mm)* | 49 (40–57) | 49 (40–55) | 50 (45–57) |
| Number of patients with known metal ion levels | 93 | 78 | 15 |
| Cobalt (μg/L)* | 13 (0–150) | 14 (0–150) | 12 (0–126) |
| Chromium (μg/L)* | 6 (0–87) | 5 (0–87) | 7 (0–60) |
| Revisions (number of hips) | 24 | 19 | 5 |
| Revision rate (%) | 13 | 13 | 12 |
| Time to revision (months)* | 45 (12–75) | 43 (12–65) | 49 (24–75) |
| Failure mode (number of hips) | |||
| Metallosis | 9 (4.7%) | 9 (6.0%) | |
| Aseptic cup loosening | 8 (4.2%) | 6 (4.0%) | 2 (4.9%) |
| Periprosthetic fracture | 2 (1.1%) | 2 (4.9%) | |
| Infection | 2 (1.1%) | 2 (1.3%) | |
| Cup malposition | 1 (0.5%) | 1 (0.7%) | |
| Aseptic femoral loosening | 1 (0.5%) | 1 (2.4%) | |
| Heterotopic ossification | 1 (0.5%) | 1 (0.7%) | |
* Values are expressed as mean, with range in parentheses.
All surgery was performed by one of two surgeons (TPV, MPB). A posterior approach with detachment of the short external rotators was utilized in all procedures. The acetabulum was prepared by underreaming by 1 mm. The desired position of the acetabular cup was 40° to 45° of inclination and 15° to 25° of anteversion, consistent with the manufacturer’s recommendations. The ASR™ acetabular component is a CoCrMo alloy one-piece cup with proprietary Porocoat® porous coating. The outer surface of the cup has this porous coating with the addition of a hydroxyapatite coating.
As part of routine clinical followup, patients were followed postoperatively at 2 weeks, 6 weeks, 6 months, 1 year, and then yearly thereafter. The primary outcome was revision. The overall average time to revision was 45 months (range, 12–75 months). Clinical evaluation performed for all patients included Harris hip score, VAS score for pain, and physical examination. The mean Harris hip score was 92 (range, 24–100), and the mean VAS score for pain was 1.9 (range, 0–8). We reviewed operative reports to obtain implant femoral head diameter for each patient. The mean implanted femoral head diameter was 49 mm (range, 40–57 mm).
Two of us (KTH, TSW) reviewed the most current AP pelvic radiographs for each patient and measured the acetabular cup inclination angle. We performed this measurement using the acetabular teardrop as a landmark reference [24, 30]. Patel et al. [27] reported the intraclass correlation coefficient (R) for this technique was 0.95 and the mean ± SD difference between observers was 1.8° ± 2.4°. The mean acetabular cup inclination angle in our radiographic evaluation was 46° (range, 35°–61°).
Blood metal ion levels can be used as a surrogate marker of articular wear [8]. Blood samples for metal ion level analysis were obtained at latest followup for 93 of the 172 patients through venous cannulation with a 21-gauge stainless steel needle (Venflon™; BD Biosciences, Franklin Lakes, NJ, USA), with the first 5 mL discarded before the definitive sample was drawn. The option of obtaining blood samples was discussed with all of the patients during followup as part of our routine care. The two major reasons patients opted to obtain blood metal ion levels were if they were experiencing negative symptoms related to the hip arthroplasty or if they wanted to have a baseline metal ion level recorded for future reference. All samples were frozen and sent to the Laboratory Corporation of America® for blinded whole-blood cobalt and chromium analysis using inductively coupled plasma mass spectrometry. All samples were analyzed at a minimum of 12 months after surgery to reflect steady-state ion concentrations from wear beyond the running-in phase [12]. The reporting limit for all samples was 1.0 μg/L and all results were verified by repeat analysis.
Patients scheduled for revision with elevated blood metal ion levels and suspicion for ARMD received a preoperative pelvic MRI using a scatter reduction protocol [35]. During revision surgery, joint fluid was sampled by inserting a cannula through the intact joint capsule before incision and drainage of the effusion. The fluid was analyzed for nucleated cell count and differentiation. Normal joint fluid has no or scant nucleated cellularity. Acetabular cup fixation status, presence of osteolysis, and other signs of ARMD were recorded intraoperatively. Soft tissue samples from three to 10 sites within the neocapsule were obtained for histologic examination. Samples were routinely processed, embedded in paraffin, stained with hematoxylin and eosin, and examined by a single pathologist (LGD). Evaluation for the presence of metallic tissue and ALVAL was performed in a matter consistent with previous reports [6, 14, 16]. A diagnosis of ALVAL was made by the pathologist if a dense perivascular inflammatory infiltrate (vasculitis-associated lesion) was observed in addition to fibrinous or necrotic exudate with an accumulation of macrophages, synovial inflammation, and hyperplasia. A diagnosis of metallic tissue was made in the presence of extensive collections of metal-stained macrophages in the periprosthetic soft tissues on histopathologic analysis. On MRI review, the presence of a pseudotumor was differentiated from a fluid collection by the presence of a well-demarcated fluid collection within a capsule in the posterior left hip joint space without evidence of extracapsular communication.
We conducted Kaplan-Meier survivorship analysis to determine survival rates in both the THA and hip resurfacing groups. Survival rates were not statistically compared. We statistically tested differences between revised and nonrevised groups in both THA and hip resurfacing. Normally distributed continuous variables (age, cup inclination, femoral head diameter) were compared with independent t-tests. Variables with skewed distributions (cobalt and chromium levels) were compared using the Wilcoxon-Mann-Whitney test. There were no missing data for the variables included this study among the research subjects. All statistical analyses were performed using SAS® Enterprise Guide® Version 4 for Windows® (SAS Institute Inc, Cary, NC, USA).
Results
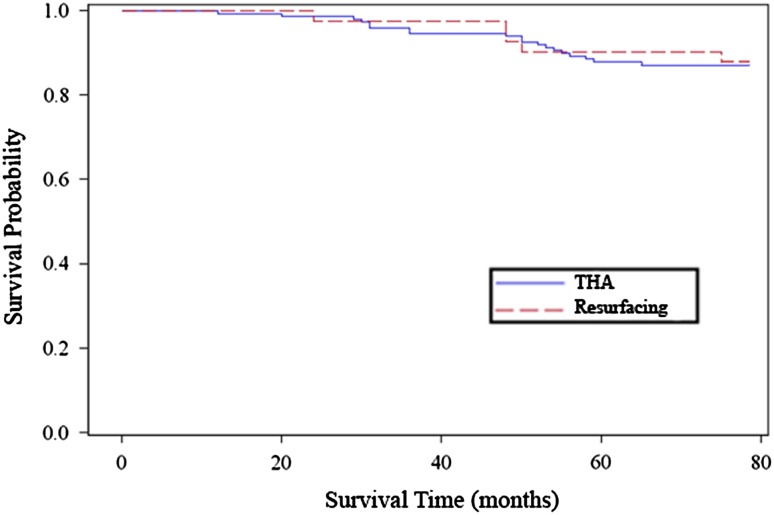
We performed 24 revisions in 18 patients with a THA and in five patients with a hip resurfacing. Therefore, 13% of the 190 hips had a revision: 13% of those with THA and 12% of those with hip resurfacing. The Kaplan-Meier survivorship rates with revision as the end point were 87% for THA and 88% for hip resurfacing (Fig. 1). There were nine revisions for elevated metal ion levels and pain concerning for metallosis (4.7% of the 190 hips), eight for aseptic acetabular component loosening (4.2%), two for infection (1.1%), one for acetabular component malposition (inclination angle: 60°) (0.5%), one for aseptic femoral component loosening (0.5%), and one for heterotopic ossification (0.5%). There were two femoral neck fractures in the hip resurfacing group. All other femoral components were well fixed at the time of revision. The patients who ultimately were revised for pain concerning for metallosis characteristically presented with moderate to severe pain predominantly in the groin with occasional audible clunking.
Fig. 1.
The Kaplan-Meier cumulative probability of survival of the ASR™ cup with an end point of revision for any reason is 0.87 for THA and 0.88 for hip resurfacing.
In the 93 patients for whom blood metal ion concentrations were obtained, the mean blood concentrations were 13 μg/L (range, 0–150 μg/L) for cobalt and 6 μg/L (range, 0–87 μg/L) for chromium. In patients with known blood metal ion concentrations, the mean prerevision blood metal ion levels were higher (p < 0.0001) in the revised group (cobalt: 48 μg/L; chromium: 18 μg/L) than in the nonrevised group (cobalt: 5 μg/L; chromium: 2 μg/L) (Table 2).
Table 2.
Results by revision status and hip procedure type
| Variable* | Overall | THA | Resurfacing | |||
|---|---|---|---|---|---|---|
| Revised | Nonrevised | Revised | Nonrevised | Revised | Nonrevised | |
| Number of hips | 24 | 166 | 19 | 130 | 5 | 36 |
| Male:female (number of hips) | 13:11 | 113:53 | 9:10 | 84:46 | 4:1 | 29:7 |
| Age (years)† | 50 (34–74) | 50 (17–78) | 51 (42–74) | 49 (17–78) | 45 (34–53) | 51 (33–65) |
| Cup inclination (°)† | 47 (38–60) | 46 (35–61) | 47 (38–55) | 46 (35–61) | 48 (45–60) | 47 (39–55) |
| Femoral head diameter (mm)† | 48 (43–57) | 49 (40–57) | 47 (43–53) | 48 (40–55) | 51 (45–57) | 50 (45–57) |
| Number of patients with known metal ion levels | 18 | 75 | 16 | 62 | 2 | 13 |
| Cobalt (ng/mL)† | 48 (3–150) | 5 (0–56)‡ | 45 (3–150) | 6 (0–56)‡ | 69 (11–126) | 3 (0–16)§ |
| Chromium (ng/mL)† | 18 (0–87) | 2 (0–18)‡ | 16 (0–87) | 2 (0–18)‡ | 35 (10–60) | 3 (0–15)‖ |
* Values at latest prerevision followup; variables with skewed distributions (cobalt and chromium levels) were compared using the Wilcoxon-Mann-Whitney test; normally distributed continuous variables (age, cup inclination, femoral head diameter) were compared with independent t-tests; †values are expressed as mean, with range in parentheses; statistical difference between revised and nonrevised groups at ‡p < 0.001, §p = 0.0024, and ‖p = 0.0018.
For 14 of the 24 revisions, the patients presented with elevated blood metal ion levels and suspicion for ARMD. The findings on the preoperative MRI, intraoperative tissue and joint fluid sampling for permanent pathology and cytology, and intraoperative assessments of acetabular fixation status, presence of osteolysis, and soft tissue pathology are summarized for these patients (Table 3). The presence of metallic tissue or ALVAL on histopathologic analysis was pervasive, occurring in all but one of the revisions with elevated blood metal ion levels suspected of ARMD. Pseudotumor was only identified on preoperative MRI in two of 14 revisions, but nonspecific fluid collections were identified in seven of the remaining 12 revisions. Evidence of osteolysis was noted intraoperatively in seven of 14 revisions.
Table 3.
Details of the revision cases for suspected ARMD
| Implant | Preoperative MRI | Histopathology | Joint fluid cytology (nucleated cell count/mm3) | Intraoperative findings | |||
|---|---|---|---|---|---|---|---|
| Metallic tissue | ALVAL | Acetabular fixation | Osteolysis | Soft tissue pathology comments | |||
| THA | Periacetabular osteolysis | Yes | Yes | 82 (71% PMN, 9% lymph) | Well fixed | Substantial metallic periacetabular osteolysis | No pathology observed |
| THA | Fluid in greater trochanteric area | Yes | Yes | 9059 (12% PMN, 46% lymph) | Well fixed | Substantial periacetabular osteolysis | No pathology observed |
| THA | Pseudotumor | Yes | Yes | 12,780 (19% PMN, 7% lymph) | Loose, no ingrowth | None | Gray fluid in deep fascia, destruction of short ER |
| THA | Posterolateral fluid collection | Yes | Yes | 1566 (9% PMN, 36% lymph) | Well fixed | None | Gray fluid in deep fascia, destruction of abductors and short ER |
| THA | Fluid collection in iliopsoas bursa | Yes | Yes | 24 (15% PMN, 8% lymph) | Well fixed | None | No pathology observed |
| THA | Normal | Yes | Yes | 46 (46% PMN, 12% lymph) | Well fixed | Metallic periacetabular and pubic osteolysis | No pathology observed |
| THA | Multiloculated posterior fluid collection | Yes | Yes | 180 (77% PMN, 3% lymph) | Well fixed | Minimal metallic periacetabular osteolysis | Gray fluid in deep fascia, destruction of short ER |
| THA | Fluid in greater trochanteric area | Yes | Yes | 389 (7% PMN, 8% lymph) | Well fixed | None | Destruction of posterior capsule short ER |
| THA | Periacetabular osteolysis, gluteus minimus/medius tendinosis | Yes | Yes | Rare histocytes and lymphocytes | Loose, no ingrowth | Substantial metallic periacetabular osteolysis | No pathology observed |
| THA | Posterior fluid collection | Yes | Yes | Scant cellularity | Well fixed | None | Gray fluid in deep fascia, destruction of short ER |
| THA | Normal | No | No | Scant cellularity | Loose, no ingrowth | None | No pathology observed |
| THA | Posterior fluid collection | Yes | Yes | Scant cellularity | Well fixed | Minimal periacetabular osteolysis | No pathology observed |
| THA | Pseudotumor | Yes | Yes | Not obtained | Well fixed | None | Pseudotumor in posterior capsule |
| Resurfacing | Normal | Yes | No | 138 (40% PMN, 60% lymph) | Well fixed (loose femoral cap) | Metallic subacetabular and pubic osteolysis | No pathology observed |
Fourteen revision procedures for suspected ARMD are shown in the table; not included are revisions for periprosthetic fracture of hip resurfacings (two), infection (two), heterotopic ossification (one), before cytology and histopathology protocols were in place (three), and performed at outside hospitals (two); ARMD = adverse reaction to metal debris; ALVAL = aseptic lymphocyte-dominant vasculitis-associated lesion; PMN = polymorphonuclear neutrophils; lymph = lymphocytes; ER = external rotators.
Discussion
Although MOM articulations possess numerous theoretical advantages in implant design, concerning revision rates have been reported in many MOM implant systems. Additionally, patients often develop elevated blood metal ion levels, the implications of which remain unclear. We therefore reported (1) revision rate, (2) blood metal ion levels, and (3) intraoperative findings for revisions related to ARMD in all ASR™ components used in THA and hip resurfacing procedures by two surgeons at a single institution.
There are several limitations to our study. First, this was a retrospective nonrandomized study. Since this study consists of all patients who received ASR™ implants during the study time, no learning curve is accounted for and some differences in surgical technique between the two surgeons must be assumed. Second, we are also limited by the fact that blood metal ion levels were obtained for only 93 of the 172 patients. The majority of blood metal ion levels obtained were in patients who had complaints at the time of clinical followup. Third, this was not a consecutive series. Although this series included every ASR™ component implanted at our medical center, many other THA procedures were performed during the same time period. Candidates for ASR™ component implantation were identified in accordance with our previously described indications. Fourth, histopathologic data were obtained for only ASR™ component revisions suspected of ARMD instead of revisions for any reason.
Numerous authors and organizations have reported increasing concern over high revision rates in MOM implant systems. Specific to the ASR™ acetabular cup, a large series from the National Joint Registry for England and Wales reported a 5-year revision rate of 12% for the hip resurfacing system and 13% for the THA system [25] (Table 4). Similarly, Steele et al. [34] recently reported an overall revision rate of 15% at a followup of only 1.6 years (range, 0.2–3.4 years) in a series of 105 hips with the ASR™ XL THA system. The joint registry data for the ASR™ cup in hip resurfacing is comparable to our revision rate of 12% at about the same followup time. Our revision rate with the THA system of 13% was similar to the reported data from the joint registry and slightly lower than the data from Steele et al. [34]. However, our reported reasons for revision (metallosis, 4.7%; aseptic acetabular component loosening, 4.2%; infection, 1.1%; acetabular component malposition, 0.5%; aseptic femoral component loosening, 0.5%) were almost identical to the data from Steele et al. [34]. Bernthal et al. [1] reported a revision rate in the ASR™ XL THA of 17.1% with a followup time between 2 and 5 years.
Table 4.
Revision rates, metal ion concentrations, and followup times for the ASR™ system reported in the literature
| Study | Revision rate (%) | Whole blood metal ion levels (μg/L)* | Followup (months)* | |
|---|---|---|---|---|
| Cobalt | Chromium | |||
| THA | ||||
| National Joint Registry for England and Wales [25] | 13 | 60 | ||
| Steele et al. [34] | 15 | 19 (2–41) | ||
| Bernthal et al. [1] | 17.1 | (24–60) | ||
| Langton et al. [16] | 6.0 | 3.26 (1.1–32) | 3.71 (2.4–22) | 41 (10–57) |
| Lavigne et al. [19] | 0 | 1.78 (0.32–7.59) | 1.78 (0.24–6.20) | 24 |
| Current study | 13 | 14 (0–150) | 5 (0–87) | 36 (12–61) |
| Resurfacing | ||||
| National Joint Registry for England and Wales [25] | 12 | 60 | ||
| Jameson et al. [13] | 5.6 | 43 (30–57) | ||
| Langton et al. [16] | 3.2 | 2.74 (0.4–271) | 4.16 (1.5–69.8) | 35 (8–57) |
| Langton et al. [17] | 1.3 | 1.89 (0.4–228.0) | 3.61 (0.6–115.0) | 26 (13–44) |
| Current study | 12 | 12 (0–126) | 7 (0–60) | 54 (12–74) |
* Values are expressed as mean, with range in parentheses.
De Smet et al. [8] demonstrated chromium ion levels of more than 17 μg/L and cobalt levels of more than 19 μg/L were associated with metallosis and elevated joint fluid ion levels. They postulated these concentrations may serve as a cutoff marker for clinical importance. We found differences in blood metal ion levels between revised and nonrevised implants in both THA and hip resurfacing. Furthermore, the blood metal ion levels in the revised implant group in this study (cobalt: 48 μg/L; chromium: 18 μg/L) exceeded the threshold for concern proposed by De Smet et al. [8]. The mechanism of production for metal wear and the differences in wear between MOM THA and hip resurfacing are currently highly debated topics [10, 13, 17, 19, 22, 38]. Due to the tremendous variability in metal ion levels in different patients, we have avoided the use of this test as a main determinant of clinical decision making to this point. However, the frequency with which we have been collecting metal ion levels in patients who are doing well after MOM implantation is increasing. We suspect, on an individual patient level, comparing steady-state asymptomatic metal ion levels to new ion levels after onset of symptoms may prove to be useful information. As our understanding of the role of blood metal ion levels in patients with MOM implants is enhanced, these tests will continue to play increasingly important diagnostic roles.
A large portion of the failures in our study (14 of 24) can be classified as ARMD. Pelvic metal artifact reduction sequence MRI has been used to identify signs of ARMD in patients with unexplained painful MOM hips [31]. In our experience, this technology has been useful for identifying pseudotumors, fluid collections, osteolytic lesions, and muscle atrophy in patients with painful MOM hips. This information can confirm the presence of ARMD and can help guide revision surgical planning. It is important to note the ARMD process is thought to take several years to develop [2]. Only one revision in this study took place within the first 2 years of the index procedure. The true incidence of ARMD in patients with MOM implants will likely be higher than what current studies report as long-term data become available. If ARMD is suspected during a revision procedure, tissue samples are histologically examined for the presence of chronic inflammation and ALVAL. In this study, tissue samples consistent with ALVAL were present in 13 of 14 revisions suspected for ARMD in which tissue samples were obtained. Briefly, ALVAL is hypothesized to be a result of an immunogenic response to the metal ions that are slowly released from the prosthetic bearing surfaces as a by-product of wear [40]. These wear particles can lead to hapten formation and elicit a Type IV hypersensitivity response in the periprosthetic tissue [11]. Our group has previously reported our experiences with histologic examination of tissue samples suspected of ALVAL [40]. Although its pathophysiology is currently poorly understood, ALVAL is becoming increasingly recognized by joint arthroplasty surgeons as a major issue in patients with MOM bearings.
Numerous studies have been published in recent years cataloging the higher-than-expected failure rates and elevated blood metal ion levels associated with MOM implants. Our study specific to the ASR™ XL THA and ASR™ hip resurfacing systems further confirmed the poor performance of both the ASR™ system itself and MOM implants as a class. Furthermore, many of the failures that we observed occurred close to the time that our study was concluded, implying premature failure was an ongoing process. We therefore expect our MOM implant revision rate and the revision rates of other groups to increase in the years to come. Even though the THA and resurfacing groups in our study had similar overall revision rates, the hip resurfacings were more prone to aseptic component loosening and periprosthetic fracture. It is possible the hip resurfacing system can fail prematurely for reasons related to ARMD and other independent design issues, and further investigation is warranted. Modern MOM implant systems gained substantial initial popularity among orthopaedic surgeons as a result of their numerous theoretical advantages, including reduced volumetric wear and increased femoral head size compared to metal-on-polyethylene components. Although current MOM implant systems are substantially flawed as a class, the design was well intended and the hypothetical benefits of implants with alternative bearing surfaces still exist. Research and innovation related to alternative bearing surfaces should be encouraged despite the major oversight of the dangers of current MOM implants. However, there is a clear and immediate need to reevaluate the device approval process and monitoring requirements to prevent similar predicaments in the future. Blood metal ion tests, MRI, and other nascent diagnostic tools can all be utilized to evaluate patients with troublesome implants and intraoperative histopathologic analysis and joint fluid cytology can help confirm ARMD at the time of revision. Continued analysis is pivotal to fully elucidate the effects of MOM hip implantation.
Acknowledgments
We thank Dr. Benjamin J. Hansen MD, Duke University Medical Center, for manuscript editing, Diane B. Covington PA, Duke University Medical Center, for data collection, and Leslie G. Dodd MD, Duke University Medical Center, for histologic review of soft tissue samples.
Footnotes
One of the authors (TPV) certifies that he, or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of more than $100,000 from DePuy Orthopaedics, Inc, a Johnson and Johnson company, Warsaw, IN, USA. One of the authors (KTH) certifies that he has received a stipend, during the study period, from Duke University’s CTSA Grant TL1RR024126 from NCCR/NIH. Each remaining author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Duke University Medical Center, Durham, NC, USA.
References
- 1.Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27:539–544. doi: 10.1016/j.arth.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Bolland BJ, Culliford DJ, Langton DJ, Millington JP, Arden NK, Latham JM. High failure rates with a large-diameter hybrid metal-on-metal total hip replacement: clinical, radiological and retrieval analysis. J Bone Joint Surg Br. 2011;93:608–615. doi: 10.1302/0301-620X.93B5.26309. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 4.Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Elevated serum cobalt with metal-on-metal articulating surfaces. J Bone Joint Surg Br. 1997;79:316–321. doi: 10.1302/0301-620X.79B2.7326. [DOI] [PubMed] [Google Scholar]
- 5.Cuckler JM, Moore KD, Lombardi AV, Jr, McPherson E, Emerson R. Large versus small femoral heads in metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19:41–44. doi: 10.1016/j.arth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 7.DePuy Orthopaedics Inc. DePuy ASR™ hip implant recall guide. 2011. Available at: http://www.depuy.com/usprofessional-depuy-hip-recall. Accessed June 29, 2011.
- 8.De Smet K, De Haan R, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C, Gill HS. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am. 2008;90(suppl 4):202–208. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- 9.Dunstan E, Ladon D, Whittingham-Jones P, Carrington R, Briggs TW. Chromosomal aberrations in the peripheral blood of patients with metal-on-metal hip bearings. J Bone Joint Surg Am. 2008;90:517–522. doi: 10.2106/JBJS.F.01435. [DOI] [PubMed] [Google Scholar]
- 10.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award. Metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. doi: 10.1302/0301-620X.83B3.9674. [DOI] [PubMed] [Google Scholar]
- 12.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg Am. 2008;90(suppl 3):125–133. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- 13.Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92:28–37. doi: 10.1302/0301-620X.92B1.22769. [DOI] [PubMed] [Google Scholar]
- 14.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty: five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 15.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 17.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 18.Langton DJ, Joyce TJ, Jameson SS, Lord J, Van Orsouw M, Holland JP, Nargol AV, De Smet KA. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164–171. doi: 10.1302/0301-620X.93B2.25099. [DOI] [PubMed] [Google Scholar]
- 19.Lavigne M, Belzile EL, Roy A, Morin F, Amzica T, Vendittoli PA. Comparison of whole-blood metal ion levels in four types of metal-on-metal large-diameter femoral head total hip arthroplasty: the potential influence of the adapter sleeve. J Bone Joint Surg Am. 2011;93(suppl 2):128–136. doi: 10.2106/JBJS.J.01885. [DOI] [PubMed] [Google Scholar]
- 20.Leslie I, Williams S, Brown C, Isaac G, Jin Z, Ingham E, Fisher J. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements: an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87:163–172. doi: 10.1002/jbm.b.31087. [DOI] [PubMed] [Google Scholar]
- 21.Malviya A, Ramaskandhan J, Holland JP, Lingard EA. Metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2010;92:1675–1683. doi: 10.2106/JBJS.I.01426. [DOI] [PubMed] [Google Scholar]
- 22.Matthies A, Underwood R, Cann P, Ilo K, Nawaz Z, Skinner J, Hart AJ. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg Br. 2011;93:307–314. doi: 10.1302/0301-620X.93B3.25551. [DOI] [PubMed] [Google Scholar]
- 23.McKee GK, Watson-Farrar J. Replacement of arthritic hips by the McKee-Farrar prosthesis. J Bone Joint Surg Br. 1966;48:245–259. [PubMed] [Google Scholar]
- 24.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–232. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 25.NJR Centre. National Joint Registry for England and Wales: 7th Annual Report, 2010. 2011. Available at: http://www.njrcentre.org.uk. Accessed June 29, 2011.
- 26.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 27.Patel SR, Toms AP, Rehman JM, Wimhurst J. A reliability study of measurement tools available on standard picture archiving and communication system workstations for the evaluation of hip radiographs following arthroplasty. J Bone Joint Surg Am. 2011;93:1712–1719. doi: 10.2106/JBJS.J.00709. [DOI] [PubMed] [Google Scholar]
- 28.Pelt CE, Bergeson AG, Anderson LA, Stoddard GJ, Peters CL. Serum metal ion concentrations after unilateral vs bilateral large-head metal-on-metal primary total hip arthroplasty. J Arthroplasty. 2011;26:1494–1500. doi: 10.1016/j.arth.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22:140–144. doi: 10.1016/j.arth.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Robb JE, Rymaszewski LA, Bentley HB, Donnan PT. Reliability of the acetabular teardrop as a landmark. Surg Radiol Anat. 1991;13:181–185. doi: 10.1007/BF01627982. [DOI] [PubMed] [Google Scholar]
- 31.Sabah SA, Mitchell AW, Henckel J, Sandison A, Skinner JA, Hart AJ. Magnetic resonance imaging findings in painful metal-on-metal hips: a prospective study. J Arthroplasty. 2011;26:71–76, 76.e1–2. [DOI] [PubMed]
- 32.Schmalzried TP, Peters PC, Maurer BT, Bragdon CR, Harris WH. Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces. J Arthroplasty. 1996;11:322–331. doi: 10.1016/S0883-5403(96)80085-4. [DOI] [PubMed] [Google Scholar]
- 33.Smith TM, Berend KR, Lombardi AV, Jr, Emerson RH, Jr, Mallory TH. Metal-on-metal total hip arthroplasty with large heads may prevent early dislocation. Clin Orthop Relat Res. 2005;441:137–142. doi: 10.1097/01.blo.0000193810.23706.73. [DOI] [PubMed] [Google Scholar]
- 34.Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of Articular Surface Replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6 suppl):14–18. doi: 10.1016/j.arth.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Toms AP, Smith-Bateman C, Malcolm PN, Cahir J, Graves M. Optimization of metal artefact reduction (MAR) sequences for MRI of total hip prostheses. Clin Radiol. 2010;65:447–452. doi: 10.1016/j.crad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration. FDA’s role and activities. 2011. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241769.htm. Accessed June 29, 2011.
- 38.Vendittoli PA, Amzica T, Roy AG, Lusignan D, Girard J, Lavigne M. Metal ion release with large-diameter metal-on-metal hip arthroplasty. J Arthroplasty. 2011;26:282–288. doi: 10.1016/j.arth.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Visuri TI, Pukkala E, Pulkkinen P, Paavolainen P. Cancer incidence and causes of death among total hip replacement patients: a review based on Nordic cohorts with a special emphasis on metal-on-metal bearings. Proc Inst Mech Eng H. 2006;220:399–407. doi: 10.1243/095441105X63282. [DOI] [PubMed] [Google Scholar]
- 40.Watters TS, Cardona DM, Menon KS, Vinson EN, Bolognesi MP, Dodd LG. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am J Clin Pathol. 2010;134:886–893. doi: 10.1309/AJCPLTNEUAH8XI4W. [DOI] [PubMed] [Google Scholar]
- 41.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 42.Zywiel MG, Sayeed SA, Johnson AJ, Schmalzried TP, Mont MA. State of the art in hard-on-hard bearings: how did we get here and what have we achieved? Expert Rev Med Devices. 2011;8:187–207. doi: 10.1586/erd.10.75. [DOI] [PubMed] [Google Scholar]