Abstract
Aims: Nicotinamide phosphoribosyltransferase (Nampt) is a key enzyme for nicotinamide adenine dinucleotide (NAD+) biosynthesis, and recent evidence indicates its role in inflammatory processes. Here, we investigated the potential effects of pharmacological Nampt inhibition with FK866 in a mouse myocardial ischemia/reperfusion model. In vivo and ex vivo mouse myocardial ischemia/reperfusion procedures were performed. Results: Treatment with FK866 reduced myocardial infarct size, neutrophil infiltration, and reactive oxygen species (ROS) generation within infarcted hearts in vivo in a mouse model of ischemia and reperfusion. The benefit of FK866 was not shown in the Langendorff model (ex vivo model of working heart without circulating leukocytes), suggesting a direct involvement of these cells in cardiac injury. Sera from FK866-treated mice showed reduced circulating levels of the neutrophil chemoattractant CXCL2 and impaired capacity to prime migration of these cells in vitro. The release of CXCL8 (human homolog of murine chemokine CXCL2) by human peripheral blood mononuclear cells (PBMCs) and Jurkat cells was also reduced by FK866, as well as by sirtuin (SIRT) inhibitors and SIRT6 silencing, implying a pivotal role for this NAD+-dependent deacetylase in the production of this chemokine. Innovation: The pharmacological inhibition of Nampt might represent an effective approach to reduce neutrophilic inflammation- and oxidative stress-mediated tissue damage in early phases of reperfusion after a myocardial infarction. Conclusions: Nampt inhibition appears as a new strategy to dampen CXCL2-induced neutrophil recruitment and thereby reduce neutrophil-mediated tissue injury in mice. Antioxid. Redox Signal. 18, 630–641.
Introduction
Initially identified as pre-B cell colony-enhancing factor (PBEF) in 1994 (33), and subsequently proposed to act as an insulin-mimetic hormone (visfatin) (8, 21), nicotinamide phosphoribosyltransferase (Nampt) has now been established as a key enzyme for nicotinamide adenine dinucleotide (NAD+) biosynthesis in mammalian cells (21, 23). Nampt is present both intracellularly and in the extracellular space (20). It catalyzes the condensation of nicotinamide and 5-phosphoribosyl-1-pyrophosphate to yield nicotinamide mononucleotide (22). The latter is subsequently converted to NAD+ by nicotinamide mononucleotide adenylyltransferase 1-3 (NMNAT1-3) enzymes. Nampt inhibition with small molecules, such as FK866 and CHS 828, markedly reduces intracellular NAD+ and, thus affects downstream metabolic pathways (4, 33, 36).
Recent studies show that Nampt-derived NAD+ might fuel pro-inflammatory and pro-immunogenic pathways by modulating the activity of NAD+-dependent enzymes and metabolic processes (4). Intracellular Nampt expression is upregulated during activation of immune cells (14, 15, 32) and adequate NAD+ concentration as well as activation of intracellular NAD+-dependent histone deacetylase (called sirtuins, which are downstream of Nampt) are required for the synthesis of pro-inflammatory cytokines (4, 35). Circulating levels of Nampt were found to be increased in patients with established inflammatory diseases (5, 18, 19, 30), and might represent a promising cardiovascular risk biomarker in diabetic patients (6). Drawing from these studies, we focused on the potential role of circulating and intracellular Nampt in the inflammatory processes underlying myocardial ischemia and reperfusion injury in mice. The potential benefit of pharmacologic Nampt inhibition with FK866 (a highly specific noncompetitive inhibitor) (10) was explored in vivo and ex vivo on myocardial infarct size, inflammatory cell infiltration, reactive oxygen species (ROS) production, and serum inflammatory chemokine expression. In addition, NAD+-dependent intracellular pathways mediating leukocyte release of CXC chemokines and ROS were investigated in vitro in inflammatory cells, previously described to play a crucial role in post-infarction inflammation (7).
Innovation.
The most important novelties of the present work are represented by the demonstration of the direct and active role of serum and intracellular Nampt in phagocyte-mediated reperfusion injury after a myocardial infarction. In particular, the pharmacological inhibition of Nampt was associated with the reduction of infarct size in vivo in a mouse model of myocardial ischemia and reperfusion. The molecular mechanisms, by which Nampt inhibition exerted its beneficial effects, were identified in: 1. The decrease of neutrophil infiltration within the infarcted myocardium in FK866-treated mice as compared to vehicle; 2. The reduction in ROS release within the infarcted myocardium; and 3. The inhibition of NAD+-dependent pathways underlying the production of the neutrophil chemoattractant CXCL2 (murine homolog of CXCL8). Thus, we concluded that Nampt might be a crucial determinant of leukocyte-mediated injury in early phases of reperfusion after a myocardial infarction in mice.
Results
Acute myocardial infarction increases serum Nampt levels
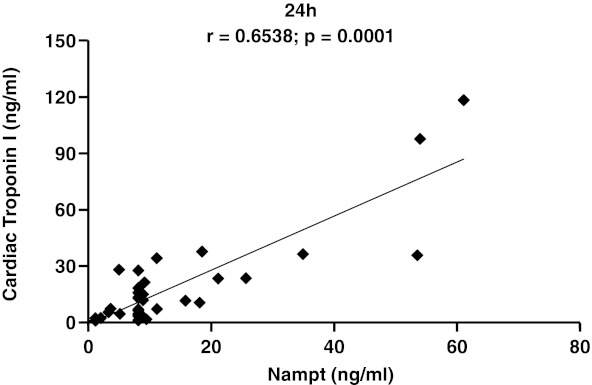
In order to investigate the possible role of Nampt in myocardial ischemia/reperfusion, serum Nampt levels were measured in sham-operated and untreated mice subjected to 30 min of myocardial ischemia, followed by different time points of reperfusion. Serum Nampt levels were significantly increased in mice with myocardial infarction from 1 h until 24 h of reperfusion as compared to sham-operated animals (Table 1). At 24 h of reperfusion, Nampt serum levels positively correlated with cTnI levels (Fig. 1). In the myocardium, a significant reduction in Nampt levels (tissue homogenates) was observed in 30-min ischemic hearts as compared to sham-operated hearts at 24 h of reperfusion (sham-operated [n=3] vs. infarcted hearts [n=10]: mean±SD: 237.50±47.08 ng/ml vs. 122.28±37.24 ng/ml, p=0.007).
Table 1.
Nampt Serum Levels During Myocardial Reperfusion
| Surgical procedure | Reperfusion time |
|---|---|
| 1 hour | |
| Sham | 2.64 (2.38–8.60) |
| Infarct | 19.66 (8.63–180.19)‡ |
| 12 hours | |
| Sham | 5.14 (3.27–8.13) |
| Infarct | 8.98 (8.22–30.65)†# |
| 24 hours | |
| Sham | 1.61 (1.26–4.37) |
| Infarct | 8.13 (8.13–61.17)*# |
Data are expressed as median (interquartile range [IQR]) of Nampt serum concentrations (ng/ml). (Sham group: n=6; Infarct group: n=7–10).
p<0.01 vs. sham.
p<0.001 vs. sham.
p<0.05 vs. sham.
N.S. vs. Infarct 1 h.
FIG. 1.
Spearman rank correlation between serum levels of Cardiac Troponin I and Nampt at 24 h of reperfusion.
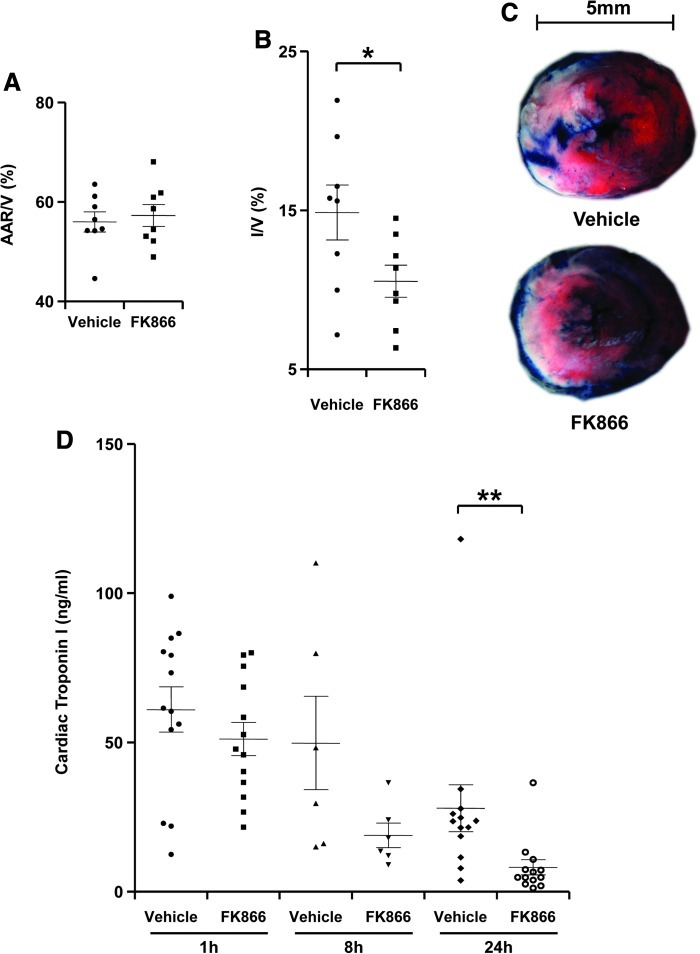
Treatment with FK866 reduces myocardial infarct size
Myocardial post-ischemic damage during reperfusion has been shown to be partially mediated by inflammatory reactions (27). Since studies showed that Nampt-derived NAD+ promotes inflammation by sustaining immune cell viability and by promoting cytokine production (4, 5, 35), we decided to evaluate whether pharmacological Nampt inhibition would block inflammation in a mouse model of myocardial ischemia/reperfusion. To this end, we made use of FK866, which effectively blocks NAD+ biosynthesis and reduces NAD+ tissue levels in vivo (4, 5, 10). Mice were subjected to myocardial ischemia (30 min), followed by 24 h of reperfusion. FK866 (30 mg/kg) or control vehicle (0.1% DMSO in PBS) were administered 5 min and 12 h after the ischemia onset, accordingly to a schedule that was determined on extensive assessments of drug activity in vivo in different types of tissues (Supplementary Table S1; supplementary data are available online at www.liebertonline/ars). The area at risk (AAR) was similar in treatment groups, indicating that ligature was reproducibly performed at the same level of the left anterior coronary artery (Fig. 2A). Treatment with FK866 reduced infarct size as compared to vehicle treatment at 24 h of reperfusion (Figs. 2B and 2C). Accordingly, treatment with FK866 significantly reduced serum cTnI levels as compared to vehicle-treated mice at 24 h after reperfusion (Fig. 2D). Apoptotic areas were not significantly reduced by FK866 treatment at the same time point of reperfusion (24 h), although a reduction trend was observed (vehicle-treated hearts vs. FK866-treated hearts: mean±SEM, n=8–9: 10.15±4.43% vs. 2.62±0.75%, p=0.0927). Interestingly, FK866 determined a significant reduction in the myocardial tissue concentrations of NAD+ and a significant increase in adenosine triphosphate (ATP) levels, as compared to control vehicle (Supplementary Table S2). No difference in mouse mortality at 24 h of reperfusion was observed in both treatment groups (1 mouse in each treatment group [n=9] died during the 24 h of reperfusion: 11.1%, p=NS). Finally, since Nampt was previously reported to promote cardiomyocyte survival, we assessed whether FK866 would have direct cytotoxic activity on cardiac myocytes (rat H2c9 cell line). Although a strong reduction in intracellular NAD+ concentration was detected in response to FK866 (vehicle vs. FK866 30 nM: mean±SEM, n=3: 45.80±0.13 nmol/mg vs. 2.00±0.18 nmol/mg, p<0.01), no effect on cell viability was documented for up to 48 h of treatment (Supplementary Fig. S1).
FIG. 2.
Treatment with FK866 reduces infarct size in vivo after 24 h of reperfusion. Vehicle or FK866 (30 mg/kg) was administered 5 min after ischemia onset and after 12 h of reperfusion. Data are expressed as scattered plots (mean±SEM, n=8 per group). (A) Quantification of area at risk (AAR) per ventricle surface (V). (B) Quantification of infarct size (I) per V: *p<0.05. (C) Representative images of TTC stained middle heart sections of vehicle- or FK866-treated mice. (D) Serum cardiac troponin I (cTnI) levels of vehicle- or 30 mg/kg FK866-treated mice after 1 h, 8 h, and 24 h of reperfusion (n=6–13 per group): **p<0.01. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
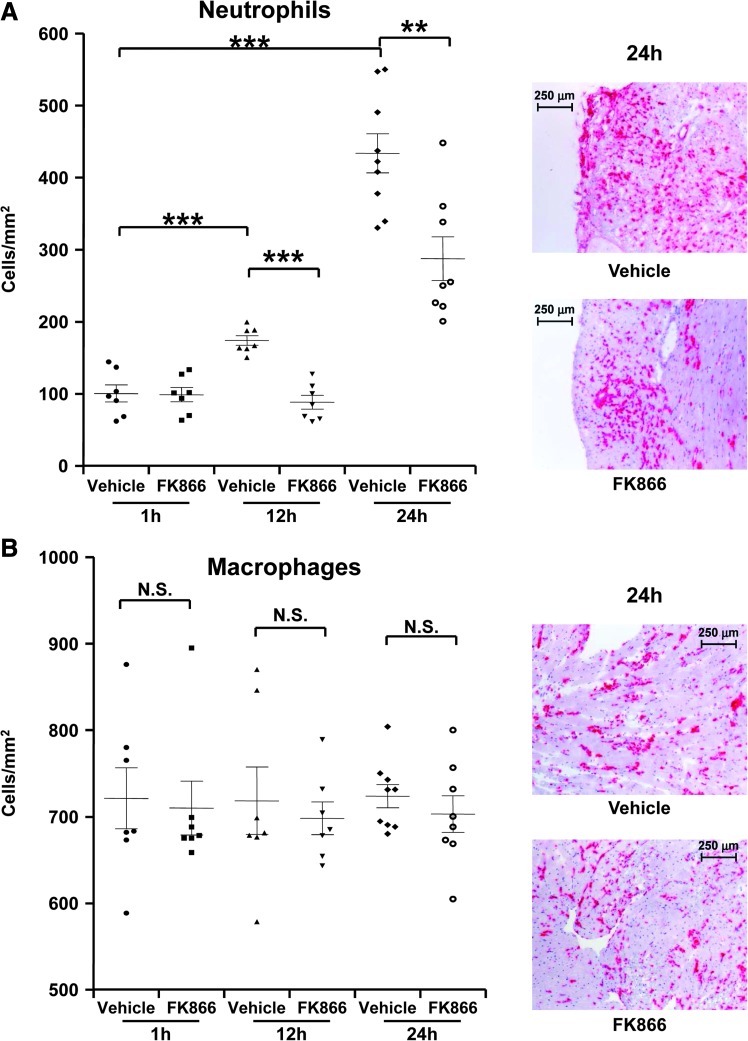
Treatment with FK866 inhibits neutrophil recruitment in infarcted hearts during reperfusion
To investigate the potential impact of Nampt inhibition on the inflammatory mechanisms underlying myocardial reperfusion injury (7, 27), we assessed leukocyte infiltration in infarcted hearts at different time points of reperfusion. FK866 treatment significantly reduced neutrophil infiltration at 12 h and 24 h after reperfusion as compared to the vehicle-treated group (Fig. 3A). Accordingly, at 8 h of reperfusion, FK866 treatment was associated with a significant reduction of neutrophil elastase (Elane) mRNA expression in mouse hearts as compared to vehicle (fold increase of Elane mRNA expression as compared to sham-operated hearts in vehicle-treated hearts vs. FK866-treated hearts: mean±SD, n=6–9: 2.07±0.89 vs. 0.88±0.32, p=0.026). In addition, FK866 capacity to reduce neutrophil infiltration in infarcted hearts was also confirmed by measuring MPO in cardiac homogenates at 24 h of reperfusion (vehicle-treated vs. FK866-treated hearts: mean±SD, n=9–10: 1269.03±442.58 ng/ml vs. 675.72±136.89 ng/ml: p=0.0076). At 24 h of incubation, FK866 did not affect human neutrophil apoptotic rate in vitro as compared to control vehicle (Supplementary Fig. S2A and B). Accordingly, in infarcted hearts from FK866-treated mice, neutrophils mostly infiltrated regions around myocardial apoptotic zones instead of co-localizing with them (Supplementary Fig. 2C). Therefore, these results suggest that FK866-mediated reduction of neutrophil locomotion was not due to increased neutrophil apoptosis.
FIG. 3.
Treatment with FK866 reduces neutrophil infiltration in infarcted hearts in vivo. (A) Quantification of infiltrated neutrophils per area in frozen sections of infarcted hearts at different reperfusion time points (1 h, 12 h, and 24 h). Data are expressed as scattered plots (mean±SEM, n=7–9 per group). *p<0.05; ***p<0.001. On the right, representative images of neutrophil (Ly-6B.2+ cell) infiltration at 24 h reperfusion are shown. (B) Quantification of infiltrated macrophages per area in frozen sections of infarcted hearts at different reperfusion time points (1 h, 12 h, and 24 h). Data are expressed as scattered plots (mean±SEM, n=7–9 per group). N.S.: not significant. On the right, representative images of macrophage (CD68+ cell) infiltration at 24 h of reperfusion are shown. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
No difference in infiltrating macrophages was detected in the two groups at any reperfusion time point investigated (Fig. 3B). Similarly, no difference in T lymphocyte (CD3+ cells) infiltration at 12 h and 24 h after reperfusion was observed (vehicle-treated hearts vs. FK866-treated hearts:, mean±SD, n=4–9: 12 h, 3.15±1.79 vs. 3.44±2.61 cells/mm2, p=NS; 24h, 0.84±0.50 vs. 1.31±0.65 cells/mm2, p=NS).
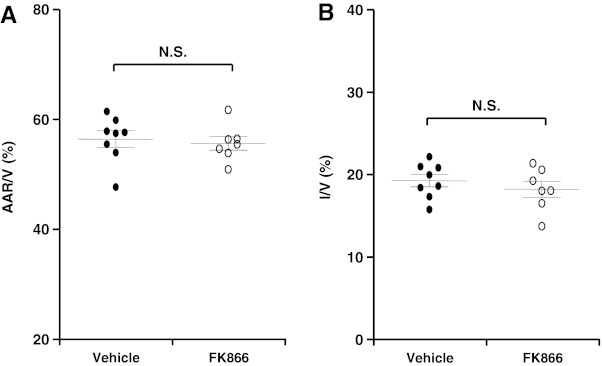
To confirm that the benefit of FK866 was due to its capacity to prevent neutrophil infiltration in the infarcted myocardium, we assessed the efficacy of this Nampt inhibitor in an ex vivo I/R model characterized by the absence of circulating leukocytes. Similarly to the in vivo protocol, buffer-perfused hearts (Langendorff system) were subjected to 30 min local ischemia. Five min after ischemia onset, FK866 or vehicle was added to the circulating perfusion system. In line with previous Langendorff protocols, infarct size was assessed after 2 h of reperfusion (27). As shown in Figure 4, AAR was similar in both treatment groups (Fig. 4A). Treatment with FK866 did not induce any effect on infarct size as compared to control vehicle (Fig. 4B).
FIG. 4.
Treatment with FK866 does not reduce infarct size in a Langendorff model without circulating leukocytes. Vehicle or FK866 (5 μg/ml) was added to the perfusion medium 5 min after ischemia onset. Data are expressed as scattered plots (mean±SEM, n=7–8 per group). (A) Quantification of area at risk (AAR) per ventricle surface (V) after 2 h of reperfusion; p: N.S. (not significant). (B) Quantification of infarct size (I) per V after 2 h of reperfusion; p: N.S. (not significant).
Treatment with FK866 reduced ROS production in infarcted hearts
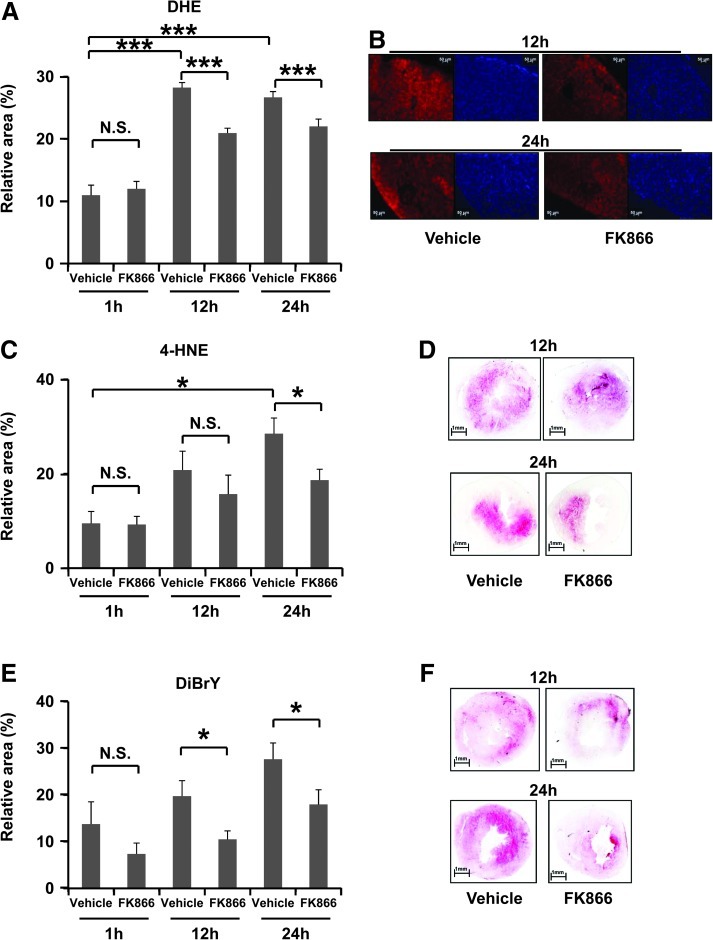
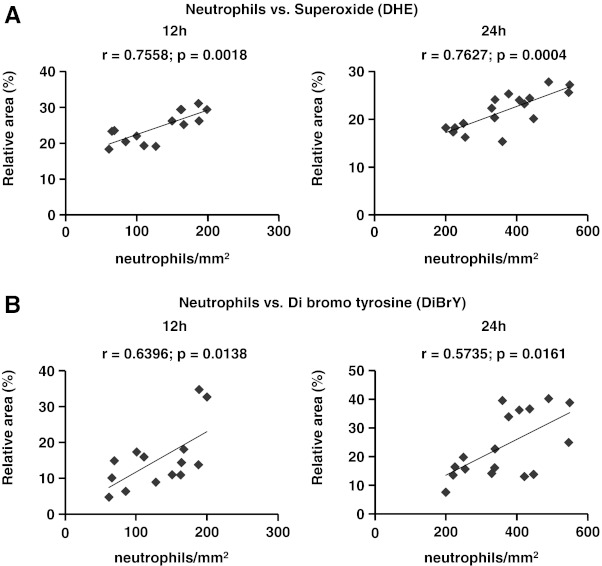
Given the association between neutrophilic inflammation and ROS release during reperfusion (26, 27), we investigated these mediators of injury within the infarcted hearts at 12 h and 24 h of reperfusion. Treatment with FK866 significantly reduced superoxide production within infarcted hearts at 12 h and 24 h reperfusion as compared to vehicle (Fig. 5A and B). In line with these results, FK866 treatment also reduced 4-HNE positive areas, which are thought to result from ROS-mediated lipid peroxidation (20) at 24 h of reperfusion (Figs. 5C and 5D). Furthermore, the levels of 3,5-dibromotyrosine (an oxidant of presumed neutrophil origin) were also decreased in FK866-treated mice as compared to control animals at both 12 h and 24 h of reperfusion (Figs. 5E and 5F) (3). As predicted, neutrophil infiltration in infarcted hearts positively correlated with superoxide production (Fig. 6A) and with dibromotyrosine-positive areas (Fig. 6B). In addition, infiltrated neutrophils co-localized with oxidant-rich areas in consecutive cryosections in infarcted hearts at 24 h of reperfusion (Supplementary Fig. S3). No difference in oxidant release was observed at 1 h of reperfusion in all staining assays (Fig. 5A). During oxidative burst, NADPH oxidase utilizes NADPH to produce superoxide which then recombines with other molecules to produce other ROS. In vitro, treatment with FK866 (100 nM for 6 h) partially reduced intracellular NAD(H) and NADP(H) content in human neutrophils. Particularly, we estimated that on average FK866 reduced NADPH levels by 35% as compared to the vehicle (Supplementary Table S3). However, this treatment did not affect TNF-α-induced superoxide production (Supplementary Table S4). Therefore, these data suggest that the reduced ROS levels detected in vivo within the infarcted hearts might be due to the reduction in the number of infiltrating neutrophils rather than to a direct inhibition of neutrophilic superoxide release during post-infarction inflammation.
FIG. 5.
Treatment with FK866 reduced superoxide production in infarcted hearts at 12 h and 24 h of reperfusion. (A) Quantification of superoxide (DHE staining) content in frozen sections of infarcted hearts at 1 h, 12 h, and 24 h of reperfusion (n=7–9 per group; ***p<0.001; p: N.S. [not significant]). (B) On the right, representative images of DHE stained middle heart sections of vehicle- or 30 mg/kg FK866-treated mice at 12 h and 24 h of reperfusion are shown. On the left, correspondent images of the same middle heart sections stained with 4',6-diamidino-2-phenylindole (DAPI), visualizing nuclear DNA. (C) Quantification of 4-HNE staining content in frozen sections of infarcted hearts at 1 h, 12 h, and 24 h of reperfusion (n=3–9 per group; *p<0.05; p: N.S. [not significant]). (D) Representative images of 4-HNE stained middle heart sections of vehicle- or 30 mg/kg FK866-treated mice at 12 h and 24 h of reperfusion are shown. (E) Dibromo tyrosine (DiBrY) content in frozen sections of infarcted hearts at 1 h, 12 h, and 24 h of reperfusion. Data are mean±SEM (n=4–10 per group; *p<0.05; p: N.S. [not significant]). (F) Representative images of DiBrY stained middle heart sections of vehicle- or 30 mg/kg FK866-treated mice at 12 h and 24 h of reperfusion are shown. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 6.
Spearman rank correlation between neutrophil infiltration and ROS production at 12 h and 24 h of reperfusion in infarcted hearts. (A) Neutrophils vs. DHE staining at 12 h and 24 h of reperfusion. (B) Neutrophils vs. DiBrY staining at 12 h and 24 h of reperfusion.
Treatment with FK866 reduces CXCL2 serum levels during reperfusion
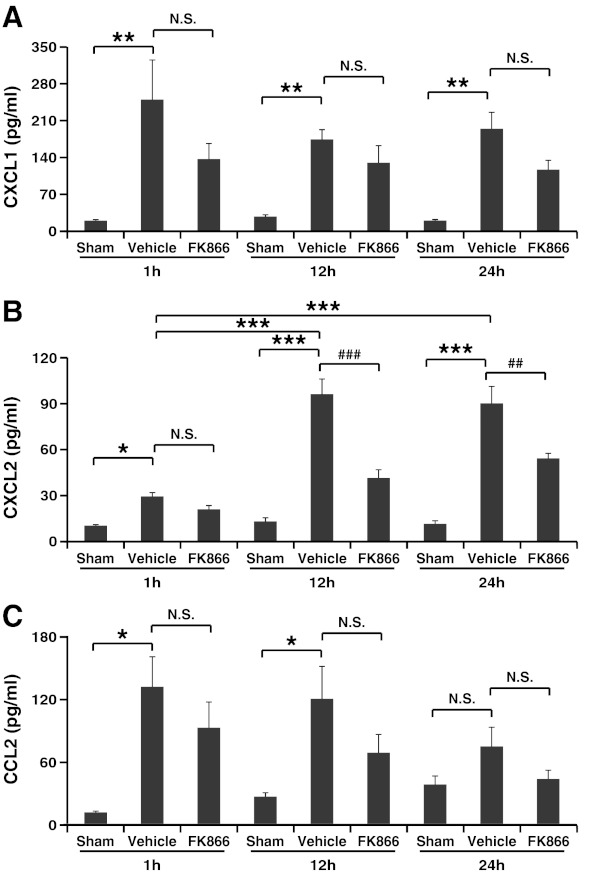
To define the molecular mechanisms underlying FK866-mediated inhibition of leukocyte recruitment within the infarcted hearts, we determined the serum levels of CXCL1, CXCL2 (known neutrophil chemoattractants) (27), and of CCL2 (monocyte/macrophage chemoattractant) (2) during reperfusion. A significant increase in the serum levels of all chemokines was observed in the first 12 h of reperfusion in vehicle-treated mice, as compared to sham-operated animals (Fig. 7A–C). FK866 treatment significantly reduced only CXCL2 serum levels at 12 h and 24 h of reperfusion as compared to vehicle-treated mice (Fig. 7B). Accordingly, only serum from vehicle-treated mice (at 12 h of reperfusion, time point at which the highest concentrations of neutrophil chemoattractants were detected) increased mouse neutrophil migration in vitro towards recombinant CXCL1 and CXCL2 as compared to control chemotaxis medium (Table 2). Conversely, incubation with serum from FK866-treated mice failed to effectively stimulate mouse neutrophil migration. Importantly, FK866 failed to directly affect neutrophil migration towards recombinant CXC chemokines (Supplementary Table S5), indicating that in vivo FK866-mediated inhibition of neutrophil recruitment within ischemic hearts was not due to impaired neutrophil migration, but instead reflected the reduction in CXCL2 serum levels.
FIG. 7.
Treatment with FK866 reduces CXCL2 serum levels during reperfusion. Leukocyte chemoattractant serum levels were measured at different reperfusion time points (1 h, 12 h, and 24 h). Data are expressed as mean±SEM (n=6–13 per group). (A) CXCL1: **p<0.01; p: N.S. (not significant). (B) CXCL2: *p<0.05, ***p<0.001; ##p<0.01, ###p<0.001; p: N.S. (not significant). (C) CCL2: *p<0.05; p: N.S. (not significant).
Table 2.
Effects of Serum from Vehicle- or FK866-Treated Mice (12 h of Reperfusion) on Mouse Neutrophil Migration
|
Polycarbonate assay (C.I.) | |||||||
|---|---|---|---|---|---|---|---|
| |
Upper well |
||||||
| |
|
Vehicle-treated serum |
FK866-treated serum |
||||
| Lower well | CTL | 1:4 | 1:2 | 1:1 | 1:4 | 1:2 | 1:1 |
| CTL | 1.00±0.00 | 1.08±0.09 | 1.66±0.16 | 2.14±0.25 | 1.10±0.04 | 0.98±0.03 | 1.47±0.29 |
| CXCL1 (200 ng/ml) | 3.67±0.33* | 3.88±0.44 | 5.23±0.48 | 6.39±0.89† | 2.98±0.35 | 3.69±0.56 | 4.97±1.12§ |
| CXCL2 (200 ng/ml) | 3.61±0.35* | 3.62±0.22 | 4.37±0.49 | 5.94±0.50‡ | 3.05±0.61 | 3.17±0.37 | 4.35±0.47|| |
Data are expressed as mean±SEM, (n=4).
p<0.05 vs. control medium (CTL)-stimulated cell migration to CTL.
p<0.05 vs. CTL-stimulated cell migration to CXCL1.
p<0.05 vs. CTL-stimulated cell migration to CXCL2.
N.S. vs. CTL-stimulated cell migration to CXCL1.
N.S. vs. CTL-stimulated cell migration to CXCL2.
Intracellular Nampt pathway is crucial for CXCL8 (human homolog of murine CXCL2) production in human lymphocytes.
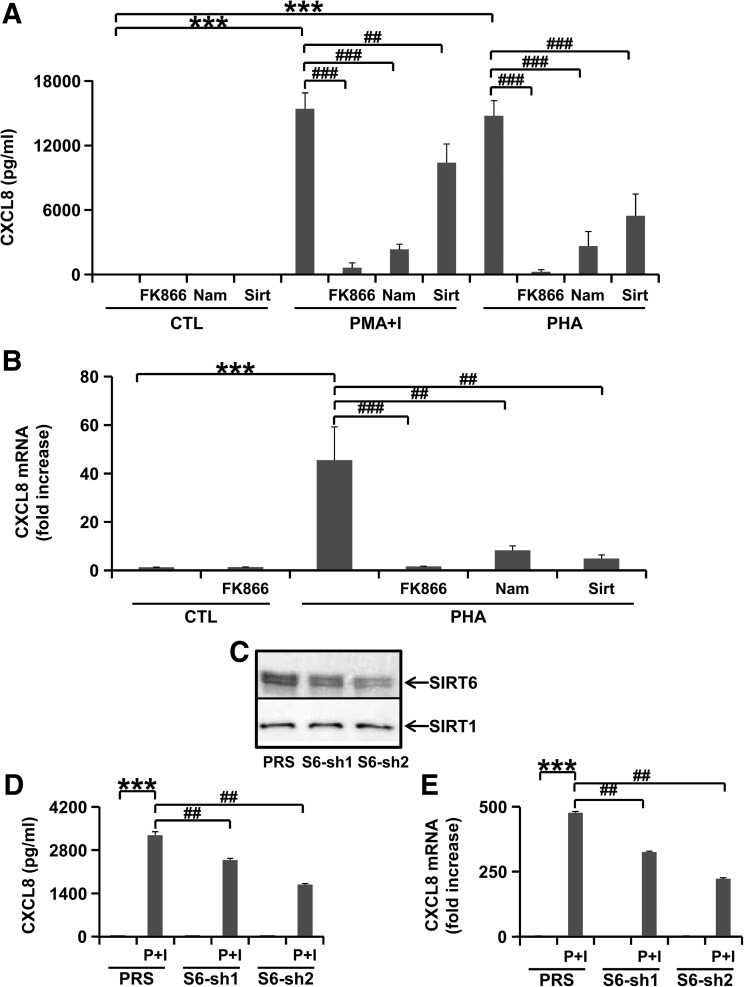
To explore the molecular mechanisms underlying the observed reduction in CXCL2 levels upon intracellular NAD+ biosynthesis inhibition with FK866, we focused on the role of the Nampt pathway in CXCL8 (human CXCL2 homolog) production by human T lymphocytes. Mitogen stimulation with PMA/Ionomycin or PHA strongly induced CXCL8 production in human PBMCs as compared to control medium (Fig. 8A). However, CXCL8 synthesis was reduced by co-incubation with FK866. Moreover, in line with a potential involvement of a sirtuin member in CXCL8 production, we found that Nam at a high concentration (10 mM) and sirtinol (nonspecific sirtuin inhibitor) also strongly reduced CXCL8 synthesis by mitogen-stimulated PBMCs (Fig. 8A and data not shown). Impaired CXCL8 production in response to FK866, sirtinol, and Nam reflected reduced CXCL8 mRNA amounts in PHA-stimulated cells (Fig. 8B). Importantly, with the exception of sirtinol (which reduced cell viability from 75% to 65%), the treatments tested did not significantly impair cell viability in any of the experiments performed (data not shown), suggesting that the observed reduction in CXCL8 synthesis in response to FK866 and sirtuin inhibition did not reflect ongoing cell demise.
FIG. 8.
Nampt inhibition reduces CXCL8 production in vitro in peripheral blood mononuclear cells (PBMCs) and Jurkat cells. (A, B) Human PBMCs were incubated in 24-well plates in the absence or presence of 30 nM FK866. After 24 h, cells were incubated with control medium (CTL), 5 μg/ml phytohemagglutinin-P (PHA), 25 ng/ml phorbol myristic acetate (PMA)/0.5 μM Ionomycin (I), 10 mM nicotinamide (Nam) or 50 μM sirtinol (Sirt, nonspecific sirtuin inhibitor) for a further 18 h. Results are means±SEM of three independent experiments with three donors. CXCL8 concentrations in the supernatants (A) ***p<0.001; ##p<0.01, ###p<0.001. CXCL8 mRNA expression in PBMCs (B) ***p<0.001; ##p<0.01, ##p<0.001. (C) Jurkat cells were engineered to express SIRT6 silencing S6-sh1, S6-sh2, or the empty pRETROSuper (PRS) vector. Thereafter, SIRT6 and SIRT1 levels were detected by immunoblotting in cell protein lysates (one representative experiment out of three is shown). (D, E) Jurkat cells expressing S6-sh1, S6-sh2, or PRS were incubated in the presence or absence of 25 ng/ml PMA and 0.5 μM ionomycin (P+I) for 24 h. Thereafter, supernatants were collected and cells were used for mRNA extraction. Results are means±SEM of three separate experiments, ***p<0.001; ##p<0.01. CXCL8 concentrations in the supernatants were detected by ELISA (D). CXCL8 mRNA induction as compared to unstimulated PRS Jurkat cells was determined by QPCR (E).
Subsequently, given its proposed role in the production of pro-inflammatory TNF-α and IFN-γ (4, 35), we explored a potential involvement of the sirtuin SIRT6 in CXCL8 biosynthesis. We silenced SIRT6 in Jurkat cells by RNAi using validated shRNAs (24). Both short-hairpins reduced SIRT6 expression while leaving SIRT1 levels unaffected (Fig. 8C). PMA/Ionomycin-induced CXCL8 up regulation (at both mRNA and protein levels) was significantly reduced in SIRT6-silenced cells as compared to cells engineered with the control vector PRS (Figs. 8D and 8E). Remarkably, CXCL8 downregulation correlated with the degree of silencing achieved by the two hairpins (S6-sh2 > S6-sh1). Spontaneous cell death in PBMC was about 10% in control medium and about 25% among cells treated with PMA-ionomycin or PHA, as previously reported (4). Therefore, these findings showed a direct role of SIRT6 in the synthesis of neutrophil chemoattractant CXCL8.
Discussion
Drawing from previous studies indicating a primary role of post-ischemic inflammation in the pathophysiology of ischemic myocardial damage (27), and based on the emerging role of Nampt-mediated NAD+ biosynthesis in inflammation (4), we decided to explore Nampt pharmacological inhibition as a therapeutic strategy in acute myocardial infarction. Our data indicated that treatment with the Nampt inhibitor FK866 in vivo was associated with a significant reduction in the infarct size at 24 h of reperfusion as compared to vehicle-treated mice. This beneficial effect was likely not due to a direct activity of FK866 on the myocardium, but rather to a reduction of neutrophil recruitment within the infarcted hearts. In particular, FK866 treatment in vivo abrogated neutrophil recruitment via the reduction of systemic CXCL2 serum levels during the early phases of reperfusion. Notably, in our study, we did not explore the potential benefit of this pharmacological invention on cardiac function preservation over long periods of time (see Supplementary Material, “Justification of the choice of methods to assess the cardiac function”), which clearly represents a limitation of our observations. Finally, despite some other limitations due to the differences in the reperfusion time in the in vivo and ex vivo models, treatment with FK866 did not induce any protective effects in the absence of circulating leukocytes (Langendorff ex vivo model).
CXCL2 and its human homologue CXCL8 represent some of the most potent neutrophil chemoattractants in mice and humans, respectively (27). We recently showed that pharmacologic inhibition of circulating CXCL2 with Evasin-3 was associated with reduced neutrophil recruitment and injury within the infarcted myocardium (27). Here, we show that a reduction of CXCL2 circulating levels induce benefits that are similar to the neutralization of its bioactivity. Direct evidence of this mechanism was shown in in vitro chemotaxis experiments by co-incubating mouse serum from FK866-treated or vehicle-treated mice. This approach showed that only serum from vehicle-treated mice at 12 h of reperfusion (time point at which the highest concentrations of CXCL2 were observed) significantly induced neutrophil migration towards CXCL1 and CXCL2, as compared to control chemotaxis medium. This effect was not detected in the presence of serum from FK866-treated mice. Since FK866 did not directly affect mouse neutrophil migration towards CXCL1 or CXCL2 in vitro, we concluded that, in vivo, FK866-mediated reduction of circulating CXCL2 levels was the major determinant of the observed reduction in neutrophil recruitment to the damaged myocardium. We also explored the potential mechanisms by which Nampt inhibition could reduce CXCL2 serum levels. Since we have previously demonstrated that FK866 administration reduced NAD+ levels in leukocytes in vivo (4), we focused on T lymphocytes (the most represented circulating leukocyte subset in mice) (25). Co-incubation with FK866 markedly reduced PHA- or PMA/Ionomycin-mediated CXCL8 mRNA expression and protein release in human PBMCs (>75% CD3+ lymphocytes). These data indicate that NAD+ availability plays a key role in CXCL8 biosynthesis. We further showed that inhibitors of sirtuins (downstream NAD+-dependent deacetylases) mimicked FK866-mediated suppression of CXCL8 synthesis in PBMCs. Furthermore, as suggested by silencing experiments in Jurkat cells, we specifically linked CXCL8 production in T lymphocytes to SIRT6 activity. SIRT6 had previously been associated with the production of TNF-α and IFN-γ (4, 35). Thus, SIRT6 appears as a master regulator of CXCL8 production in human T lymphocytes in response to pro-inflammatory stimuli. The use of human primary PBMCs and of a leukemia cell line represents an important limitation of our study. However, we believe that the relevance of our data on the regulatory role of Nampt on CXCL8 production remains attractive. The finding that a Nampt inhibitor would be beneficial for the treatment of myocardial infarction was surprising since Hsu and colleagues and Lym and co-workers reported that Nampt has cardioprotective activities (12, 13, 17). The authors showed that Nampt downregulation in cardiomyocytes reduces intracellular NAD+ and ATP, and predisposes myocytes to apoptosis. On the other hand, cardiac-specific Nampt overexpression reduced the size of myocardial infarction and apoptosis in response to ischemia. A relevant protective role might be mediated by cardiac SIRT1 (a NAD+-dependent deacetylase downstream Nampt) (13). Thus, the authors proposed that myocyte Nampt would prevent cell death by ensuring that adequate NAD+ and ATP levels are maintained. The apparently conflicting results between the present article and the work of Hsu and co-workers might be explained by both different methodological issues and mouse models used. In particular, we used a reduced duration of myocardial ischemia (30 min vs. 45–120 min). Prolonged ischemia has been shown to increase cardiac injury and might activate different salvage pathways in the cardiac cells (34). In addition, Hsu and co-workers did not perform in vivo pharmacological inhibition of Nampt. Instead, they made use of a selective cardiac transgenic upregulation of this enzyme. Notably, Hsu and colleagues specifically evaluated the effect of Nampt and of its inhibition/upregulation in cardiomyocytes, without considering the impact of global Nampt inhibition and, in particular, the possible role of Nampt-dependent pro-inflammatory pathways in AMI.
The present research shows that Nampt (at both systemic and intracellular levels) might be considered as a promising target in acute myocardial infarction and reperfusion injury in mice. Interestingly, we found increased Nampt serum levels in infarcted mice as compared to sham-operated animals. Increased levels of plasma Nampt were previously shown also in humans few days after AMI (18). In mice, we observed an earlier increase of Nampt (already at 1 h of reperfusion). Nampt plasma levels were similar to those observed by Beiser and co-workers after 90 min of hemorrhagic shock in mice (1). Interestingly, we also showed a positive correlation between Nampt serum levels and Cardiac Troponin I (a well-validated parameter of myocardial necrosis selectively released by cardiomyocytes) (9, 27). Given the rapid increase in Nampt serum levels in response to myocardial ischemia (also confirmed by a concomitant decrease in cardiac Nampt levels in infarcted hearts), we believe that these changes might reflect Nampt leakage from damaged heart tissue, instead of de novo protein synthesis. However, Nampt production by other tissues (including inflammatory cells) cannot in principle be excluded.
We investigated the potential mechanisms underlying neutrophil-mediated injury, such as ROS. These oxidative compounds are generated within the myocardium during ischemia/reperfusion also by infiltrating inflammatory cells (16), and they directly contribute to cardiac injury, thereby favoring cardiomyocyte death (11). FK866 treatment reduced ROS production in the infarcted myocardium at 12 h and 24 h of reperfusion. This effect strongly correlated with the reduced neutrophil infiltration in the ischemic area, suggesting that FK866-mediated benefit would result from inhibition of CXCL2-induced neutrophil infiltration. We did not observe any direct effect of pre-incubation with FK866 on TNF-α-mediated release of superoxide anion in human neutrophils. Thus, these in vitro experiments indicate that treatment with FK866 inhibited ROS production in vivo by reducing the number of infiltrating neutrophils rather than by preventing neutrophil activation and superoxide release.
Finally, in contrast to what observed by Hsu and colleagues (12), at 24 h of reperfusion, we observed that FK866 treatment led to increased ATP levels in the myocardium of infarcted mice as compared to vehicle-treated animals. Higher tissue concentrations of ATP could be due to the ATP saved as a result of blocked of NAD+ biosynthesis (which is per se an ATP-consuming process) (29). Alternatively, they could reflect the persistence of more viable myocardium in FK866-treated animals. Either way, the relevance of this increase in myocardial ATP concentration may be marginal as shown by the lack of FK866-mediated benefits in the Langendorff model.
In conclusion, this study sheds new light on Nampt's role in AMI. Our data indicate that pharmacological Nampt inhibition might represent an effective strategy to reduce post-infarction neutrophilic inflammation within the injured myocardium. However, since our experiments were mainly performed using in vivo, ex vivo, and in vitro mouse models, the relevance of our discovery is limited and caution should be used for its potential translational application in humans. We believe that further evaluations of Nampt inhibitors for clinical applications in humans are warranted.
Materials and Methods
In vivo I/R protocol
Male C57Bl/6 mice (8–12 weeks of age) were obtained from the University Medical Centre animal facility of the University of Geneva. The method is described in detail in the online supplementary material The dose and the treatment schedule were planned on the basis of recently published experiments in mice and in vitro (4, 31) and in vivo results on systemic NAD+ depletion in FK866-treated mice (Supplementary Table 1).
Ex vivo I/R protocol
The technique of Langendorff isolated buffer-perfused mouse heart preparation was used (28). Animals were anesthetized with 4% isoflurane and sacrificed by neck dislocation. The heart was rapidly excised and placed in ice-cold Krebs-Henseleit bicarbonate (KHB) buffer consisting of (in mmol/L): 118.5 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 5 glucose, and equilibrated with 95% O2/5% CO2 (pH 7.4). The detailed method is reported in the online supplementary material.
Area at risk and infarct size assessment
To assess area at risk (AAR) and infarct size (I) in in vivo I/R protocol, mice were anaesthetized and sacrificed after 24 h of reperfusion, as previously described (27). The method is described in detail in the online supplementary material.
Justification of the choice of methods to assess the cardiac function
This topic is discussed in detail in the online supplementary material.
Determination of mouse heart content of Nampt and myeloperoxidase
Mouse heart content of Nampt and MPO was measured by colorimetric enzyme-linked immunosorbent assay (ELISA), as described in the online supplementary material.
Immunostaining
Hearts from animals sacrificed after 1 h, 12 h, or 24 h of reperfusion were frozen in OCT and cut serially from the occlusion locus to the apex in 7 μm sections. The method is described in detail in the online supplementary material.
Apoptotic cell measurement within infarcted hearts
Apoptotic areas within the infarcted hearts were measured after 24 h of reperfusion. The method is described in detail in the online supplementary material.
Oxidative stress determination
Measurement of superoxide in myocardium submitted to I/R was performed using the superoxide-sensitive dye dihydroethidium staining (DHE, Molecular Probes), 4-hydroxy-2-nonenal (mouse anti-4-HNE monoclonal antibody, Oxis International Inc, Foster City, CA) and 3,5-dibromotyrosine (mouse anti-Di bromo tyrosine monoclonal antibody, AMS Biotechnology, LTD, Abingdon, UK) immunostaining. The methods are described in detail in the online supplementary material.
Neutrophil (phagocytic oxidase) versus cardiomyocyte (mitochondria) driven ROS formation
This topic is discussed in detail in the online supplementary material.
Determination of the intracellular NAD+ and ATP levels in mouse hearts
Mouse hearts were flash frozen in liquid nitrogen, and NAD+ and ATP levels were determined as described in detail in the online supplementary material.
Serum Nampt, cardiac troponin I, and neutrophil chemoattractant level detection
Serum Nampt, cardiac troponin I (cTnI), and neutrophil chemoattractant levels were measured by colorimetric enzyme-linked immunosorbent assay (ELISA), as described in the online supplementary material.
Mouse peritoneal neutrophil isolation and migration assay
Mouse neutrophils were obtained as previously described (31). The detailed method of mouse neutrophil migration is described in the online supplementary material.
Isolation, culture, and NAD+/NADP+ determination in human primary neutrophils
Neutrophils were obtained from healthy volunteers after signature of an informed consent. Neutrophil isolation and cultures are described in detail in the online supplementary material. The method to determine the intracellular content of NAD/NADP is described in the online supplementary material.
Human peripheral blood mononuclear cell isolation and culture
PBMCs (>75% CD3+ lymphocytes) were isolated and cultured as described in the online supplementary material.
Jurkat cell culture and SIRT6 silencing
The T cell leukemia cell line Jurkat was grown in RPMI 1640-based medium supplemented with 10% FBS, penicillin (10,000 U/ml), and streptomycin (10,000 μg/ml). Retroviral transgenesis was then performed as described in detail in the online supplementary material.
Cardiac cell line culture, viability assay, and NAD+ determination
H2c9 cardiomyocyte culture and viability test are discussed in detail in the online supplementary material.
Real time RT PCR
Total RNA was extracted from mouse tissues and human cells as detailed in the online supplementary material.
Immunoblotting
Cell lysates were generated from 1.5×106 cells by directly resuspending cell pellets in sodium dodecyl sulfate (SDS) sample buffer (Tris-HCl 0.25 M, pH 6.8, SDS 2%, glycerol 10%, β-mercaptoethanol 2%, bromophenol blue 0.005%; Boston Bioproducts, Boston, MA). The method is described in detail in the online supplementary material.
Statistical analysis
Statistics were performed with GraphPad Instat software version 3.05 (GraphPad Software) using one-way ANOVA for multiple group comparison or unpaired t test (two-tailed) for two group comparison. Results are expressed as mean±SEM. For continuous variables, results are expressed as medians (interquartile range [IQR]). The Mann-Whitney nonparametric test was used for comparisons of continuous variables. Spearman's rank correlation coefficients were used to assess correlations. P values below 0.05 were considered significant.
Supplementary Material
Abbreviations Used
- DHE
dihydroethidium staining
- Elane
neutrophil elastase
- NAD+
nicotinamide adenine dinucleotide
- Nampt
nicotinamide phosphoribosyltransferase
- PBEF
pre-B cell colony-enhancing factor
- PBMCs
peripheral blood mononuclear cells
- ROS
reactive oxygen species
- SIRT
sirtuin
- TNF-α
tumor necrosis factor-alpha
Acknowledgments
This research was funded by EU FP7, Grant number 201668, AtheroRemo and by Novartis Foundation to Mach, by grants from the Swiss National Science Foundation (to Mach [# 310030B-133127] and Montecucco [# 32003B-134963/1]), by the Associazione Italiana per la Ricerca sul Cancro (to Nencioni and Bauer), by the Associazione Italiana per la Lotta alle Leucemie/Linfomi e al Mieloma, and by the University of Genoa.
Disclosure Statement
No conflicts of interest exist for any of the authors.
References
- 1.Beiser DG. Wang H. Li J. Wang X. Yordanova V. Das A. Mirzapoiazova T. Garcia JG. Stern SA. Vanden Hoek TL. Plasma and myocardial visfatin expression changes are associated with therapeutic hypothermia protection during murine hemorrhagic shock/resuscitation. Resuscitation. 2010;81:742–748. doi: 10.1016/j.resuscitation.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunersreuther V. Mach F. Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. [PubMed] [Google Scholar]
- 3.Braunersreuther V. Pellieux C. Pelli G. Burger F. Steffens S. Montessuit C. Weber C. Proudfoot A. Mach F. Arnaud C. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J Mol Cell Cardiol. 2010;48:789–798. doi: 10.1016/j.yjmcc.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzone S. Fruscione F. Morando S. Ferrando T. Poggi A. Garuti A. D'Urso A. Selmo M. Benvenuto F. Cea M. Zoppoli G. Moran E. Soncini D. Ballestrero A. Sordat B. Patrone F. Mostoslavsky R. Uccelli A. Nencioni A. Catastrophic NAD+ depletion in activated T lymphocytes through NAMPT inhibition reduces demyelination and disability in EAE. PLoS One. 2009;4:e7897. doi: 10.1371/journal.pone.0007897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busso N. Karababa M. Nobile M. Rolaz A. Van Gool F. Galli M. Leo O. So A. De Smedt T. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008;3:e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MP. Chung FM. Chang DM. Tsai JC. Huang HF. Shin SJ. Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. Smith CW. Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 8.Fukuhara A. Matsuda M. Nishizawa M. Segawa K. Tanaka M. Kishimoto K. Matsuki Y. Murakami M. Ichisaka T. Murakami H. Watanabe E. Takagi T. Akiyoshi M. Ohtsubo T. Kihara S. Yamashita S. Makishima M. Funahashi T. Yamanaka S. Hiramatsu R. Matsuzawa Y. Shimomura I. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 9.Haider KH. Stimson WH. Cardiac troponin-I: A biochemical marker for cardiac cell necrosis. Dis Markers. 1993;11:205–215. doi: 10.1155/1993/901687. [DOI] [PubMed] [Google Scholar]
- 10.Hasmann M. Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 11.Hori M. Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CP. Oka S. Shao D. Hariharan N. Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu CP. Zhai P. Yamamoto T. Maejima Y. Matsushima S. Hariharan N. Shao D. Takagi H. Oka S. Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q. Liu D. Majewski P. Schulte LC. Korn JM. Young RA. Lander ES. Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal J. Zaidi M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem Biophys Res Commun. 2006;342:1312–1318. doi: 10.1016/j.bbrc.2006.02.109. [DOI] [PubMed] [Google Scholar]
- 16.Kaminski KA. Bonda TA. Korecki J. Musial WJ. Oxidative stress and neutrophil activation—The two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 17.Lim SY. Davidson SM. Paramanathan AJ. Smith CC. Yellon DM. Hausenloy DJ. The novel adipocytokine visfatin exerts direct cardioprotective effects. J Cell Mol Med. 2008;12:1395–1403. doi: 10.1111/j.1582-4934.2008.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu SW. Qiao SB. Yuan JS. Liu DQ. Association of plasma visfatin levels with inflammation, atherosclerosis and acute coronary syndromes (ACS) in humans. Clin Endocrinol (Oxf) 2009;71:202–207. doi: 10.1111/j.1365-2265.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu X. Ji Y. Chen J. Li S. Luo F. Circulating visfatin in chronic obstructive pulmonary disease. Nutrition. 2009;25:373–378. doi: 10.1016/j.nut.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Liu YH. Carretero OA. Cingolani OH. Liao TD. Sun Y. Xu J. Li LY. Pagano PJ. Yang JJ. Yang XP. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:H2616–2623. doi: 10.1152/ajpheart.00546.2005. [DOI] [PubMed] [Google Scholar]
- 21.Luk T. Malam Z. Marshall JC. Pre-B cell colony-enhancing factor (NAMPT)/visfatin: A novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 22.Martin PR. Shea RJ. Mulks MH. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol. 2001;183:1168–1174. doi: 10.1128/JB.183.4.1168-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlothlin JR. Gao L. Lavoie T. Simon BA. Easley RB. Ma SF. Rumala BB. Garcia JG. Ye SQ. Molecular cloning and characterization of canine pre-B-cell colony-enhancing factor. Biochem Genet. 2005;43:127–141. doi: 10.1007/s10528-005-1505-2. [DOI] [PubMed] [Google Scholar]
- 24.Michishita E. McCord RA. Berber E. Kioi M. Padilla-Nash H. Damian M. Cheung P. Kusumoto R. Kawahara TL. Barrett JC. Chang HY. Bohr VA. Ried T. Gozani O. Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller TA. Schaefer FW., 3rd Changes in mouse circulating leukocyte numbers in C57BL/6 mice immunosuppressed with dexamethasone for Cryptosporidium parvum oocyst production. Vet Parasitol. 2007;149:147–157. doi: 10.1016/j.vetpar.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Montecucco F. Lenglet S. Braunersreuther V. Burger F. Pelli G. Bertolotto M. Mach F. Steffens S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612–620. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Montecucco F. Lenglet S. Braunersreuther V. Pelli G. Pellieux C. Montessuit C. Lerch R. Deruaz M. Proudfoot AE. Mach F. Single administration of the CXC chemokine-binding protein Evasin-3 during ischemia prevents myocardial reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1371–1377. doi: 10.1161/ATVBAHA.110.206011. [DOI] [PubMed] [Google Scholar]
- 28.Pellieux C. Aasum E. Larsen TS. Montessuit C. Papageorgiou I. Pedrazzini T. Lerch R. Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol. 2006;41:459–466. doi: 10.1016/j.yjmcc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Pittelli M. Formentini L. Faraco G. Lapucci A. Rapizzi E. Cialdai F. Romano G. Moneti G. Moroni F. Chiarugi A. Inhibition of nicotinamide phosphoribosyltransferase: Cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem. 2010;285:34106–34114. doi: 10.1074/jbc.M110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rho YH. Solus J. Sokka T. Oeser A. Chung CP. Gebretsadik T. Shintani A. Pincus T. Stein CM. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60:1906–1914. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rongvaux A. Galli M. Denanglaire S. Van Gool F. Drèze PL. Szpirer C. Bureau F. Andris F. Leo O. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 32.Rongvaux A. Shea RJ. Mulks MH. Gigot D. Urbain J. Leo O. Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Samal B. Sun Y. Stearns G. Xie C. Suggs S. McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takagi H. Hsu CP. Kajimoto K. Shao D. Yang Y. Maejima Y. Zhai P. Yehia G. Yamada C. Zablocki D. Sadoshima J. Activation of PKN mediates survival of cardiac myocytes in the heart during ischemia/reperfusion. Circ Res. 2010;107:642–649. doi: 10.1161/CIRCRESAHA.110.217554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Gool F. Gallí M. Gueydan C. Kruys V. Prevot PP. Bedalov A. Mostoslavsky R. Alt FW. De Smedt T. Leo O. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Heideman A. Berglund A. Larsson R. Nygren P. Safety and efficacy of NAD depleting cancer drugs: Results of a phase I clinical trial of CHS 828 and overview of published data. Cancer Chemother Pharmacol. 2010;65:1165–1172. doi: 10.1007/s00280-009-1125-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.