Abstract
Quinic acid (QA) is an active ingredient of Cat's Claw (Uncaria tomentosa), which is found to be active in enhancing DNA repair and immunity in model systems and able to generate neuroprotective effects in neurons. However, QA's role in improving survival is not well studied. Here we report that QA can provide protection in Caenorhabidits elegans and improve worm survival under stress. Under heat stress and oxidative stress, QA-treated wild-type C. elegans N2 (N2) survived 17.8% and 29.7% longer, respectively, than the control worms. Our data suggest that under heat stress, QA can upregulate the expression of the small heat shock protein hsp-16.2 gene, which could help the worms survive a longer time. We also found that QA extended the C. elegans mutant VC475 [hsp-16.2 (gk249)] life span by 15.7% under normal culture conditions. However, under normal culture conditions, QA did not affect hsp-16.2 expression, but upregulated the expression of daf-16 and sod-3 in a DAF-16–dependent manner, and downregulated the level of reactive oxygen species (ROS), suggesting that under normal conditions QA acts in different pathways. As a natural product, QA demonstrates great potential as a rejuvenating compound.
Introduction
Natural products have been playing an important role in antiaging research. For example, resveratrol was reported to be able to extend the life span of model organisms, including the yeast Saccharomyces cerevisiae,1 the nematode worm Caenorhabditis elegans, and the fruit fly Drosophila melanogaster.2 Although the life span–extending effect of resveratrol remains controversial,3 more evidence has been obtained in support of the antiaging effect of resveratrol.4,5
Researchers interested in generating an antiaging effect by natural products are always trying to find new candidate natural compounds. In recent years, a water-soluble Cat's Claw extract called C-Med-100 was found to possess versatile protective activities; it can inhibit cell growth without cell death, thus providing enhanced opportunities for DNA repair6–8 and the consequences thereof, such as immune stimulation,7,9 antiinflammation,10 and cancer prevention.6,11
We found that quinic acid (QA), an active ingredient of Cat's Claw, could significantly improve the survival of N2 worms under both normal conditions and stress and significantly extend the life span of the C. elegans mutant VC475 [hsp-16.2 (gk249)]. Here we report that QA can upregulate the expression of, but does not depend on, hsp-16.2 to extend the worm life span and protect worms under stress. The upregulating effects of QA on the expression of daf-16 and sod-3 and the free radical scavenging effects of QA may jointly contribute to its beneficial properties.
Experimental Procedures
Reagents
QA (98%; Sigma) was stored in water solution at −20°C. Floxuridine (FUDR; 98%; Sigma) was used as a reproductive suppressant to prevent the accumulation of worm progeny. Pyrogallol (Sigma) was used as a free radical provider. H2DCF-DA (2′,7′-dichlorodihydro-fluorescein diacetate) (Sigma) was used as a fluorescence probe. Juglone (5-hydroxy-1,4- naphthoquinone), a reactive oxygen species (ROS)-generating compound, was used to induce oxidative stress in worms.
Worm strains and maintenance
Standard nematode growth medium (NGM)12 was used for C. elegans growth and maintenance at 20°C. Unless stated otherwise, plates were seeded with live E. coli.12 Bristol N2 (Caenorhabditis Genetics Center [CGC]) was used as the wild-type strain. The mutant strains CF1553 (muIs84), CF1588 [daf-16(mu86) I; daf-2(e1370) III; muIs84] containing the superoxide dismutase-34::green fluorescent protein (SOD-3::GFP)-linked reporter, used to visualize the SOD-3 expression, were from the CGC. The mutant CF1038 [daf-16(mu86) I] was from the CGC. The CL2070 (dvIs70), containing the heat shock protein-16.2 (HSP-16.2)::GFP-linked reporter, used to visualize the HSP-16.2 expression, was a generous gift from Dr. Luo Yuan of University of Maryland (College Park, MD). Mutant VC475 [hsp-16.2 (gk249)] was a generous gift from Dr. Cynthia Kenyon of the University of California (San Francisco, CA).
Life span assays and stress-resistance
Treatment plates were standard NGM media. Throughout the trials, we used the reproductive suppressant FUDR (Sigma, 100 mg/L) to prevent the accumulation of progeny on the treatment plates.2 The timing of adding FUDR is important to avoid developmental abnormalities of C. elegans.13,14 We found that if FUDR was added on the second day after the worms reached adulthood, the worm life span was not affected, whereas the worms had a shorter life span if FUDR was administrated during worm development (data not shown). Use of FUDR could cause some life span variations in mutant but not wild-type C. elegans.14 The possible interaction between FUDR and QA was ruled out because the preliminary life span assays of both N2 worms and mutants were done without FUDR, and similar results were generated by assays with FUDR treatment (data not shown).
Life span assays were performed at 20°C. Synchronized hermaphrodite N2 animals were transferred to treatment plates when young adults began to lay eggs of the indicated genotypes. The worms were then transferred to fresh treatment plates every 2 days for the first 10 days of the assays. Treatment plates were prepared by spreading different concentrations of QA stocks, 0 mg/mL, 0.05 mg/ml (0.26 μM), 0.1 mg/mL (0.52 μM), 0.2 mg/mL(1.04 μM), and 0.4 mg/mL (2.08 μM) diluted into live E. coli suspension, on the surface of the dry plates. Every experiment was repeated at least three times, and double blinds were conducted. For mutant VC475 [hsp-16.2 (gk249)] and CF1038 [daf-16(mu86) I], life span assays were carried out at the same conditions, but only under the selected concentration of 0.1 mg/mL of QA.
Heat shock assays were performed at 35°C using 2-day-old adults. The worms, shortly after reaching adulthood, were treated on treatment plates containing 0 mg/mL or 0.1 mg/mL QA for 2 days, and then transferred to an incubator with the temperature set to 35°C. The number of dead worms was recorded every hour.15,16 Every experiment was repeated three times and double blinds were conducted.
The fluorescence intensity of HSP-16.2::GFP in CL2070 (dvIs70) worms was detected by fluorescence microscopy. The worms were treated with 0 mg/mL or 0.1 mg/mL QA for 2 days, followed by heat shock (treatment at 25°C and 30°C for half an hour, respectively, and then 35°C for 1 hr) and recovery for 24 hr.17,18 Worms were fed on QA treatment plates during the heat shock and recovery periods.
Juglone sensitivity was assessed at 20°C using 2-day-old adults. L4 larvae were incubated on treatment plates containing 0 mg/mL or 0.1 mg/mL QA for 2 days, and then transferred to plates with 500 μM juglone. The juglone was diluted in absolute ethanol and added to NGM medium balanced at 55°C. The NGM plates were dried and used 3 hr after the preparation. The number of dead worms was counted and recorded every hour. Each treatment was repeated three times by double blinds.18
Pyrogallol self-oxidation assay
The in vitro superoxide anion–scavenging effect of QA was measured by detecting the chemiluminescence in the pyrogallol–luminol system. All reagents were equilibrated in a water bath at a constant temperature of 25°C, then added to a glass luminescence tube (1×5 cm) in a water bath in the following order: 50 μL 1 mmol/L pyrogallol and 950 μL 0.1 mmol/L luminol (in sodium carbonate buffer, pH 10.2). The final concentration of QA was 0.1 mL/mL. Light emission was detected after a 15-sec delay at 25°C.19
GSH/GSSG ratio assay
The adult N2 worms were treated with or without 0.1 mg/mL QA for 2 days. The reduced glutathione/oxidized glutathione (GSH/GSSG) ratio of C. elegans nematodes was determined using an enzymatic method with the GSH and GSSG Assay Kit (product no. S0053, Beyotime Institute of Biotechnology) following the manufacturer's manual. GSH in analyte could be oxidized to GSSG by chromophoric substrate 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), which reacts with GSH to form a spectrophotometrically detectable product at 412 nm, through which the GSH content from analyte can be determined directly. GSSG can be recycled to GSH by the GSH reductase, through which the total content of GSH, and GSSG can be determined indirectly through reaction. The GSSG content can be subsequently calculated from the difference between the total content of GSH and GSSG and the GSH content.20
Measurement of intracellular ROS in C. elegans
Intracellular ROS in C. elegans were measured with 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCF-DA) as the molecular probe. For ROS detection at normal culture conditions, the worms that had just reached adulthood were treated with or without QA (0.1 mg/mL) for 2 days. For ROS detection under oxidative stress, worms that had just reached adulthood were treated with 300 μM juglone for 1 hr and then treated with or without QA (0.1 mg/mL) for 2 days. At the end of the specified treatment times, the C. elegans were collected into 100 μL of phosphate-buffered saline with 1% Tween-20 (PBST) in eppendorf tubes. The worms were then sonicated (Branson Sonifier 250, VWR Scientific, Suwanee, GA) and pipetted into the wells of 96-well plates containing H2DCF-DA (final concentration 50 μM in PBS). Samples were read every 20 min for 2 hr in a FLx800 Microplate Fluorescent Reader (Bio-Tek Instruments, Winooski, VT) at 37°C at excitation 485 nm and emission 530 nm.21,22
Fluorescence quantification and visualization
Overall GFP fluorescence of GFP-expressing populations was assayed using a Thermolabsystems Fluoroskan Ascent microplate reader. Adult worms were treated with or without 0.1 mg/mL QA for 2 days. Twenty control or treated adult animals of the indicated age were transferred in 100 μL of S1 buffer to a well of a 96-well microtiter plate (black, clear, flat-bottomed wells), and total GFP fluorescence was measured using 485-nm excitation and 530-nm emission filters.23 Quadruple populations were used for each determination.
For fluorescence microscopy, the worms were mounted with a drop of heavy mineral oil placed on a cover slip covered with 2% agarose. The GFP photographs of transgenic worms were taken using an Olympus FluoViewTM FV500 microscope.18,23
Quantitative real-time PCR
Adult worms were treated with or without 0.1 mg/mL QA for 2 days. Total RNA was extracted from adult worms with TRIzol™ reagent (Invitrogen), and cDNA was produced by oligo(dT) priming. The RT-PCR primers were as follows:
daf-16 (NM_001026247), 5′-TTTCCGTCCCCGAACTCAA-3′, and 5′-ATTCGCCAACCCATGATGG-3′;
sod-3 (NM_078363), 5′-AGCATCATGCCACCTACGTGA-3′, and 5′-CACCACCATTGAATTTCAGCG-3′;
ama-1, 5′-CTGACCCAAAGAACACGGTGA-3′ and 5′-TCCAATTCGATCCGAAGAAGC-3′.
ama-1 was used as the internal control. The expression of mRNA was assessed by quantitative real-time PCR on a Bio-Rad IQ5 Multicolor Realtime-PCR Detection System using SYBR Green as the detection method. The gene expression data were analyzed using the comparative 2−ΔΔCt method,24 taking ama-1 mRNA as the normalizer.
Statistical tools
The data of life span assays and stress resistance assays were processed with Kaplan–Meier survival analysis of SPSS 13.0. Accidentally lost worms were calculated as censored. p values were calculated by Kaplan–Meier log-rank pairwise comparison between the control and the treated groups. Data of three individual experiments were taken together to draw the survival. Other data were analyzed with Origin 7.0. The error bars in the figures are means±standard error (SE) (*p<0.05; **p<0.01).
Results
QA extends the life span of C. elegans under normal culture conditions
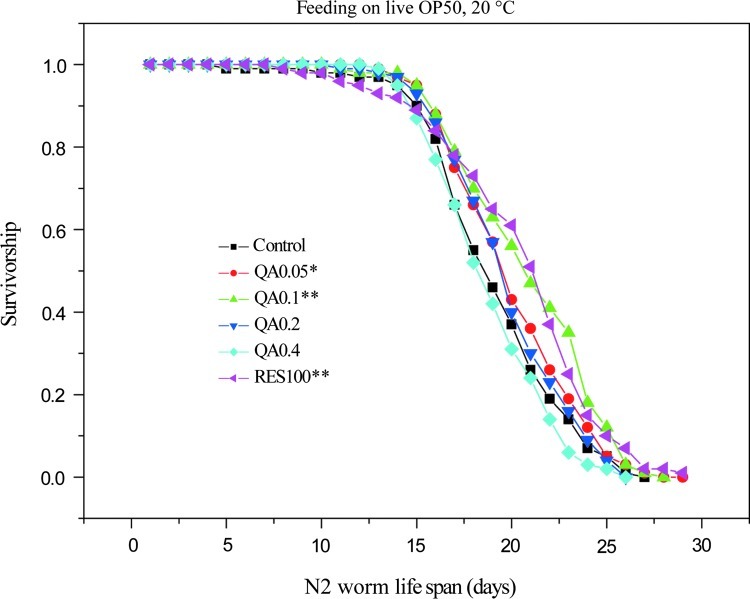
To investigate whether QA is able to extend worm life span under normal culture conditions, we exposed wild-type C. elegans (N2) (feeding on live E. coli OP50, hereafter E. coli) to different concentrations of QA (0, 0.05, 0.1, 0.2, 0.4 mg/mL) at 20°C. Resveratrol, which has been widely studied for its life span–extending effects and other antiaging properties,2,4,25 was used at 100 μM as a positive control. At 20°C, QA showed dose-related life span–extending effects, with the best effect at 0.1 mg/mL (extending 6.9% of the worm life span, p<0.001), whereas the positive control resveratrol extended the worm life span by 5.2% (p<0.001) (Fig. 1; Table 1). Heat-killed E. coli was also used for life span experiments to rule out the possibility that QA acted only to extend life span after being metabolized by live E. coli, and similar results were generated (data not shown). Apparently, QA's life span–extending effect is statistically significant, but not convincing enough. Therefore, we went further to test its protective effect in worms under stress.
FIG. 1.
Effect of quinic acid (QA) on the life span of N2 worms under normal culture conditions. At 20°C, QA exhibited different effects at different concentrations: 0.05 mg/mL (3.2%, n=258), 0.1 mg/mL (6.9%, n=193), 0.2 mg/mL (2.2%, n=179), 0.4 mg/mL (−3.6%, n=194); resveratrol (100 μM, positive control, 5.2%, n=212). Rates of increase were calculated in comparison with the control (n=183). Worms were fed on live E. coli strain OP50. Please refer to Table 1 for statistical data. (*) p<0.05; (**) p<0.01.
Table 1.
Life Span–Extending Effect of Quinic Acid on N2 Worms Feeding on Live E. coli OP50 at 20°C
| |
|
|
|
Mean |
|
||
|---|---|---|---|---|---|---|---|
| Trial | Treatment | Total n | Censored n | Survival (days) | SE | % Extendeda | Log rank test sig. (p) |
| 1 | Control | 59 | 0 | 19.6 | 0.42 | ||
| QA0.05 | 72 | 0 | 21.0 | 0.43 | 7.0 | 0.0276 | |
| QA0.1 | 60 | 5 | 21.2 | 0.42 | 8.3 | 0.0192 | |
| QA0.2 | 45 | 0 | 21.2 | 0.40 | 8.3 | 0.0656 | |
| QA0.4 | 57 | 0 | 19.4 | 0.43 | −1.1 | 0.6820 | |
| RES100 | 70 | 22 | 20.9 | 0.56 | 6.6 | 0.0310 | |
| 2 | Control | 66 | 0 | 20.1 | 0.46 | ||
| QA0.05 | 80 | 0 | 20.3 | 0.37 | 1.0 | 0.5025 | |
| QA0.1 | 56 | 3 | 21.2 | 0.48 | 5.5 | 0.0032 | |
| QA0.2 | 69 | 1 | 19.6 | 0.40 | −2.5 | 0.0267 | |
| QA0.4 | 66 | 0 | 18.4 | 0.32 | −8.5 | 0.0006 | |
| RES100 | 71 | 17 | 20.5 | 0.66 | 2.0 | 0.0168 | |
| 3 | Control | 58 | 3 | 19.3 | 0.42 | ||
| QA0.05 | 106 | 1 | 19.7 | 0.28 | 1.7 | 0.6632 | |
| QA0.1 | 77 | 0 | 20.7 | 0.46 | 7.3 | 0.0092 | |
| QA0.2 | 65 | 1 | 19.6 | 0.37 | 1.3 | 0.8262 | |
| QA0.4 | 71 | 12 | 19.2 | 0.39 | −0.6 | 0.7843 | |
| RES100 | 71 | 8 | 20.8 | 0.47 | 7.7 | 0.0108 | |
| Total | Control | 183 | 3 | 19.7 | 0.26 | ||
| QA0.05 | 258 | 1 | 20.3 | 0.20 | 3.2 | 0.0201 | |
| QA0.1 | 193 | 8 | 21.0 | 0.27 | 6.9 | 0.0000 | |
| QA0.2 | 179 | 2 | 20.1 | 0.24 | 2.2 | 0.2708 | |
| QA0.4 | 194 | 12 | 19.0 | 0.22 | −3.6 | 0.1013 | |
| RES100 | 212 | 47 | 20.7 | 0.32 | 5.2 | 0.0002 | |
Percentage is relative to control.
SE, Standard error.
QA improves the worm survival under heat stress
In the thermotolerance assay, N2 worms were pretreated shortly after reaching adulthood, with QA at 0.1 mg/mL for 48 hr before being exposed to heat shock at 35°C. During the thermal stress treatment, dead worms were counted every hour. Results showed that QA pretreatment enhanced the worm's resistance to heat stress, and thus brought an increased survival rate under the heat shock. The mean survival rate was significantly increased, up to 17.8% by QA at 0.1 mg/mL (P=0.0001, relative to the control) (Fig. 2A; Table 2).
FIG. 2.
Quinic acid (QA) provides protection for C. elegans under heat stress. (A) Under heat stress (35°C), QA at 0.1 mg/mL significantly improves the worm survival time by 17.8% (n=267), compared to the control (n=271). Please refer to Table 2 for statistical data. (B) There was no significant difference between the QA (0.1 mg/mL)-treated group and the control before heat stress and 4 hr after heat stress. (C) Representative heat shock protein-16.2::green fluorescent protein (HSP-16.2::GFP) picture of the control group, after 24-hr recovery following heat stress. (D) Representative HSP-16.2::GFP picture of the QA (0.1 mg/mL)-treated group, after 24-hr recovery following heat stress, stronger than C. (E) HSP-16.2::GFP intensity comparison between the control and QA (0.1 mg/mL)-treated group, after 24-hr recovery following heat stress. There was a significant increase of HSP-16.2::GFP intensity in the QA-treated group. Results were averaged from four single experiments, with 20 worms in every experiment, and were detected on a Thermolabsystems Fluoroskan Ascent microplate reader. (F) QA significantly extended the life span of mutant VC475 [hsp-16.2 (gk249)] by 15.7%. QA-treated group, n=173. Control group, n=170. Please refer to Table 3 for statistical data. The error bars indicate±standard error SE). (*) p<0.05; (**) p<0.01. OP50, E. coli strain OP50.
Table 2.
Effect of Quinic Acid on the Thermotolerance of N2 Worms Feeding on Live E. coli OP50 at 35°C
| |
|
|
|
Mean |
|
||
|---|---|---|---|---|---|---|---|
| Trial | Treatment | Total n | Censored n | survival (hours) | SE | % Extendeda | Log rank rest sig. (p) |
| 1 | Control | 81 | 0 | 5.4 | 0.27 | ||
| QA0.1 | 70 | 0 | 6.3 | 0.30 | 14.2 | 0.0349 | |
| 2 | Control | 55 | 0 | 5.0 | 0.24 | ||
| QA0.1 | 80 | 0 | 5.7 | 0.24 | 12.1 | 0.0290 | |
| 3 | Control | 135 | 0 | 4.6 | 0.19 | ||
| QA0.1 | 117 | 0 | 5.5 | 0.19 | 16.7 | 0.0076 | |
| Total | Control | 271 | 0 | 4.9 | 0.13 | ||
| QA0.1 | 267 | 0 | 5.8 | 0.14 | 17.8 | 0.0001 | |
Percentage is relative to control.
SE, Standard error.
Because QA could improve the thermotolerance, of C. elegans, we predicted QA might upregulate the expression of HSP-16.2, which is a downstream effector of DAF-16 and can serve as a stress-sensitive reporter to predict longevity in C. elegans, with a higher level of HSP-16.2 predicting longer life span.17,26 The reporter transgene HSP-16.2::GFP in transgenic strain CL2070 (dvIs70) was used to visualize hsp-16.2 expression. CL2070 (dvIs70) mutants were treated with heat shock at 35°C for 1 hr and allowed to recover at 20°C for 24 hr. HSP-16.2::GFP fluorescence intensity was investigated at three different time frames—before, shortly (4 hr) after, and 24 hr after heat stress. Before heat stress, the HSP-16.2::GFP fluorescence intensity was hardly detected, whereas 4 hr after heat stress, there was an increase but no significant difference between the control and the QA-treated group (Fig. 2B). Twenty-four hours after heat shock, HSP-16.2::GFP fluorescence intensity was increased significantly, and a significant difference was observed in worms treated with QA at the concentration of 0.1 mg/mL (p<0.01, compared to the control) (Fig. 2C–E).
In other words, QA upregulates the HSP-16.2::GFP signal under heat stress but does not do so without heat stress. It seems that under normal culture conditions, hsp-16.2 might not be involved in life span regulation by QA. Therefore, it would be very interesting to investigate whether QA could still extend the C. elegans life span when hsp-16.2 was mutated. Mutant VC475 [hsp-16.2 (gk249)] was used to test this idea. It is found that mutant VC475 [hsp-16.2 (gk249)] worms had a shorter life span than the wild type (data now shown), which was consistent with previous reports.17,26 The mean life span of VC475 [hsp-16.2 (gk249)] was extended up to 15.7% by 0.1 mg/mL QA (p=3.4×10−6, compared to the control) (Fig. 2F; Table 3). This suggests that although the upregulation of HSP-16.2 is important in the survival-enhancing effect of QA under heat stress, HSP-16.2 is not indispensable for QA to extend worm life span under normal culture conditions.
Table 3.
Effect of Quinic Acid on the Life Span of Mutant VC475 [hsp-16.2 (gk249)] Feeding on Live E. coli OP50 at 25°C
| |
|
|
|
Mean |
|
||
|---|---|---|---|---|---|---|---|
| Trial | Treatment | Total n | Censored n | Survival (days) | SE | % Extendeda | Log rank test sig. (p) |
| 1 | Control | 85 | 0 | 9.6 | 0.28 | ||
| QA0.1 | 38 | 0 | 10.9 | 0.40 | 12.7 | 0.0618 | |
| 2 | Control | 61 | 0 | 9.0 | 0.36 | ||
| QA0.1 | 61 | 0 | 10.7 | 0.31 | 18.5 | 0.0055 | |
| 3 | Control | 24 | 0 | 10.5 | 0.54 | ||
| QA0.1 | 74 | 0 | 11.4 | 0.35 | 8.8 | 0.0468 | |
| Total | Control | 170 | 0 | 9.5 | 0.21 | ||
| QA0.1 | 173 | 0 | 11.0 | 0.21 | 15.7 | 0.0000 | |
Percentage is relative to control.
SE, Standard error.
QA upregulates sod-3 expression in a DAF-16–dependent manner
sod-3 is one of the target genes of DAF-16 that is critical for life span and stress resistance regulation, and it plays an important role in the aging process of C. elegans.26 We also wanted to know whether QA could modulate the expression of sod-3. The C. elegans strain CF1553 (muIs84) containing the SOD-3::GFP reporter gene was used to visualize the effect of QA on sod-3. In CF1553 (muIs84), QA at the concentration of 0.1 mg/mL significantly upregulated the fluorescence intensity of SOD-3::GFP (p<0.05 compared to the control) (Fig. 3A–C). In addition, SOD-3::GFP fluorescence intensity in CF1553 (muIs84) was investigated in a 9-day time period to confirm the modulating effect of QA on sod-3. It was found that QA could keep the SOD-3::GFP fluorescent signal at a higher level, compared to the control, although QA could not cease the reducing tendency of SOD-3::GFP fluorescence intensity during the 9 days of the worm adulthood (Fig. 3D). At the mRNA level, the upregulation of QA on sod-3 was also observed by quantitative real-time PCR (p<0.05 compared to the control) (Fig. 3E).
FIG. 3.
Quinic acid (QA) upregulates the expression of the sod-3 gene in a daf-16–dependent manner. (A) Representative green fluorescent protein (GFP) photograph demonstrating the fluorescence intensity of superoxide dismutase-3::green fluorescent protein (SOD-3::GFP) in adult mutant CF1553 (muIs84) in the control group. (B) Representative GFP photograph demonstrating the fluorescence intensity of SOD-3::GFP in adult mutant CF1553 (muIs84) in QA (0.1 mg/mL)-treated group (2 days treatment), stronger than A. (C) QA (0.1 mg/mL) could significantly upregulate fluorescence intensity of SOD-3::GFP in CF1553 (muIs84), but not in mutant CF1588 [daf-16(mu86) I; daf-2(e1370) III; muIs84] when daf-16 was mutated. (D) SOD-3::GFP intensity (±SE) in adult CF1553 (muIs84) with or without 0.1 mg/mL QA over a period of 9 days. QA could keep the SOD-3::GFP fluorescence intensity at a higher level than the control. (C and D) Data were averaged from four single experiments, with 20 worms in every experiment, and were detected on a Thermolabsystems Fluoroskan Ascent microplate reader. (E) QA at 0.1 mg/mL could significantly upregulate the expression of sod-3 and daf-16 in N2 worms. Gene expression was detected with quantitative real-time PCR. (F) QA (0.1 mg/mL) could not extend the life span of mutant CF1038 [daf-16(mu86) I], when daf-16 was mutated. For the QA-treated group, n=359; for the control group, n=320. Please refer to Table 4 for statistical data. The error bars indicate±standard error (SE). (*) p<0.05.
DAF-16/forkhead transcription factor, the downstream target of the insulin-like signaling in C. elegans, is indispensable for both life span regulation and stress resistance under many conditions.27 Therefore, daf-16 might be required for the beneficial effects of QA. Quantitative real-time PCR results showed that the expression of daf-16 was upregulated significantly by 0.1 mg/mL QA (Fig. 3E).
Because sod-3 is a target gene of DAF-16, we further investigated whether QA regulated sod-3 expression through daf-16. Because DAF-16 is the main target of the DAF-2 pathway,28,29 we used the double mutant CF1588 [daf-16(mu86) I; daf-2(e1370) III; muIs84] to test this relationship. We did not have the single mutant for daf-16 constructed with SOD-3::GFP, but because DAF-16 is the main target of DAF-2 pathway,29,30 we therefore used the double mutant CF1588 [daf-16(mu86) I; daf-2(e1370) III; muIs84] to test this relationship. We found that when daf-16 was mutated, in the mutant CF1588 fluorescence intensity of SOD-3::GFP could not be upregulated by QA treatment (Fig. 3C), suggesting that QA upregulated sod-3 expression through DAF-16.
We wanted to further confirm the indispensable role of daf-16 for the beneficial effects of QA. Mutant CF1038 [daf-16(mu86) I] was used to test the necessity of daf-16 in the life span–extending effect of QA. Whereas QA could extend the life span of wild-type C. elegans, as we previously mentioned, no significant difference was observed between the control and 0.1 mg/mL QA-treated CF1038 [daf-16(mu86) I] worms (Fig. 3F; Table 4), suggesting that daf-16 was required in the life span–extending effect of QA.
Table 4.
Effect of Quinic Acid on the Life Span of Mutant CF1038 [daf-16(mu86) I] Feeding on Live E. coli OP50 at 25°C
| |
|
|
|
Mean |
|
||
|---|---|---|---|---|---|---|---|
| Trial | Treatment | Total n | Censored n | survival (days) | SE | % Extendeda | Log rank test sig. (p) |
| 1 | Control | 108 | 0 | 16.8 | 0.26 | ||
| QA0.1 | 131 | 0 | 17.5 | 0.23 | 4.2 | 0.0803 | |
| 2 | Control | 120 | 0 | 18.3 | 0.23 | ||
| QA0.1 | 129 | 0 | 18.2 | 0.23 | −0.3 | 0.4678 | |
| 3 | Control | 92 | 0 | 18.8 | 0.28 | ||
| QA0.1 | 99 | 0 | 17.6 | 0.21 | −6.0 | 0.0493 | |
| Total | Control | 320 | 0 | 17.9 | 0.15 | ||
| QA0.1 | 359 | 0 | 17.8 | 0.13 | −0.7 | 0.0625 | |
Percentage is relative to control.
SE, Standard error.
QA keeps reactive oxygen species at a lower level in C. elegans
In 1954, Harman initially advanced the free radical theory of aging and hypothesized that free radical species cause deterioration of an organism.31 On one hand, aerobic organisms have developed a cellular metabolism that takes oxygen as an electron acceptor, but they continuously generate ROS, specifically, hydroxyl radicals, superoxide anions, and hydrogen peroxide; on the other hand, they possess antioxidant defense systems that can effectively remove these ROS.31
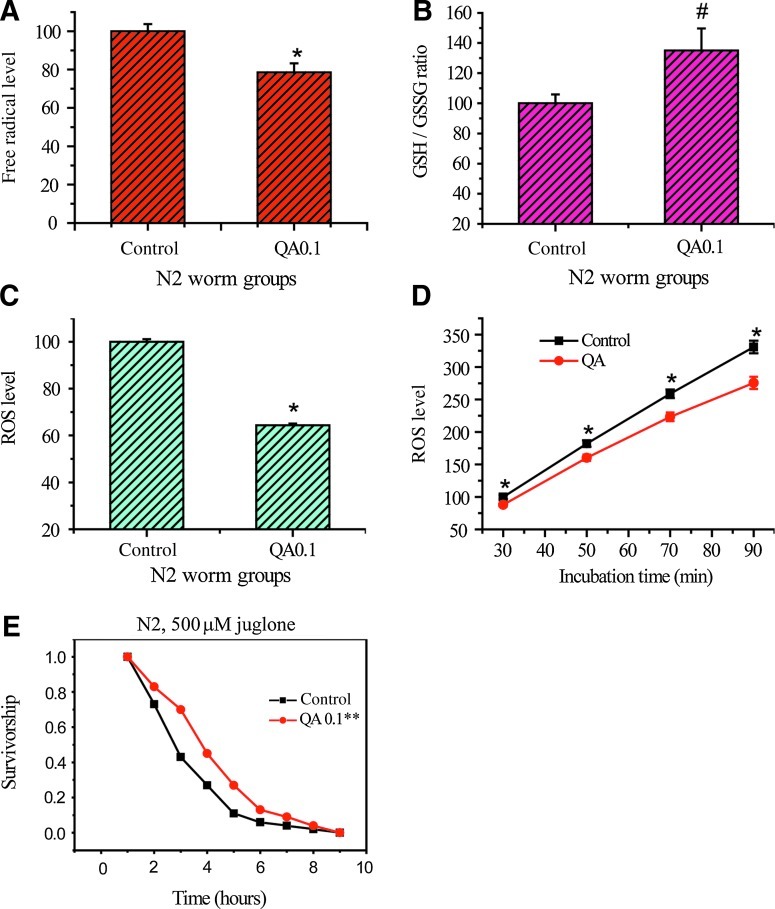
Because we found QA could upregulate the expression of hsp-16.2 (Fig. 2) and sod-3 (Fig. 3), we wondered whether QA could subsequently downregulate the level of free radicals. QA was investigated for antioxidant properties and demonstrated a free radical–scavenging effect in vitro and in vivo. In a chemical system, the free radicals produced by pyrogallol self-oxidation were efficiently scavenged by QA at 0.1 mg/mL in vitro (demonstrated 21.5% reduction) (Fig. 4A).
FIG. 4.
Quinic acid (QA) scavenges free radicals directly and indirectly. (A) In vitro free radical scavenging effect of QA (0.1 mg/mL). (B) QA (0.1 mg/mL) increased the reduced glutathione to oxidized glutathione ratio (GSH/GSSG) in N2 worms. (C) In vivo free radical scavenging effect of QA (0.1 mg/mL) under normal culture conditions, detected after 30 min of incubation at 37°C. (D) In vivo free radical scavenging effect of QA (0.1 mg/mL) under oxidative stress generated by 500 μM juglone, detected after 30 min of incubation at 37°C, and lasted 90 min. (E) Under oxidative stress (500 μM juglone), QA at 0.1 mg/mL significantly improved the N2 worm survival time by 29.7% (n=269), compared to the control (n=201). Please refer to Table 5 for statistical data. Every data point is the average value of three trials. The error bars indicate±standard error (SE). (*) p<0.05; (**) p<0.01.
The GSH/GSSG ratio was then evaluated in vivo. Reduced GSH, a tripeptide (γ-glutamylcysteinylglycine) with a free thiol group, is a major antioxidant that provides reducing equivalents for the GSH–peroxidase-catalyzed reduction of hydrogen peroxide and lipid hydroperoxides to water and the corresponding alcohol, during which GSH becomes GSSG.32 When worms are exposed to increased oxidative stress, the ratio of GSH/GSSG will decrease as a consequence of GSSG accumulation. The GSH/GSSG ratio is a useful indicator of oxidative stress and can be used to monitor the effectiveness of antioxidant intervention strategies.33 In this study, worms treated with QA (0.1 mg/mL) showed a GSH/GSSG ratio with a 34.9 % increase compared to the control (Fig. 4B).
If QA could downregulate the level of free radicals directly by scavenging free radicals in vivo, as the upregulated GSH/GSSG ratio implies, and indirectly by upregulating the expression of hsp-16.2 (Fig. 2) and sod-3 (Fig. 3), the intracellular ROS level in C. elegans should be lower in the QA-treated worms than that of the control. Consistently, under normal culture conditions, QA at the concentration 0.1 mg/mL could inhibit the production of ROS significantly by 35.7% in vivo (Fig.4C). In the following assays, the ROS levels were detected in N2 worms treated with juglone (300 μM). Data showed that, under juglone-generated oxidative stress, QA could effectively reduce the ROS accumulation, detected in a 90-min time course (Fig. 4D). The data from intracellular ROS levels are consistent with those from chemical system and GSH/GSSG assays.
This was further confirmed when we evaluated the protective effect of QA to oxidative stress. N2 worms were exposed to juglone (500 μM), following 48 hr of pretreatment with QA at 0.1 mg/mL shortly after reaching adulthood. Juglone, a prooxidant that can be reduced with nicotinamide adenine dinucleotide phosphate (NAD[P]H) by diaphorases, converts oxygen to superoxide anion, and consequently increases intracellular oxidative stress.18,34,35 The results showed that QA pretreatment improved the worm's resistance against oxidative stress induced by juglone. The mean survival rate was significantly increased up to 29.7% by QA (Fig.4E; Table 5).
Table 5.
Protective Effect of Quinic Acid on N2 Worms Feeding on Live E. coli OP50 at 20°C Under Oxidative Stress
| |
|
|
|
Mean |
|
||
|---|---|---|---|---|---|---|---|
| Trial | Treatment juglone (500 μM) | Total n | Censored n | Survival (hours) | SE | % Extendeda | Log rank test sig. (p) |
| 1 | Control | 70 | 0 | 2.5 | 0.17 | ||
| QA0.1 | 88 | 0 | 3.5 | 0.20 | 40.0 | 0.0003 | |
| 2 | Control | 64 | 0 | 2.7 | 0.19 | ||
| QA0.1 | 113 | 0 | 3.4 | 0.18 | 25.9 | 0.0078 | |
| 3 | Control | 67 | 0 | 2.8 | 0.24 | ||
| QA0.1 | 68 | 0 | 3.7 | 0.20 | 33.0 | 0.0174 | |
| Total | Control | 201 | 0 | 2.7 | 0.12 | ||
| QA0.1 | 269 | 0 | 3.5 | 0.11 | 29.7 | 0.0000 | |
Percentage is relative to control.
SE, Standard error.
Discussion
QA can significantly extend the life span of N2 worms under normal culture conditions; however, the life span extension rate is not extremely convincing. We evaluated the antiaging effect of QA under various culture conditions. Under heat stress, QA could significantly improve the survival of N2 worms by 17.8%. This might be generated by the upregulative effect of QA on the expression of heat shock protein HSP-16.2, which could improve the stress resistance and serve as a stress-sensitive reporter to predict longevity in C. elegans.17,26 When exposed to toxic compounds or stress conditions, the expression of HSP-16.2 in C. elegans could be induced,36,37 which would help the worms survive.
In our study, the hsp-16.2 expression was upregulated by QA treatment. Was this due to conditional stress caused by QA-treatment? If so, we should be able to observe that the expression of hsp-16.2 was induced by QA treatment without heat induction. However, in our study, hsp-16.2 expression was induced only after heat stress (Fig. 2B). So QA did not generate stress on the worms to induce hsp-16.2 expression. More importantly, QA could upregulate the hsp-16.2 expression after heat stress, and we suggested here that this could contribute much to the significantly improved thermotolerance of QA-treated C. elegans.
However, in a similar study, Strayer et al have found EGb 761, a standardized extract of Ginkgo biloba leaves, which has been shown to have antioxidative properties, can increase stress resistance by suppressing hsp-16.2 gene expression.17 They interpreted the suppression of HSP-16.2::GFP fluorescence intensity as an indication that EGb 761 decreases cellular stress resulting from exogenous treatments, therefore leading to a decreased transcriptional induction of the reporter transgene that was induced by the prooxidant juglone and by heat shock.17 We suggest that QA acted differently from EGb761 and provided protection not through decreasing the internal level of stress caused by thermal stress, but rather by increasing the expression of stress-responsive genes, such as daf-16, hsp-16.2, and sod-3. In addition, we observed the upregulatory effect of QA on these genes.
In addition, mutant VC475 [hsp-16.2 (gk249)] was used to investigate whether hsp-16.2 was indispensable for QA to take effect as an antiaging agent. Results suggested that when hsp-16.2 was mutated, QA could still extend the worm life span, with an increase of 15.7% in the mean lifespan of QA-treated VC475 [hsp-16.2 (gk249)] worms (Fig. 2F; Table 3). This indicates that HSP-16.2 is not an indispensable effector but one of the DAF-16–targeted effectors resulting in extended longevity and enhanced stress resistance for C. elegans. Interestingly, this is consistent with the hsp-16.2 expression in the QA-treated N2 worms under normal culture conditions, which showed that QA only generated an effect on the hsp-16.2 expression under stress.
Therefore, under heat stress, hsp-16.2 was induced by QA treatment to express more to improve survival, whereas under normal culture conditions, hsp-16.2 expression was not changed by QA treatment, which could explain why QA could extend the life span of mutant VC475 [hsp-16.2 (gk249)] worms when hsp-16.2 was mutated.
Thus, QA must work to demonstrate its beneficial effects through other pathways besides hsp-16.2. We investigated the free radical–scavenging effects of QA in vitro and in vivo. QA was found to be capable of scavenging free radicals in both the chemical system and living worms (Fig. 4A,C,D). Moreover, the GSH/GSSG ratio (Fig. 4B) and the expression of sod-3 (Fig. 3) were both upregulated by QA, which would consequently downregulate the ROS level in worms. Consistently, the total ROS level in normal worms and juglone-treated worms were both reduced by QA (Fig. 4C,D). This was confirmed when we found that QA could improve the N2 worm survival under oxidative stress (Fig. 4E).
Meanwhile, the upregulation of daf-16 possibly, but not certainly, modulate the expression of the DAF-16–targeted genes, such as sod-3 and hsp-16.2, which are both important for life span and stress-resistance regulation.17,26,27 And the effects of QA on the expression of daf-16, sod-3, and hsp-16.2 are consistent (Figs. 2 and 3).
Therefore, we conclude that under heat stress, QA could improve worm survival by upregulating the expression of hsp-16.2. Under normal culture conditions, QA would extend the life span of N2 worms and more significantly extend the life span of mutant VC475 [hsp-16.2 (gk249)] worms by upregulating the daf-16 expression, which would consequently regulate its targeted genes such as sod-3, by improving DNA repair as reported previously.9–11 As a natural in vivo antioxidant, QA is a safe and reasonable antiaging candidate with great potential.
Acknowledgments
The authors are grateful to Dr. Cynthia Kenyon of the University of California, San Francisco, for her critical reading and comments on the manuscript, valuable input, and discussions. We thank Dr. Cynthia Kenyon and her lab for providing us with C. elegans mutants and the isogenic N2 strain; Dr. Luo Yuan from the University of Maryland for providing research materials and technical instructions; Mr. Paul Watkins of BioMarker for his valuable input; and William Wilson for his thorough editing. The GFP pictures were taken with the assistance of Teng Yan from the Institute of Biophysics, CAS. We also thank Zhao Xudong, for equipment support, from Core Facilities of the Institute of Biophysics, CAS. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center. This work was supported by BioMarker Pharmaceuticals, Inc. (BM-103), USA, LifeExtension Foundation, USA, and by grants from the National Natural Science Foundation of China (30170239, 30370361 and 30930036).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Howitz KT. Bitterman KJ. Cohen HY. Lamming DW. Lavu S. Wood JG. Zipkin RE. Chung P. Kisielewski A. Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 2.Wood JG. Rogina B. Lavu S. Howitz K. Helfand SL. Tatar M. Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 3.Bass TM. Weinkove D. Houthoofd K. Gems D. Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Valenzano DR. Terzibasi E. Genade T. Cattaneo A. Domenici L. Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Baur JA. Pearson KJ. Price NL. Jamieson HA. Lerin C. Kalra A. Prabhu VV. Allard JS. Lopez-Lluch G. Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheng Y. Pero RW. Amiri A. Bryngelsson C. Induction of apoptosis and inhibition of proliferation in human tumor cells treated with extracts of Uncaria tomentosa. Anticancer Res. 1998;18:3363–3368. [PubMed] [Google Scholar]
- 7.Sheng Y. Bryngelsson C. Pero RW. Enhanced DNA repair, immune function and reduced toxicity of C-MED-100, a novel aqueous extract from Uncaria tomentosa. J Ethnopharmacol. 2000;69:115–126. doi: 10.1016/s0378-8741(99)00070-7. [DOI] [PubMed] [Google Scholar]
- 8.Sheng Y. Li L. Holmgren K. Pero RW. DNA repair enhancement of aqueous extracts of Uncaria tomentosa in a human volunteer study. Phytomedicine. 2001;8:275–282. doi: 10.1078/0944-7113-00045. [DOI] [PubMed] [Google Scholar]
- 9.Pero RW. Amiri A. Sheng Y. Welther M. Rich M. Formulation and in vitro/in vivo evaluation of combining DNA repair and immune enhancing nutritional supplements. Phytomedicine. 2005;12:255–263. doi: 10.1016/j.phymed.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Mammone T. Akesson C. Gan D. Giampapa V. Pero RW. A water soluble extract from Uncaria tomentosa (Cat's Claw) is a potent enhancer of DNA repair in primary organ cultures of human skin. Phytother Res. 2006;20:178–183. doi: 10.1002/ptr.1827. [DOI] [PubMed] [Google Scholar]
- 11.Sheng Y. Akesson C. Holmgren K. Bryngelsson C. Giamapa V. Pero RW. An active ingredient of Cat's Claw water extracts identification and efficacy of quinic acid. J Ethnopharmacol. 2005;96:577–584. doi: 10.1016/j.jep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi S. Santelli J. Mitchell DH. Stiles JW. Sanadi DR. A simple method for maintaining large, aging populations of Caenorhabditis elegans. Mech Ageing Dev. 1980;12:137–150. doi: 10.1016/0047-6374(80)90090-1. [DOI] [PubMed] [Google Scholar]
- 14.Aitlhadj L. Sturzenbaum SR. The use of FUdR can cause prolonged longevity in mutant nematodes. Mech Ageing Dev. 2010;131:364–365. doi: 10.1016/j.mad.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Hansen M. Hsu AL. Dillin A. Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lithgow GJ. White TM. Melov S. Johnson TE. Thermotolerance, extended life-span conferred by single-gene mutations, induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rea SL. Wu D. Cypser JR. Vaupel JW. Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strayer A. Wu Z. Christen Y. Link CD. Luo Y. Expression of the small heat-shock protein Hsp16-2 in Caenorhabditis elegans is suppressed by Ginkgo biloba extract EGb 761. FASEB J. 2003;17:2305–2307. doi: 10.1096/fj.03-0376fje. [DOI] [PubMed] [Google Scholar]
- 19.Jian L. Increased carbon disulfide-stimulated chemiluminescence in the pyrogallol-luminol system. Luminescence. 2001;16:281–283. doi: 10.1002/bio.653. [DOI] [PubMed] [Google Scholar]
- 20.Arkblad EL. Tuck S. Pestov NB. Dmitriev RI. Kostina MB. Stenvall J. Tranberg M. Rydstrom J. A Caenorhabditis elegans mutant lacking functional nicotinamide nucleotide transhydrogenase displays increased sensitivity to oxidative stress. Free Radical Biol Med. 2005;38:1518–1525. doi: 10.1016/j.freeradbiomed.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Bass DA. Parce JW. Dechatelet LR. Szejda P. Seeds MC. Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 22.Gutierrez-Zepeda A. Santell R. Wu Z. Brown M. Wu Y. Khan I. Link CD. Zhao B. Luo Y. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci. 2005;6:54. doi: 10.1186/1471-2202-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraynov VS. Chamberlain C. Bokoch GM. Schwartz MA. Slabaugh S. Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Seve M. Chimienti F. Devergnas S. Aouffen M. Douki T. Chantegrel J. Cadet J. Favier A. Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem. 2005;1:629–633. doi: 10.2174/157340605774598144. [DOI] [PubMed] [Google Scholar]
- 26.Hsu AL. Murphy CT. Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 27.Tissenbaum HA. Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 28.Lin K. Hsin H. Libina N. Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 29.Lin K. Dorman JB. Rodan A. Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb S. Ruvkun G. daf-2, daf-16 and daf-23: Genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 32.Jones DP. Redefining oxidative stress. Antiox Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 33.Rahman I. Biswas SK. Jimenez LA. Torres M. Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antiox Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 34.Burmeister C. Luersen K. Heinick A. Hussein A. Domagalski M. Walter RD. Liebau E. Oxidative stress in Caenorhabditis elegans: Protective effects of the Omega class glutathione transferase (GSTO-1) FASEB J. 2007;22:343–354. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- 35.Kampkotter A. Gombitang Nkwonkam C. Zurawski RF. Timpel C. Chovolou Y. Watjen W. Kahl R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch Toxicol. 2007;81:849–858. doi: 10.1007/s00204-007-0215-4. [DOI] [PubMed] [Google Scholar]
- 36.Dengg M. van Meel JC. Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J Pharmacol Toxicol Methods. 2004;50:209–214. doi: 10.1016/j.vascn.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Link CD. Cypser JR. Johnson CJ. Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperon. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]