Abstract
The epithelial-derived cytokine thymic stromal lymphopoietin (TSLP) has been associated with the promotion of type 2 inflammation and the induction of allergic disease. In humans TSLP is elevated in the lungs of asthma patients and in the lesional skin of individuals with atopic dermatitis, whereas mice lacking TSLP responses are refractory to models of Th2-driven allergic disease. Although several cell types, including dendritic cells, basophils, and CD4 T cells, have been shown to respond to TSLP, its role in macrophage differentiation has not been studied. Type 2 cytokines (i.e., IL-4 and IL-13) can drive the differentiation of macrophages into alternatively activated macrophages (aaMϕs, also referred to as M2 macrophages). This population of macrophages is associated with allergic inflammation. We therefore reasoned that TSLP/TSLPR signaling may be involved in the differentiation and activation of aaMϕs during allergic airway inflammation. In this study, we report that TSLP changes the quiescent phenotype of pulmonary macrophages toward an aaMϕ phenotype during TSLP-induced airway inflammation. This differentiation of airway macrophages was IL-13–, but not IL-4–, dependent. Taken together, we demonstrate in this study that TSLP/TSLPR plays a significant role in the amplification of aaMΦ polarization and chemokine production, thereby contributing to allergic inflammation.
Introduction
Macrophages are innate immune cells with well-established roles in primary responses to pathogens, tissue homeostasis, coordination of adaptive immune responses, inflammation, resolution, and tissue repair (1, 2). Depending on the microenvironment, macrophage differentiation and function are governed by numerous cell-extrinsic factors, including cytokines, chemokines, and TLRs (3). For example, classically known macrophage (M1 macrophage) activation is induced by IFN-γ, which triggers the proinflammatory responses required to kill intracellular pathogens, including Leishmania and Salmonella (4). In contrast, macrophages can undergo alternative activation by IL-4 and IL-13, triggering an immune response important for parasite elimination. These Th2 cytokines promote the differentiation of alternatively activated macrophages (aaMϕs), characterized by abundant expression of mannose receptor (MR, or CD206), with increased levels of MHC class II and a panel of signature genes, including arginase 1 (Arg1), chitinase-like molecules (Ym1/2 and AMCase), and resistin-like molecule α (2, 5–9). Although the recruitment of aaMϕs is a characteristic inflammatory feature of parasitic infection, allergy, diabetes, and cancer (10–14), the potential roles of aaMϕs in influencing the development, severity, or resolution of inflammatory responses have remained controversial. Furthermore, IL-4 and IL-13 selectively induce CCL17 (TARC), CCL22 (MDC), and CCL24 (eotaxin-2) in aaMϕs but are inhibited by IFN-γ (15–17). The CCR4 agonist CCL17, produced by aaMϕs, along with IL-10 inhibits the generation of classically activated macrophages (18).
Recently, thymic stromal lymphopoietin (TSLP), an IL-7–related cytokine, has emerged as an important potential contributor to allergic inflammation (19). The involvement of TSLP in dysregulated Th2 responses was first demonstrated in atopic dermatitis (AD) patients, as skin biopsies from AD lesions showed greatly enhanced levels of TSLP protein compared with normal and nonlesional skin (20). Additionally, human asthmatics have increased concentrations of TSLP in their airways, which correlates with Th2-attracting chemokine expression and disease severity (21). A more causative relationship between TSLP and Th2 cytokine–mediated disease was established with transgenic overexpression models. Induced epidermal TSLP expression leads to a spontaneous AD-like disease characterized by many of the hallmark features of human AD (22). In contrast, TSLPR-deficient mice exhibited a dramatically reduced Th2 inflammatory response in an FITC-mediated contact hypersensitivity model of AD (23). Mice expressing lung-specific TSLP developed severe airway inflammation (24) whereas mice deficient in the TSLPR failed to develop asthma in the classic OVA prime/challenge model (24, 25).
Although it is becoming clear that TSLP is an important determinant in allergic inflammation, the mechanisms underlying this effect remain unclear. In this study we demonstrate that TSLP is a hitherto unrecognized amplifier of aaMϕ polarization, and that TSLP-activated aaMϕs significantly contribute to type 2 immune responses both locally and systemically.
Materials and Methods
Mice
Six- to 8-wk-old female BALB/c mice were obtained from Charles River Laboratories. SPC-TSLP mice were generated as described previously (24). K5-TSLP mice were generated as described previously (22, 26). IL-4 knockout (KO; Il4−/−), IL-4Rα KO (Il4rα−/−), and STAT6 KO (Stat6−/−) mice were purchased from The Jackson Laboratory. IL-13 KO (Il13−/−) mice were provided by Dr. Andrew McKenzie (Medical Research Council, Cambridge, U.K.). All animals were housed in specific pathogen-free conditions at the Benaroya Research Institute (Seattle, WA) animal facility. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Benaroya Research Institute.
Preparation of peritoneal macrophages and bone marrow–derived macrophages
Peritoneal cells were obtained by sterile lavage with RPMI 1640 supplemented with 2% heat-inactivated FCS (HyClone Laboratories, Logan, UT) and antibiotics. Peritoneal macrophages were isolated and sorted according to the phenotype F4/80+ and CD19−.
Bone marrow cells were isolated from femurs and cultured with RPMI 1640 supplemented with 10% FBS and 10% L929 conditioned medium. Culture fluid was exchanged for fresh culture medium every 4 d. Under these conditions, adherent macrophage monolayers were obtained within 8–10 d. Cells were washed extensively to remove L cell conditioned medium, and murine bone marrow–derived macrophages (BMDMs) were then stimulated with IL-4, IL-13, IFN-γ (all 10 ng/ml; PeproTech), LPS (10 ng/ml; Sigma-Aldrich), and TSLP (20 ng/ml; gift of Amgen), or in combination.
OVA sensitization and airway challenge
Wild-type (WT) and Tslpr−/− mice were sensitized on days 1 and 14 by i.p. injection of 50 μg OVA (A7642; Sigma-Aldrich) emulsified in 1.3 mg aluminum hydroxide (Alum) gel (Sigma-Aldrich) in a total volume of 200 μl. Anesthetized mice were challenged intranasally (i.n.) with 50 μg OVA in 40 μl PBS on days 21, 22, and 23. Mice were sacrificed 3 h following Ag challenge on days 21 and 22 as well as 24 and 48 h after final challenge. Serum, bronchoalveolar lavage (BAL) cells, and lungs were harvested and analyzed.
Intranasal treatment
Anesthetized animals were administered 500 ng TSLP i.n. or 25 μg mouse serum albumin (MSA; Sigma-Aldrich) with 25 μg OVA (A7642; Sigma-Aldrich) in a total volume of 40 μl in PBS. Following i.n. administration, mice were suspended in an upright position for 10 min to ensure complete uptake of the treatment solution (27).
Ab treatment
M1 Ab, which is against IL-4Rα (referred to as M1), was used to block both IL-4 and IL-13 signaling pathways (28). This Ab was given twice weekly via i.p. injection (1 mg/mouse) as described before (29). Anti–IL-4 mAb (mAb clone 11B11) (National Institutes of Health, Bethesda, MD) was used to block IL-4 signaling pathway and was given once a week i.p. (1.5 mg/mouse). For control animals, an equivalent dose of normal control IgG (Sigma-Aldrich) was used.
BAL, tissue fixation, and staining
Mice were euthanized by i.p. injection of 1 ml 2.5% Avertin in PBS. The lungs were subjected to BAL extraction four times, each using 1 ml PBS through a tracheal cannula. The BAL was centrifuged at 1400 × g for 5 min to collect the cell pellet. BAL cells were resuspended in PBS containing 1% BSA and counted using a hemocytometer. Differential cell counts were performed on cytospin cell preparations stained with a modified Wright-Giemsa stain (Diff-Quik stain set; Siemens). After BAL extraction, lungs were excised completely from the chest cavity, inflated with 10% neutral buffered formalin (Fisher BioTech), and fixed at room temperature overnight in the same solution. Tissues were embedded in paraffin and sectioned and stained with periodic acid-Schiff.
Digestion of lung tissue
Immediately after lavage, the lung vascular bed was perfused with PBS, and the lungs excised, minced, and digested enzymatically with digestion solution (RPMI 1640, 0.13 mg/ml Liberase Blendzyme, and 20 U/ml DNase) at 37°C for 30 min. The suspension was dispersed by repeated aspiration through a 10-ml syringe, and erythrocytes were lysed by suspension in erythrocyte lysis buffer. The cells were then washed twice with HBSS, resuspended in RPMI 1640, and filtered through a 100-μm cell strainer.
Quantitative RT-PCR
Total RNA was isolated using the GenElute mammalian total RNA miniprep kit (Sigma-Aldrich) and cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) according to the manufacturers’ instructions. Quantitative PCR was performed with Platinum SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen) and a 7900HT PCR system (Applied Biosystems). All data were analyzed on SDS software (Applied Biosystems) and gene expression was determined as an average of triplicate samples after drawing standard curve and normalized to GAPDH gene expression.
Detection of IgE, Ag-specific IgE, and CCL17/22/24 by ELISA
High-binding 96-well ELISA plates (Costar, Corning, NY) were coated with 100 ng/well anti-mouse IgE Ab (BD Pharmingen, San Diego, CA) overnight at 4°C. The plates were washed three times with PBS containing 0.5% Tween 20 and blocked with PBS containing 1% BSA for 1 h at 37°C. Plates were washed, and purified IgE (BD Pharmingen) or sera were incubated in duplicate for 2 h at 37°C. Plates were washed, and a biotinylated anti-mouse IgE was added for 2 h at 37°C. Plates were washed and incubated for 0.5 h at 37°C in the presence of streptavidin-HRP (Sigma-Aldrich). Finally, substrate (tetramethylbenzidine) was added and incubated at room temperature in the dark. The reaction was stopped with 2 N H2SO4. OD was measured at 450 nm.
For OVA-specific IgE, plates were coated overnight with 0.2 mg/ml OVA in PBS. Plates were washed and blocked with 1% BSA-PBS for 1 h at 37°C. Serum samples were added and plates were incubated overnight at 4°C. Plates were washed, and biotin-conjugated anti-mouse IgE was added at a 1/500 dilution and incubated for 1 h at 37°C. Plates were washed, incubated, and read as previously described. To detect BSA-specific IgE, plates were coated overnight with BSA and blocked with 1% FBS.
CCL17/22/24 ELISA reagents were purchased from R&D Systems. ELISA was performed according to the manufacturer’s instructions.
BrdU labeling of proliferating cells in vivo
Mice were i.p. injected with 100 μl 10 mg/ml BrdU in Dulbecco’s PBS 3 h prior to experimental end point.
Flow cytometric analysis
Abs for flow cytometry were purchased from BD Biosciences, eBioscience, and Serotec. Cells were washed in ice-cold flow cytometry buffer (1% [v/v] BSA and 2 mM EDTA in PBS, pH 7.5), then incubated with each Ab for 15 min and washed twice with flow cytometry buffer. MR was stained following membrane permeabilization with a Leucoperm kit (Serotec) according to the manufacturer’s instructions. For detection of BrdU incorporation, cells were stained for surface markers, then fixed and permeabilized using a staining buffer set (BD Bioscience). Cells were then stained with anti-BrdU for 30 min at room temperature, or incubated first with or without DNase for 30 min at 37°C before staining with anti-BrdU Ab. Data were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo (Tree Star).
Statistics
All statistical analysis was performed using GraphPad Prism 5. Unless otherwise indicated, all statistical tests are one-way ANOVA with a Tukey posthoc test with significance between groups represented as p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001.
Results
Overexpression of TSLP enhances recruitment and activation of aaMϕs in vivo
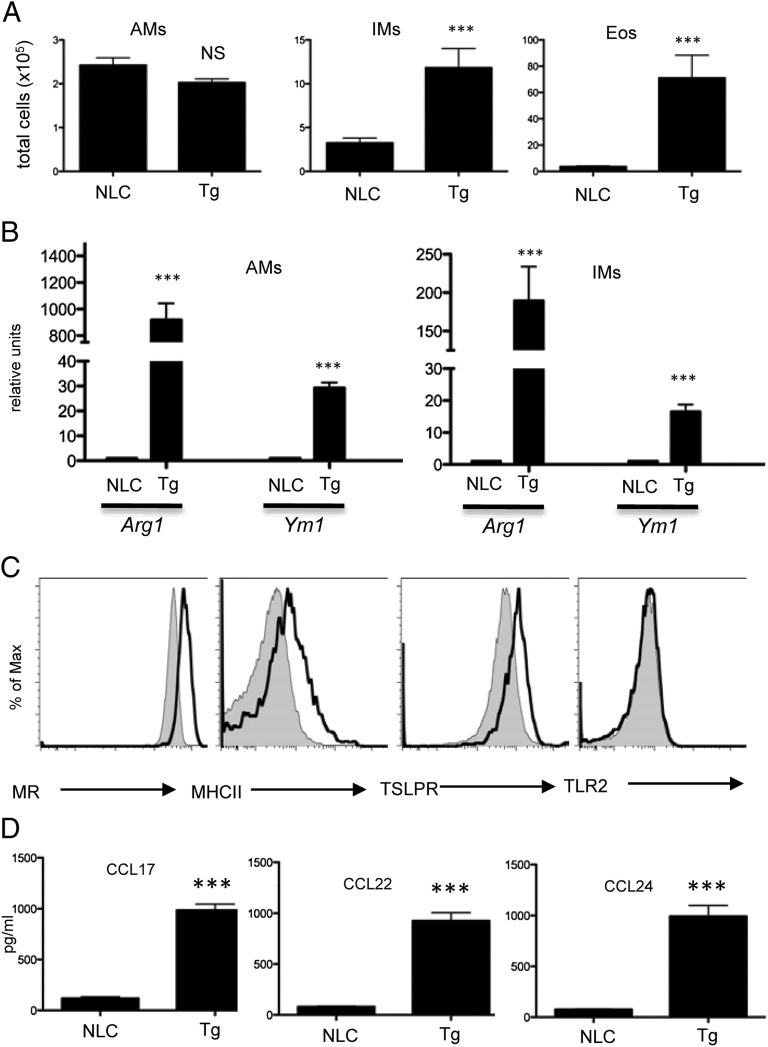
Our previous data indicated that elevated TSLP expression in the lung was sufficient for the development of an asthma-like chronic inflammatory airway disease (24). Mice with a lung-specific TSLP transgene (referred to as SPC-TSLP) showed increased BAL cell counts as well as marked cellular infiltration compared with control mice (24). Indeed, we found that SPC-TSLP mice recruited interstitial macrophages (IMs; Siglec-F−CD11c−CD11b+F4/80+) and eosinophils (Eos; Siglec-F+CD11c−), but not alveolar macrophages (AMs; Siglec-F+CD11c+CD11bintF4/80+), to the site of inflammation (Fig. 1A, Supplemental Fig. 1). Further analysis of SPC-TSLP mice showed markedly elevated expression of aaMϕ signature markers Arg1 and Ym1 from both AMs and IMs (Fig. 1B). Additionally, AMs were polarized to an aaMϕ phenotype with a marked increase in the expression of MR. MHC class II and TSLPR were also increased. In contrast, TSLP did not affect the expression of M1 associated receptor, TLR2 (Fig. 1C). Reflecting the degree of cellular infiltrate, high levels of type 2 chemokines were detected, including CCL17 (memory Th2 and NKT cell chemoattractants), CCL22, and CCL24 (Eos, basophil, and mast cell chemoattractants; Fig. 1D).
FIGURE 1.
TSLP enhances the polarization of aaMϕs in vivo. (A) Numbers of lung AMs (Siglec-F+CD11c+CD11bintF4/80+), IMs (Siglec-F−CD11c-CD11b+F4/80+), and Eos (Siglec-F+CD11c−) harvested from WT or SPC-TSLP transgenic mice. (B) Quantitative RT-PCR analyses of Arg1 and Ym1 in FACS-sorted AMs and IMs. (C) Cell surface markers of AMs of WT (filled) or SPC-TSLP (open) mice as analyzed by FACS. One of three representative experiments in which similar results were obtained is shown. (D) CCL17, CCL22, and CCL24 production in BAL fluid by ELISA. Data are representative of two independent experiments (n = 4 mice/group). Groups of animals were compared using Student t tests. ***p ≤ 0.001. NLC, normal littermate control; Tg, SPC-TSLP.
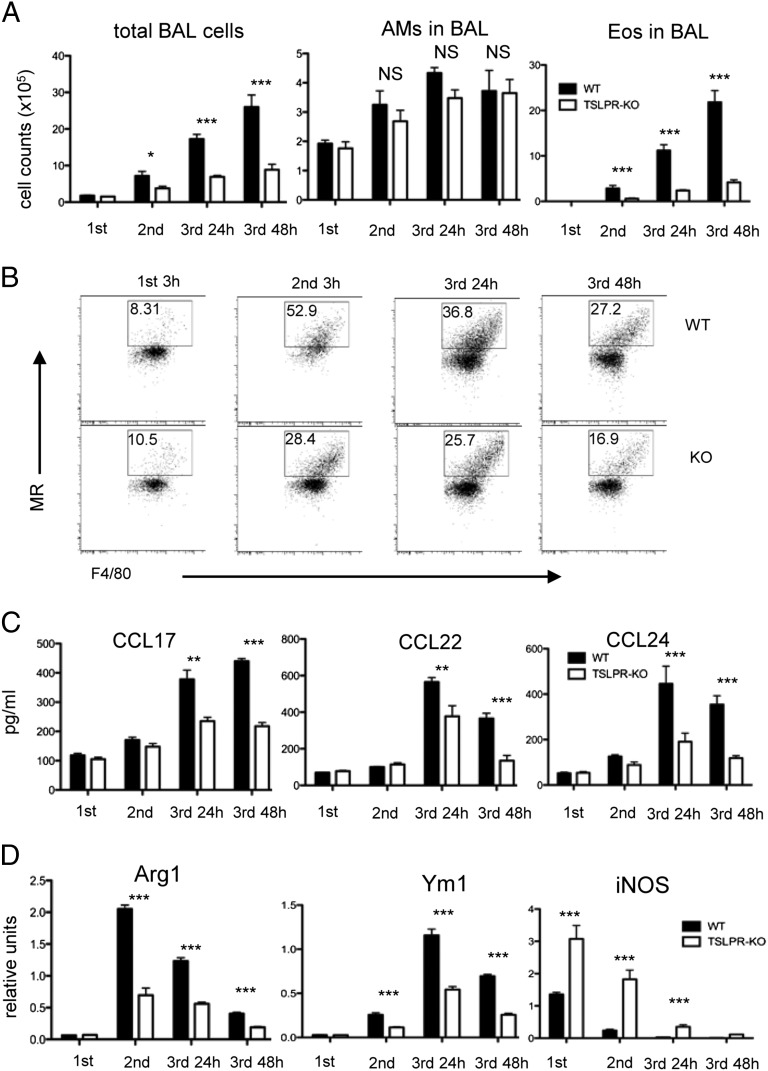
Tslpr−/− mice have impaired aaMϕ polarization during allergic airway inflammation
We and other groups have shown that mice deficient in the TSLPR (Tslpr−/−) fail to develop asthma in response to inhaled Ag (24, 25). We next investigated the hypothesis that TSLP/TSLPR regulation of aaMϕ differentiation and type 2 chemokine production significantly contributes to allergic airway inflammation. To test this possibility, WT and Tslpr−/− mice were sensitized with OVA/Alum and later challenged for 3 consecutive days with either i.n. OVA or PBS. BAL fluids were analyzed after each challenge and chemokines were examined after the final challenge. As we reported previously (24), there was a less pronounced increase in total cells and Eos in the BAL fluid of Tslpr−/− compared with WT mice (Fig. 2A). Tissue interstitial macrophage recruitment to the lung, as well as MR expression upon OVA challenge, was decreased in Tslpr−/− mice (Fig. 2B and data not shown). WT mice sensitized and challenged with OVA had increased levels of CCL17, CCL22, and CCL24 in the BAL fluids. In contrast, there was little or no increased expression of these chemokines in OVA-sensitized and -challenged Tslpr−/− mice (Fig. 2C). Next, we isolated RNA from lungs of WT and Tslpr−/− mice 3 h after the first and second challenges, and 24 and 48 h after the final challenge, and assayed by quantitative PCR for classically and aaMϕ-associated genes. Arg1 and Ym1 expression was markedly reduced at all time points in Tslpr−/− mice, whereas M1 macrophage markers such as inducible NO synthase (iNOS) were increased (Fig. 2D). Taken together, these data indicate that TSLP/TSLPR signaling is pivotal in the amplification of aaMϕ polarization and chemokine production in allergic airway inflammation.
FIGURE 2.
TSLPR−/− mice show impaired aaMϕ development during allergic airway inflammation. WT and Tslpr−/− mice sensitized i.p. with 50 μg OVA in 1.3 mg Alum on days 1 and 14 and challenged i.n. on days 21, 22, and 23 with 50 μg OVA. (A) Differential cell counts in BAL fluid performed 3 h after first and second OVA challenges and 24 and 48 h after final OVA challenge. (B) Cell surface markers of IMs in digested lungs analyzed by FACS. Upper panel, WT mice; lower panel, Tslpr−/− mice. One of three representative experiments in which similar results were obtained is shown. (C) CCL17, CCL22, and CCL24 concentrations in BAL fluid analyzed by ELISA. (D) Three hours after first and second OVA challenges and 24 and 48 h after final challenge, Arg1, Ym1, and iNOS expression in lungs of BALB/c and Tslpr−/− mice was analyzed by quantitative PCR. Data are representative of two independent experiments (n = 5/group). Groups of animals were compared using Student t tests. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
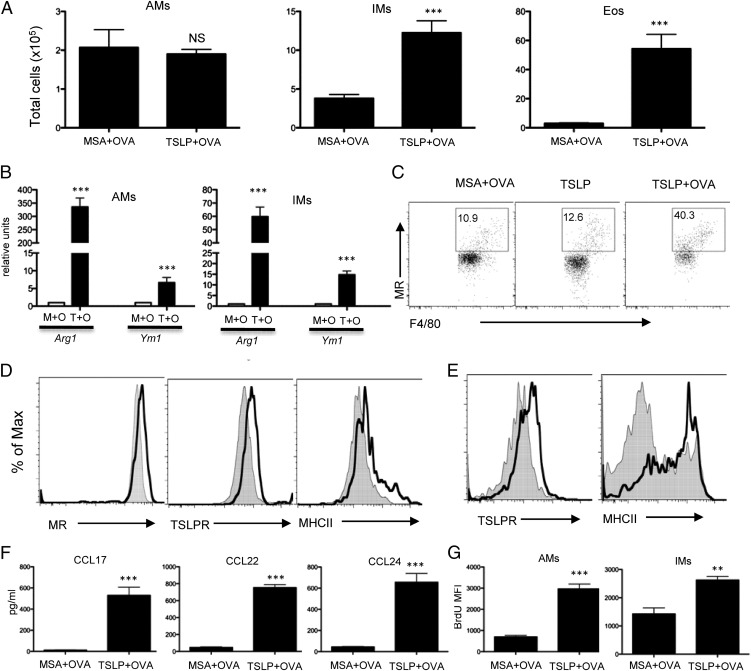
TSLP administration enhances aaMϕ recruitment and activation in vivo
We have developed an acute model of TSLP-induced airway inflammation in which BALB/c mice are given i.n. TSLP in the presence of OVA every other day for 2 wk. Mice receiving TSLP plus OVA display significant increases across all disease parameters (27). Although LPS signaling through TLR4 can induce Th2 responses to allergen in some models of mouse allergic inflammation (30), this model is independent of LPS stimulation (27). TSLP also triggered recruitment of IMs and Eos (Fig. 3A) and significant increases in aaMϕ signature markers (Fig. 3B). Additionally, TSLP administration induced MR expression from IMs, whereas control protein MSA had no effect (Fig. 3C). TSLP administration in the absence of Ag failed to alter BAL cellular composition (27) or macrophage activation (Fig. 3C). Following TSLP plus OVA administration, AMs polarized to an aaMϕ phenotype with marked increase in MR expression, whereas TSLPR and MHC class II were also increased (Fig. 3D). Increased expression of TSLPR and MHC class II was also observed on IMs from mice treated with TSLP plus OVA (Fig. 3E). Reflecting the cellular infiltrates, high levels of type 2 chemokines were detected. CCL17, CCL22, and CCL24 were significantly increased in mice treated with TSLP plus OVA compared with the control group (Fig. 3F). This would suggest that TSLP changes the quiescent phenotype of lung macrophages into aaMϕs in vivo.
FIGURE 3.
TSLP administration enhances aaMϕ recruitment and activation in vivo. (A) Numbers of lung AMs, IMs, and Eos harvested from BALB/c mice treated i.n. with MSA plus OVA or TSLP plus OVA for 2 wk. Quantitative RT-PCR analyses of cytokines and chemokines in lungs (n = 4 mice/group). (B) Quantitative RT-PCR analyses of Arg1 and Ym1 in FACS-sorted AMs and IMs. (C) Cell surface markers of IMs in digested lungs analyzed by FACS. (D) Cell surface markers of AMs from mice treated with MSA plus OVA (filled) or TSLP plus OVA (open) analyzed by FACS. (E) Cell surface markers of IMs from mice treated with MSA plus OVA (filled) or TSLP plus OVA (open) analyzed by FACS. One of three representative experiments in which similar results were obtained is shown. (F) CCL17, CCL22, and CCL24 concentrations in BAL fluid were analyzed by ELISA. (G) Mean fluorescent intensity (MFI) of BrdU in AMs and IMs measured by flow cytometry. BrdU administered 3 h before analysis. Data are representative of two independent experiments (n = 4 mice/group). Groups of animals were compared using Student t tests. ***p ≤ 0.001. M+O, MSA plus OVA; T+O, TSLP plus OVA.
Although terminal differentiation of most mammalian cells typically results in reduced proliferative capacity, recent studies suggest that macrophage proliferation is characteristically common to various type 2 inflammatory settings (31–33). Our data raised the possibility that the large increase in macrophages seen during inflammation is the result of resident population expansion. Indeed, TSLP plus OVA treatment resulted in a dramatically increased frequency of BrdU+ AMs and IMs (Fig. 3G), as measured by a 3-h pulse of BrdU.
TSLP-induced airway inflammation and aaMϕ polarization is IL-13–, but not IL-4–, dependent
The results from our experiments suggested that the effect of TSLP on aaMϕs differentiation was not direct, but rather was a consequence of its ability to drive Th2 type responses. We next addressed whether blockade of these responses in a therapeutic model could control or reverse TSLP-induced disease progression and aaMϕ differentiation. We used an Ab specific for the IL-4Rα-chain (M1), capable of blocking the biologic activity of both IL-4 and IL-13 (28), or an IL-4 neutralizing Ab (11B11) to block these Th2 cytokine activities during TSLP plus OVA i.n. treatment. As expected, both total and Ag-specific IgE were dramatically decreased following both M1 and 11B11 Ab treatment (Supplemental Fig. 2B). Surprisingly, BAL cellularity and Eos counts were reduced after M1 but not 11B11 treatment (Supplemental Fig. 2A). Additionally, goblet cell metaplasia, mucus overproduction, as well as overall lung inflammation were suppressed only following M1 Ab treatment (data not shown). Similarly, no differences were seen for CCL17, CCL22, or CCL24 expression following 11B11 treatment (Supplemental Fig. 2C). Analysis of lung RNA levels showed that, compared with rIgG, 11B11 led to similar expression of Arg1 and Ym1 (Supplemental Fig. 2D). Additionally, aaMϕ bias was shifted back in M1-treated animals with suppressed expression of CCL17, CCL22, and CCL24 (Supplemental Fig. 2C). Lung RNA analysis showed that M1 treatment reduced the expression of Arg1 and Ym1 (Supplemental Fig. 2E). These data suggest that aside from IgE production IL-4 does not play a role in TSLP-induced airway inflammation. We conclude that IL-13, but not IL-4, is required for TSLP-induced airway inflammation and differentiation of AMs toward aaMϕs.
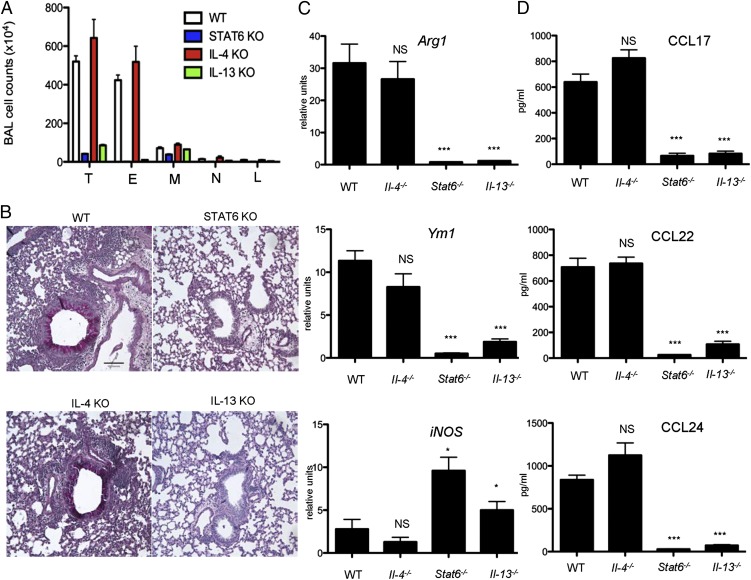
To confirm the Ab blockade studies we used mice with targeted deletions in Il4, Il13, and Stat6 genes. Stat6 is activated following either IL-4 or IL-13 exposure and is essential for the function of both cytokines and for Th2 development (34). To determine whether IL-4 or IL-13 is required for TSLP-induced pathogenesis, mice deficient in Stat6, IL-4, and IL-13 were given TSLP plus OVA as previously described. Consistent with Ab treatment, Stat6−/− mice were completely devoid of any symptoms of TSLP-mediated airway inflammation (Fig. 4A, 4B). Lungs from these same mice lacked inflammatory infiltrates, goblet cell metaplasia, and mucus overproduction (Fig. 4B). RNA analysis showed that these mice produced less Arg1 and Ym1 (Fig. 4C) as well as CCL17, CCL22, and CCL24 in the BAL (Fig. 4D). Similar results were obtained from Il13−/− mice.
FIGURE 4.
STAT6 and IL-13– but not IL-4–deficient mice exhibit attenuated airway inflammation and aaMϕ activation. (A) Differential cell counts in BAL fluid. (B) Representative lung tissue cross-sections stained with periodic acid-Schiff to visualize mucus-producing goblet cells and airway inflammation. Scale bar, 100 μm. (C) Quantitative RT-PCR analyses of Arg1, Ym1, and iNOS in lungs. (D) CCL17, CCL22, and CCL24 concentrations in BAL fluid were analyzed by ELISA (n = 5 mice per group). *p ≤ 0.05, ***p ≤ 0.001. E, Eosinophils; L, lymphocytes; M, macrophages; N, neutrophils; T, total cells.
In contrast to Stat6- and Il13-deficient mice, Il4−/− mice developed severe TSLP-mediated airway inflammation (Fig. 4A, 4B). In comparison with WT mice, lungs from Il4−/− mice showed inflammatory infiltrates, goblet cell metaplasia, and mucus overproduction (Fig. 4B). They also exhibited similar expression of Arg1 and Ym1 in the lung (Fig. 4C), as well as CCL17, CCL22, and CCL24 in the BAL (Fig. 4D). Taken together, our data suggest IL-13 but not IL-4 is indispensable for TSLP-mediated airway inflammation and aaMϕ activation.
TSLP amplification of aaMϕ polarization is IL-4R–dependent
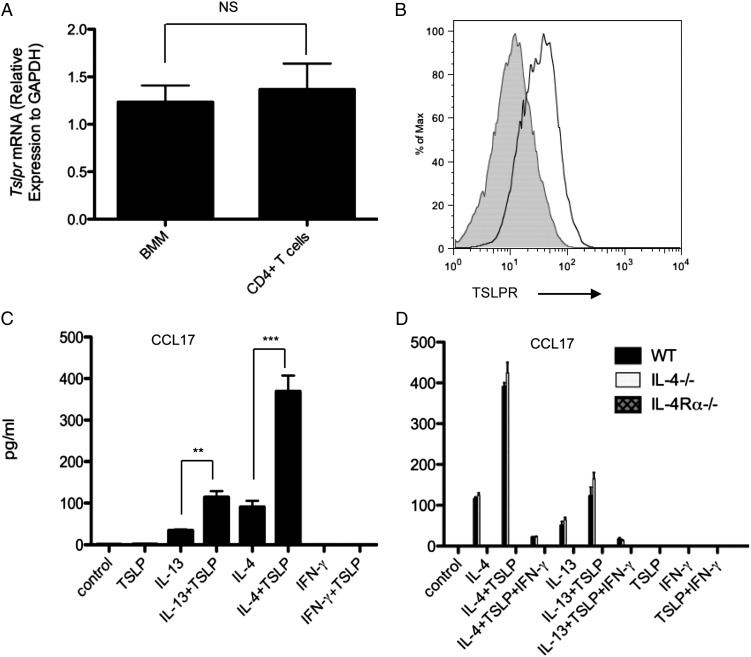
The expression of the TSLPR was examined on macrophages at the mRNA and protein levels. Quantitative RT-PCR showed that BMDMs expressed similar Tslpr mRNA levels as CD4+ T cells (Fig. 5A). Consistently, flow cytometric analysis detected TSLPR on BMDMs (Fig. 5B). Given the well-established roles of IL-4, IL-13, and the IL-4Rα-chain in aaMϕ differentiation (5), we evaluated the contribution of these factors to TSLP-amplified aaMϕ development by culturing BMDMs from WT, Il4−/−, or Il4rα−/− mice. TSLP alone did not induce CCL17 production; however, in the presence of IL-13 or IL-4, TSLP dramatically increased chemokine production in WT and Il4−/− but not in Il4rα−/− macrophages (Fig. 5C). Additionally, the synergistic effect of IL-13/IL-4 with TSLP on chemokine production was inhibited by IFN-γ. Taken together, these data demonstrate that the synergistic effect of TSLP and IL-13/IL-4 on aaMϕ polarization is IL-4R–dependent.
FIGURE 5.
IL-4 and IL-13 increase macrophage responsiveness to TSLP. (A) Quantitative PCR analysis of Tslpr in BMDMs and CD4+ T cells. Groups of animals were compared using Student t tests. (B) Protein level of TSLPR determined by flow cytometry. Filled histograms represent isotype controls. One of three representative experiments in which similar results were obtained is shown. (C and D) CCL17 production by BMDMs from WT BALB/c (C, D), Il4−/−, or Il4rα−/− (D) mice. Harvested BMDMs were stimulated for 48 h with IL-13 (10 ng/ml), IL-4 (10 ng/ml), IFN-γ (10 ng/ml), murine TSLP (20 ng/ml), or combinations of these reagents. CCL17 production analyzed by ELISA. Data represent one of three independent experiments. **p ≤ 0.01, ***p ≤ 0.001.
Enhanced induction of AAMϕ signature genes in K5-TSLP macrophages
To assess whether the ability of TSLP to promote aaMϕ polarization is restricted to lung macrophage populations, we analyzed macrophages from K5-TSLP mice in which TSLP is increased systemically following treatment with doxycycline (26). Analysis of cells isolated from the peritoneum of K5-TSLP mice showed an ∼10-fold increase in total cellularity, with eosinophils being the predominant cell type (Supplemental Fig. 3A). Peritoneal macrophages from doxycycline-treated K5-TSLP mice were considerably enlarged (data not shown) and displayed increased expression of MHC class II and B7-1, suggesting activation. K5-TSLP macrophages also had significant increases in MR expression (Supplemental Fig. 3B). F4/80+CD19− peritoneal macrophages were FACS-purified, after which total RNA was extracted and expression of Ym1, Arg1, and CCL17 analyzed by quantitative RT-PCR. These three genes, known to promote Th2 cytokine expression and regulate allergic inflammation (2, 5–9), were highly elevated in peritoneal macrophages isolated from doxycycline-treated transgenic mice (Supplemental Fig. 3C).
Discussion
The innate immune system is increasingly being associated with airway disease induction and progression. The local mesenchyme can also interpose signals to fine tune immune responses according to local microenvironment needs (14, 35). Macrophages are an important part of the innate immune system, but their contribution to asthma pathogenesis is not yet clear. Recent work has associated aaMϕs and their products with asthma (10). Macrophage differentiation into this phenotype occurs following exposure to IL-4, IL-13, or both. Therefore, it is not surprising that markers of alternative activation, such as AMCase, arginase, and mannose receptors, are associated with asthma (10). In a murine model of asthma, aaMϕs can contribute to disease development (36, 37). Mice with asthma have higher numbers of aaMϕs in lung tissue than do control animals, and increasing aaMϕ numbers in lung tissue by intratracheal instillation prior to inducing allergic inflammation amplifies the development of airway inflammation. Similarly, in humans, increased numbers of aaMϕs were found in the BAL fluid of patients with severe asthma compared with healthy control subjects (14). The notion of aaMϕs as an asthma modifier is also consistent with a report that demonstrated transfer of IL-4Rα+ macrophages is sufficient to promote allergic inflammation (38). However, this result was questioned in a subsequent study (39).
Emerging studies implicate TSLP, IL-25, and IL-33 as critical regulators of innate and adaptive immune responses associated with Th2 cytokine-mediated inflammation at mucosal sites (40). Although IL-4 and IL-13 are the prototypical direct inducers of aaMϕs, other cytokines such as IL-33 and IL-25 amplify aaMϕ induction indirectly through Th2 cells (41). IL-33 binds to ST2, a receptor for IL-33, to amplify aaMϕ induction (42). IL-25, an IL-17 family member that induces production of multipotent hemopoietic progenitors in bone marrow and GALT, gives rise to cells of monocyte/macrophage and granulocyte lineages (43). Furthermore, IL-25 reduces renal injury and inhibits colitis with induction of aaMϕs (44, 45).
Given the presence of TSLP in asthma patients, it is likely that TSLP-driven macrophage activation may contribute to clinical disease. Data presented in this study demonstrate that TSLP is a powerful enhancer of aaMϕ development during innate and adaptive immune responses. It is thought that alveolar macrophages produce suppressive cytokines such as TGF-β and inhibit the initiation of adaptive immunity to harmless Ags to avoid alveolar damage (46). However, in the presence of pathogens or damaged tissues, AMs are likely responsible for initiating inflammatory responses (47–50). In this study, we show that TSLP synergizes with endogenous IL-13/IL-4 signaling to induce the polarization of macrophages toward aaMϕs, promoting type 2 inflammation in the lung. It is also likely that parenchymal macrophages differentiate into aaMϕs in the presence of TSLP, as TSLP-treated mice show enhanced MR, Arg1, and Ym1 expression. Of interest was the finding that mice with Stat6 or Il13 deficiency, but not Il4 deficiency, were completely protected from the development of TSLP-induced airway inflammation upon repeated challenge with TSLP plus OVA. Moreover, the failure to develop airway inflammation in these animals was associated with their inability to produce Th2-type cytokines or promote aaMϕ activation. To our knowledge, the present study is the first to demonstrate that signaling of IL-13, but not IL-4, plays an essential role in the development of airway inflammation and aaMϕ polarization in TSLP-induced experimental asthma. Consistent with our data, many of the observed effects seen in mice with elevated TSLP in their lungs, including fibrosis, mucus secretion, and parasitic expulsion, depend on IL-13 rather than IL-4 (1, 51). Interestingly, it has been demonstrated that Arg1 and Ym1 are dependent on IL-13Rα1 after allergen challenge but are independent of IL-13Rα1 after IL-4 administration (52). Although recent studies have revealed considerable new information regarding the role of innate lymphoid cells in producing cytokines associated with Th2 cells that induce allergic inflammation, including IL-5 and IL-13 (53–56), their role in promoting aaMϕ induction is unclear.
Macrophage activation is a dynamic process, with the same cells initially taking part in proinflammatory and cytotoxic reactions later participating in the resolution of inflammation and wound healing (57–60). This illustrates the plasticity of macrophage differentiation and the abilities of macrophages to modulate their responses consequent to a changing microenvironment (57, 58, 61). The TSLP-enhanced polarization of macrophages toward the aaMϕ phenotype and the subsequent production of type 2 chemokines further empowers aaMϕs to recruit inflammatory cells to heighten the immune response. This process, together with the contribution of TSLP to the activation of B cells (62), dendritic cells (63, 64), Th2 cells (65, 66), basophils (67), mast cells, and NK T cells (68–70), can lead to a pathogenic outcome such as excessive type 2 inflammation or AHR.
In summary, in this study we define a previously unrecognized role for TSLP/TSLPR signaling in type 2 immunity as an important enhancer for the development of aaMϕs as well as for chemokine production. In vivo, TSLP is likely induced by a variety of antigenic and environmental stimuli in the epithelial barrier. Our findings may therefore have implications for a wide range of immune responses whereby persistent aaMϕ activation is found to play a crucial role. Because activation and recruitment of aaMϕs are dominant features in inflammatory responses associated with diseases as diverse as cancer, diabetes, and asthma, the manipulation of TSLP expression may offer novel therapeutic strategies for the treatment of multiple inflammatory conditions.
Acknowledgments
We thank the members of the Ziegler Laboratory for discussions. We specifically thank Masayuki Kitajima and Jen-Feng Lai for the valuable suggestions on FACS analysis. We thank Sylvia McCarty for administrative assistance in the preparation of the manuscript.
This work was supported in part by National Institutes of Health Grants AI68731, AR55695, AR56113, and HL098067 and by a grant from the Asthma and Allergy Foundation of America to S.F.Z.
The online version of this article contains supplemental material.
- aaMϕ
- alternatively activated macrophage
- AD
- atopic dermatitis
- Alum
- aluminum hydroxide
- AM
- alveolar macrophage
- Arg1
- arginase 1
- BAL
- bronchoalveolar lavage
- BMDM
- bone marrow–derived macrophage
- Eos
- eosinophil
- IM
- interstitial macrophage
- i.n.
- intranasal(ly)
- iNOS
- inducible NO synthase
- KO
- knockout
- MR
- mannose receptor
- MSA
- mouse serum albumin
- TSLP
- thymic stromal lymphopoietin
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Martinez F. O., Helming L., Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27: 451–483 [DOI] [PubMed] [Google Scholar]
- 2.Stein M., Keshav S., Harris N., Gordon S. 1992. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176: 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor P. R., Martinez-Pomares L., Stacey M., Lin H. H., Brown G. D., Gordon S. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23: 901–944 [DOI] [PubMed] [Google Scholar]
- 4.MacMicking J., Xie Q. W., Nathan C. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15: 323–350 [DOI] [PubMed] [Google Scholar]
- 5.Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3: 23–35 [DOI] [PubMed] [Google Scholar]
- 6.Nair M. G., Guild K. J., Artis D. 2006. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J. Immunol. 177: 1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loke P., Nair M. G., Parkinson J., Guiliano D., Blaxter M., Allen J. E. 2002. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raes G., De Baetselier P., Noël W., Beschin A., Brombacher F., Hassanzadeh Gh G. 2002. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 71: 597–602 [PubMed] [Google Scholar]
- 9.Nair M. G., Du Y., Perrigoue J. G., Zaph C., Taylor J. J., Goldschmidt M., Swain G. P., Yancopoulos G. D., Valenzuela D. M., Murphy A., et al. 2009. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206: 937–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z., Zheng T., Homer R. J., Kim Y. K., Chen N. Y., Cohn L., Hamid Q., Elias J. A. 2004. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304: 1678–1682 [DOI] [PubMed] [Google Scholar]
- 11.Anthony R. M., Urban J. F., Jr., Alem F., Hamed H. A., Rozo C. T., Boucher J. L., Van Rooijen N., Gause W. C. 2006. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12: 955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condeelis J., Pollard J. W. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124: 263–266 [DOI] [PubMed] [Google Scholar]
- 13.Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. 2007. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447: 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E. Y., Battaile J. T., Patel A. C., You Y., Agapov E., Grayson M. H., Benoit L. A., Byers D. E., Alevy Y., Tucker J., et al. 2008. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 14: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonecchi R., Sozzani S., Stine J. T., Luini W., D’Amico G., Allavena P., Chantry D., Mantovani A. 1998. Divergent effects of interleukin-4 and interferon-γ on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood 92: 2668–2671 [PubMed] [Google Scholar]
- 16.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25: 677–686 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K., Jose P. J., Rankin S. M. 2002. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J. Immunol. 168: 1911–1918 [DOI] [PubMed] [Google Scholar]
- 18.Katakura T., Miyazaki M., Kobayashi M., Herndon D. N., Suzuki F. 2004. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J. Immunol. 172: 1407–1413 [DOI] [PubMed] [Google Scholar]
- 19.Ziegler S. F., Artis D. 2010. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 11: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soumelis V., Reche P. A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3: 673–680 [DOI] [PubMed] [Google Scholar]
- 21.Ying S., O’Connor B., Ratoff J., Meng Q., Mallett K., Cousins D., Robinson D., Zhang G., Zhao J., Lee T. H., Corrigan C. 2005. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 174: 8183–8190 [DOI] [PubMed] [Google Scholar]
- 22.Yoo J., Omori M., Gyarmati D., Zhou B., Aye T., Brewer A., Comeau M. R., Campbell D. J., Ziegler S. F. 2005. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 202: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson R. P., Zimmerli S. C., Comeau M. R., Itano A., Omori M., Iseki M., Hauser C., Ziegler S. F. 2010. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J. Immunol. 184: 2974–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B., Comeau M. R., De Smedt T., Liggitt H. D., Dahl M. E., Lewis D. B., Gyarmati D., Aye T., Campbell D. J., Ziegler S. F. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6: 1047–1053 [DOI] [PubMed] [Google Scholar]
- 25.Al-Shami A., Spolski R., Kelly J., Keane-Myers A., Leonard W. J. 2005. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202: 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astrakhan A., Omori M., Nguyen T., Becker-Herman S., Iseki M., Aye T., Hudkins K., Dooley J., Farr A., Alpers C. E., et al. 2007. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat. Immunol. 8: 522–531 [DOI] [PubMed] [Google Scholar]
- 27.Headley M. B., Zhou B., Shih W. X., Aye T., Comeau M. R., Ziegler S. F. 2009. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J. Immunol. 182: 1641–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckmann M. P., Schooley K. A., Gallis B., Vanden Bos T., Friend D., Alpert A. R., Raunio R., Prickett K. S., Baker P. E., Park L. S. 1990. Monoclonal antibodies block murine IL-4 receptor function. J. Immunol. 144: 4212–4217 [PubMed] [Google Scholar]
- 29.Zhou B., Headley M. B., Aye T., Tocker J., Comeau M. R., Ziegler S. F. 2008. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J. Immunol. 181: 6557–6562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenbarth S. C., Piggott D. A., Huleatt J. W., Visintin I., Herrick C. A., Bottomly K. 2002. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196: 1645–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siracusa M. C., Reece J. J., Urban J. F., Jr., Scott A. L. 2008. Dynamics of lung macrophage activation in response to helminth infection. J. Leukoc. Biol. 84: 1422–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chorro L., Sarde A., Li M., Woollard K. J., Chambon P., Malissen B., Kissenpfennig A., Barbaroux J. B., Groves R., Geissmann F. 2009. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 206: 3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins S. J., Ruckerl D., Cook P. C., Jones L. H., Finkelman F. D., van Rooijen N., MacDonald A. S., Allen J. E. 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan M. H., Schindler U., Smiley S. T., Grusby M. J. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4: 313–319 [DOI] [PubMed] [Google Scholar]
- 35.Holt P. G., Strickland D. H. 2010. Interactions between innate and adaptive immunity in asthma pathogenesis: new perspectives from studies on acute exacerbations. J. Allergy Clin. Immunol. 125: 963–972, quiz 973–974 [DOI] [PubMed] [Google Scholar]
- 36.Melgert B. N., Oriss T. B., Qi Z., Dixon-McCarthy B., Geerlings M., Hylkema M. N., Ray A. 2010. Macrophages: regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol. 42: 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melgert B. N., Ten Hacken N. H., Rutgers B., Timens W., Postma D. S., Hylkema M. N. 2010. 2011. More alternative activation of macrophages in lungs of asthmatic patients. J. Allergy Clin. Immunol. 127: 831–833 [DOI] [PubMed] [Google Scholar]
- 38.Ford A. Q., Dasgupta P., Mikhailenko I., Smith E. M., Noben-Trauth N., Keegan A. D. 2012. Adoptive transfer of IL-4Rα+ macrophages is sufficient to enhance eosinophilic inflammation in a mouse model of allergic lung inflammation. BMC Immunol. 13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwenhuizen N. E., Kirstein F., Jayakumar J., Emedi B., Hurdayal R., Horsnell W. G., Lopata A. L., Brombacher F. 2012. Allergic airway disease is unaffected by the absence of IL-4Rα-dependent alternatively activated macrophages. J. Allergy Clin. Immunol. 130: 743–750, e8 [DOI] [PubMed] [Google Scholar]
- 40.Saenz S. A., Taylor B. C., Artis D. 2008. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226: 172–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon S., Martinez F. O. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604 [DOI] [PubMed] [Google Scholar]
- 42.Kurowska-Stolarska M., Stolarski B., Kewin P., Murphy G., Corrigan C. J., Ying S., Pitman N., Mirchandani A., Rana B., van Rooijen N., et al. 2009. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183: 6469–6477 [DOI] [PubMed] [Google Scholar]
- 43.Saenz S. A., Siracusa M. C., Perrigoue J. G., Spencer S. P., Urban J. F., Jr., Tocker J. E., Budelsky A. L., Kleinschek M. A., Kastelein R. A., Kambayashi T., et al. 2010. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464: 1362–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Q., Wang C., Zheng D., Wang Y., Lee V. W., Wang Y. M., Zheng G., Tan T. K., Yu D., Alexander S. I., et al. 2011. IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease. J. Am. Soc. Nephrol. 22: 1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzo A., Monteleone I., Fina D., Stolfi C., Caruso R., Fantini M. C., Franzè E., Schwendener R., Pallone F., Monteleone G. 2012. Inhibition of colitis by IL-25 associates with induction of alternatively activated macrophages. Inflamm. Bowel Dis. 18: 449–459 [DOI] [PubMed] [Google Scholar]
- 46.Morris D. G., Huang X., Kaminski N., Wang Y., Shapiro S. D., Dolganov G., Glick A., Sheppard D. 2003. Loss of integrin αvβ6-mediated TGF-β activation causes Mmp12-dependent emphysema. Nature 422: 169–173 [DOI] [PubMed] [Google Scholar]
- 47.Song C., Luo L., Lei Z., Li B., Liang Z., Liu G., Li D., Zhang G., Huang B., Feng Z. H. 2008. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 181: 6117–6124 [DOI] [PubMed] [Google Scholar]
- 48.Pribul P. K., Harker J., Wang B., Wang H., Tregoning J. S., Schwarze J., Openshaw P. J. 2008. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 82: 4441–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao M., Fernandez L. G., Doctor A., Sharma A. K., Zarbock A., Tribble C. G., Kron I. L., Laubach V. E. 2006. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 291: L1018–L1026 [DOI] [PubMed] [Google Scholar]
- 50.Broug-Holub E., Toews G. B., van Iwaarden J. F., Strieter R. M., Kunkel S. L., Paine R., III, Standiford T. J. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65: 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wynn T. A. 2004. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 4: 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munitz A., Brandt E. B., Mingler M., Finkelman F. D., Rothenberg M. E. 2008. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl. Acad. Sci. USA 105: 7240–7245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monticelli L. A., Sonnenberg G. F., Abt M. C., Alenghat T., Ziegler C. G., Doering T. A., Angelosanto J. M., Laidlaw B. J., Yang C. Y., Sathaliyawala T., et al. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12: 1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H., Koyasu S. 2010. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463: 540–544 [DOI] [PubMed] [Google Scholar]
- 55.Neill D. R., Wong S. H., Bellosi A., Flynn R. J., Daly M., Langford T. K., Bucks C., Kane C. M., Fallon P. G., Pannell R., et al. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price A. E., Liang H. E., Sullivan B. M., Reinhardt R. L., Eisley C. J., Erle D. J., Locksley R. M. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107: 11489–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez F. O., Sica A., Mantovani A., Locati M. 2008. Macrophage activation and polarization. Front. Biosci. 13: 453–461 [DOI] [PubMed] [Google Scholar]
- 58.Porcheray F., Viaud S., Rimaniol A. C., Léone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. 2005. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142: 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosser D. M., Edwards J. P. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8: 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laskin D. L., Sunil V. R., Gardner C. R., Laskin J. D. 2011. Macrophages and tissue injury: agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 51: 267–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stout R. D., Suttles J. 2004. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 76: 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray R. J., Furlonger C., Williams D. E., Paige C. J. 1996. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur. J. Immunol. 26: 10–16 [DOI] [PubMed] [Google Scholar]
- 63.Watanabe N., Hanabuchi S., Soumelis V., Yuan W., Ho S., de Waal Malefyt R., Liu Y. J. 2004. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 5: 426–434 [DOI] [PubMed] [Google Scholar]
- 64.Ito T., Wang Y. H., Duramad O., Hori T., Delespesse G. J., Watanabe N., Qin F. X., Yao Z., Cao W., Liu Y. J. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202: 1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Shami A., Spolski R., Kelly J., Fry T., Schwartzberg P. L., Pandey A., Mackall C. L., Leonard W. J. 2004. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 200: 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Omori M., Ziegler S. 2007. Induction of IL-4 expression in CD4+ T cells by thymic stromal lymphopoietin. J. Immunol. 178: 1396–1404 [DOI] [PubMed] [Google Scholar]
- 67.Perrigoue J. G., Saenz S. A., Siracusa M. C., Allenspach E. J., Taylor B. C., Giacomin P. R., Nair M. G., Du Y., Zaph C., van Rooijen N., et al. 2009. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 10: 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allakhverdi Z., Comeau M. R., Jessup H. K., Delespesse G. 2009. Thymic stromal lymphopoietin as a mediator of crosstalk between bronchial smooth muscles and mast cells. J. Allergy Clin. Immunol. 123: 958–960, e2 [DOI] [PubMed] [Google Scholar]
- 69.Allakhverdi Z., Comeau M. R., Jessup H. K., Yoon B. R., Brewer A., Chartier S., Paquette N., Ziegler S. F., Sarfati M., Delespesse G. 2007. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagata Y., Kamijuku H., Taniguchi M., Ziegler S., Seino K. 2007. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int. Arch. Allergy Immunol. 144: 305–314 [DOI] [PubMed] [Google Scholar]