Abstract
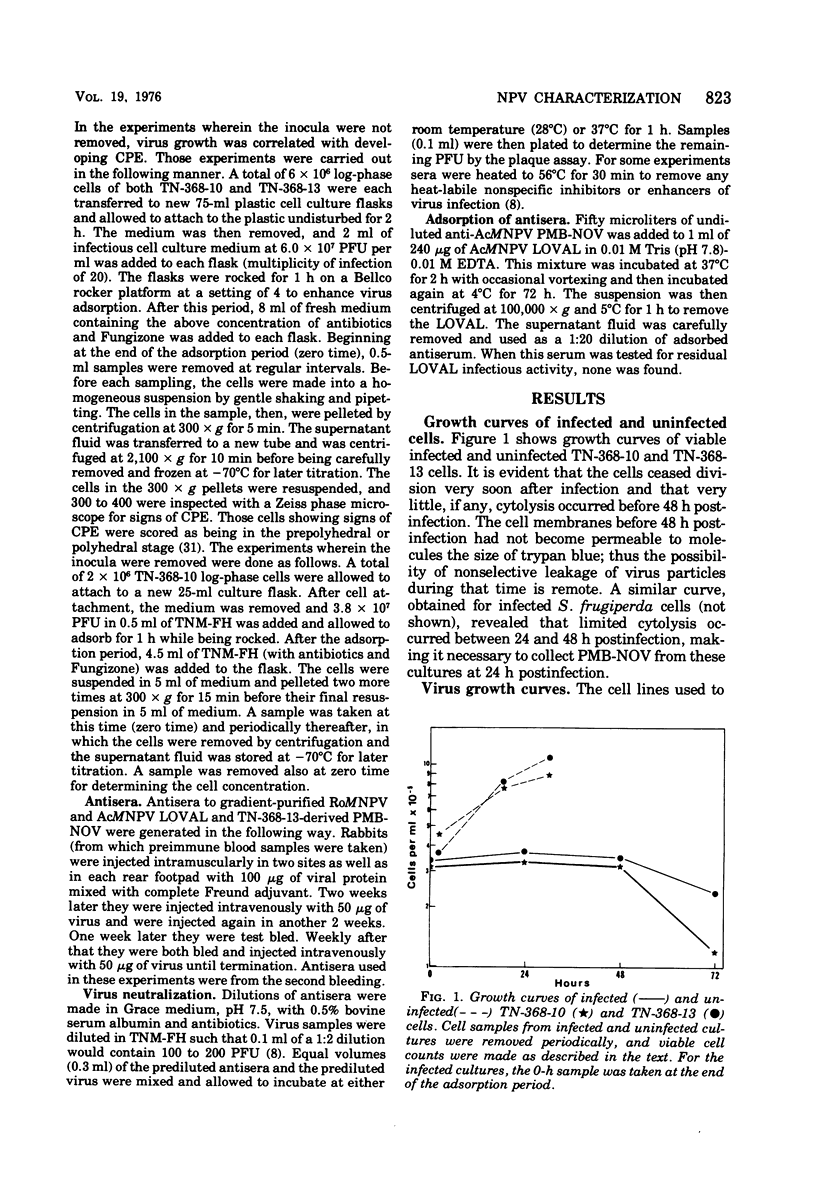
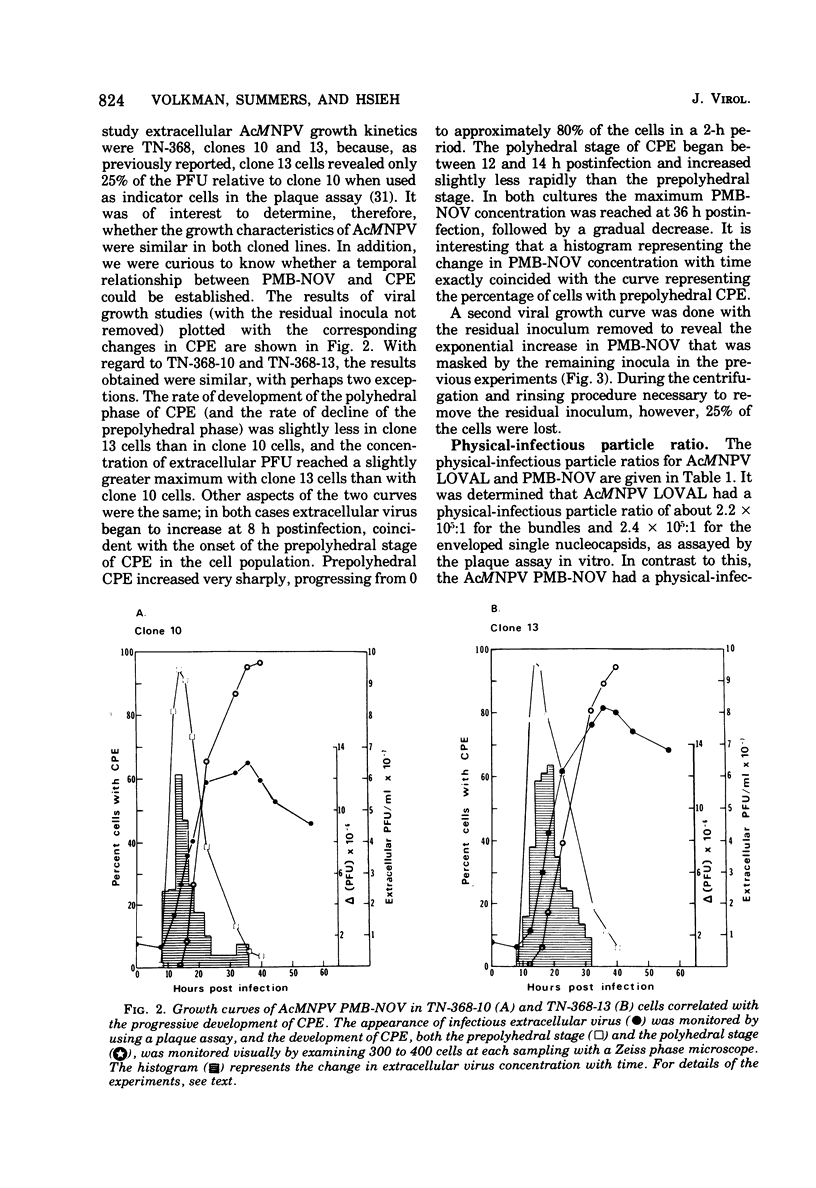
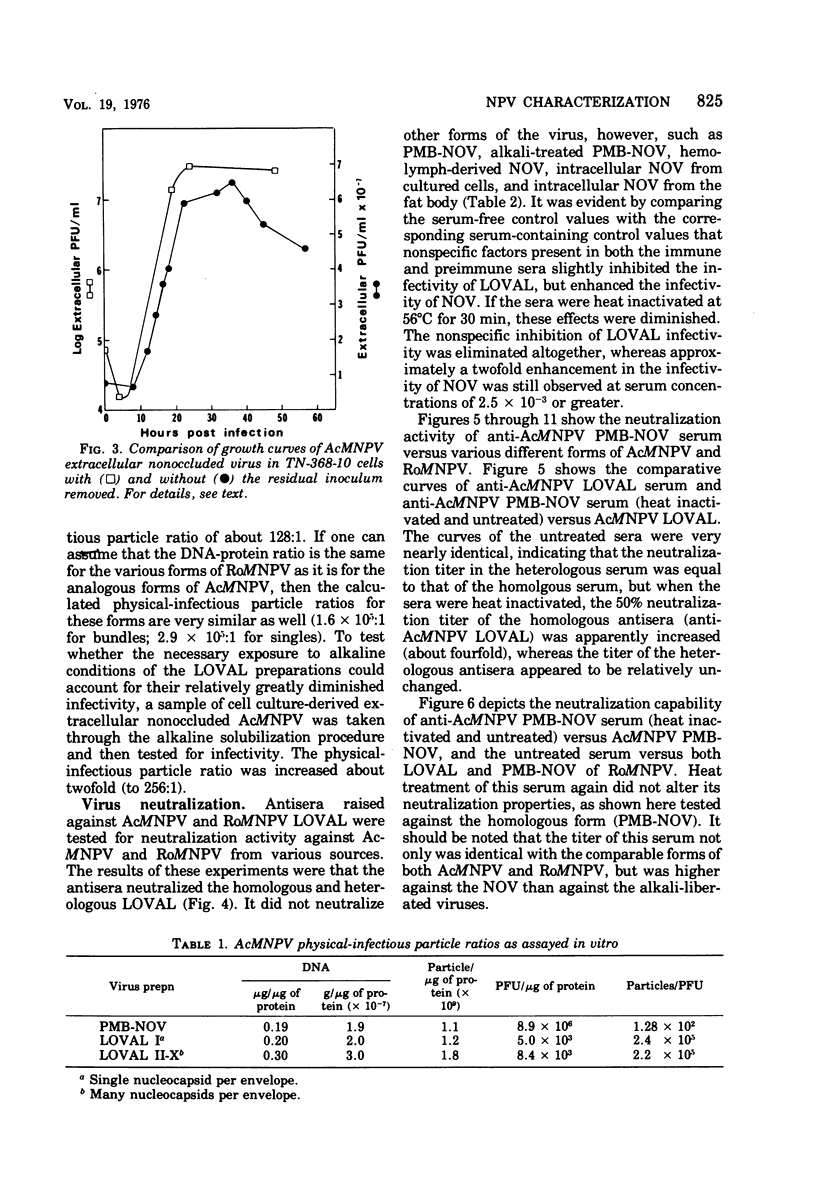
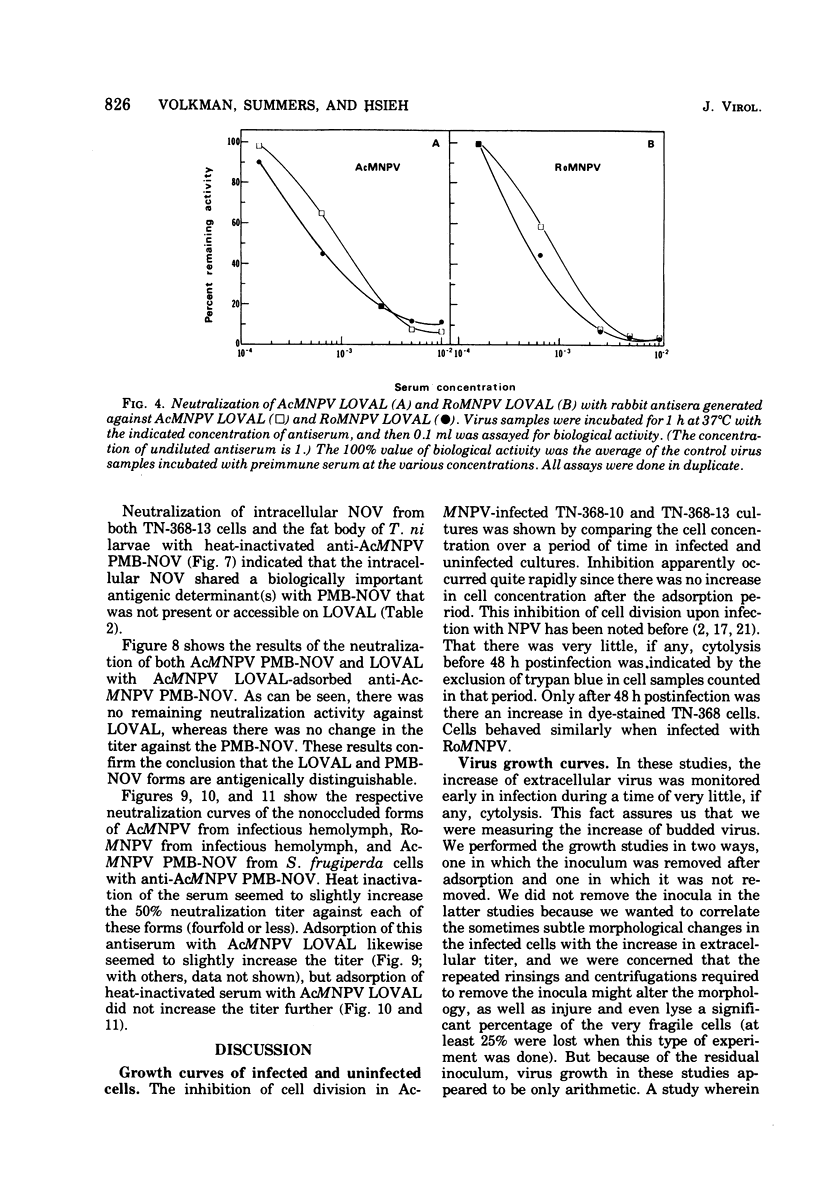
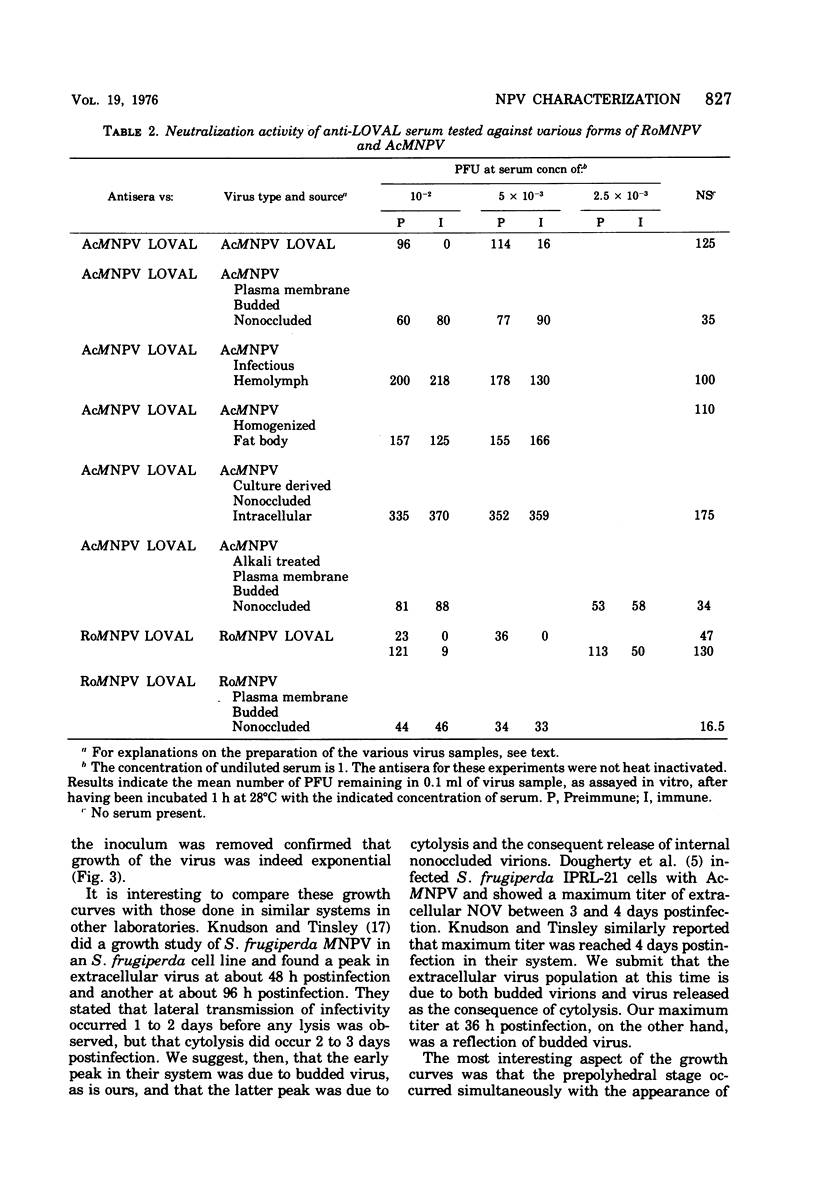
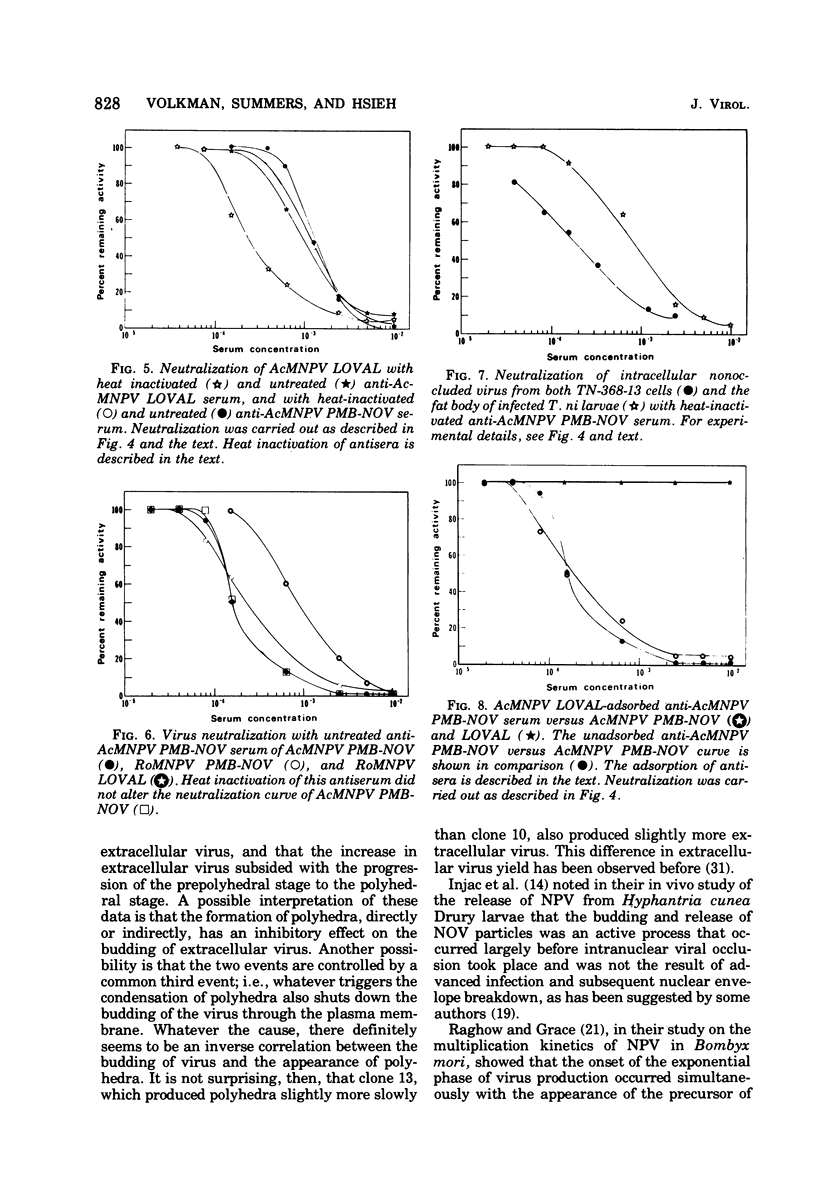
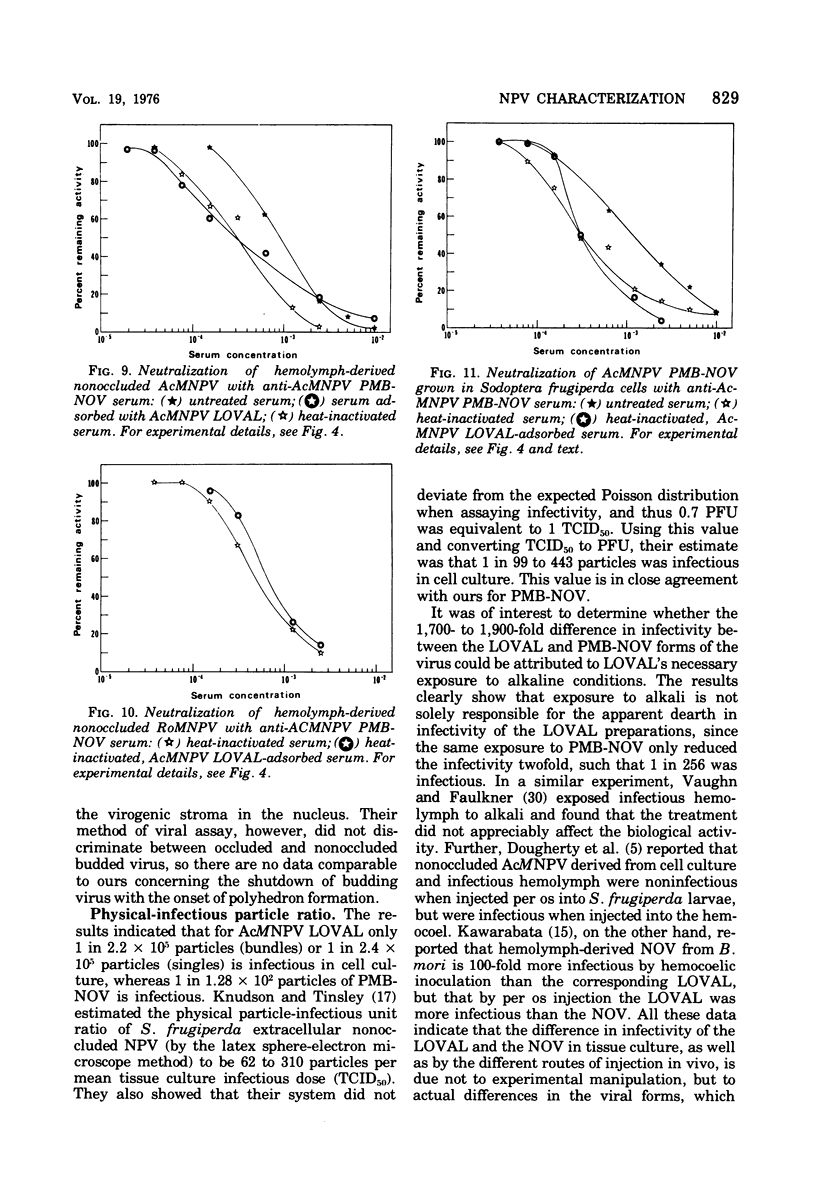
Nuclear polyhedrosis virus infections of lepidopteran cells often result in the production of both occluded and nonoccluded virus. The characterization of these two different forms has been the subject of several papers. We have divided the nonoccluded virus (NOV) category further into plasma membrane-budded non-occluded virus (PMB-NOV), intracellular NOV, and hemolymph-derived NOV, and have done additional studies investigating the differences between these nonoccluded forms and the alkali-liberated forms from occlusions of the nuclear polyhedrosis viruses of Autographa californica and Rachiplusa ou. The methods used to discern differences and similarities among the forms were serological, biochemical, and visual, all related to their biological acitivity. Neutralization studies revealed that alkali-liberated virus and PMB-NOV had both similar and different antigens. Antisera raised against alkali-liberated virus from occlusions neutralized the alkali-liberated form of the virus, but did not neutralize the intracellular or extracellular nonoccluded forms. Antisera raised against the TN-368-13 PMB-NOV, however, neutralized the alkali-liberated forms as well as all forms of the NOV. Adsorption of this antisera with alkali-liberated virus did not diminish the neutralization titer against the nonoccluded forms, thus confirming the antigenic differences between the alkali-liberated and nonoccluded forms of the virus. Physical-infectious particle ratio calculations indicated that the PMB-NOV of Autographa californica are about 1,900-fold more infectious than the single-nucleocapsid-per-envelope alkali-liberated particles and about 1,700-fold more infectious than the multiple-nucleocapsid-per-envelope particles, as assayed in vitro. In addition, a study of viral growth kinetics monitored concurrently with the appearance of polyhedra showed that PMB-NOV production is shut down with the onset of polyhedron formation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E. A., Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Dougherty E. M., Vaughn J. L., Reichelderfer C. F. Characteristics of the non-occluded form of a nuclear polyhedrosis virus. Intervirology. 1975;5(3-4):109–121. doi: 10.1159/000149889. [DOI] [PubMed] [Google Scholar]
- EASTERBROOK K. B. The multiplication of vaccinia virus in suspended KB cells. Virology. 1961 Dec;15:404–416. doi: 10.1016/0042-6822(61)90108-8. [DOI] [PubMed] [Google Scholar]
- FURNESS G., YOUNGNER J. S. One-step growth curves for vaccinia virus in cultures of monkey kidney cells. Virology. 1959 Nov;9:386–395. doi: 10.1016/0042-6822(59)90130-8. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Faulkner P., MacKinnon E. A. Some biophysical properties of virus present in tissue cultures infected with the nuclear polyhedrosis virus of Trichoplusia ni. J Gen Virol. 1974 Jan;22(1):143–146. doi: 10.1099/0022-1317-22-1-143. [DOI] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Kawarabata T. Highly infectious free virions in the hemolymph of the silkworm (Bombyx mori) infected with a nuclear polyhedrosis virus. J Invertebr Pathol. 1974 Sep;24(2):196–200. doi: 10.1016/0022-2011(74)90011-1. [DOI] [PubMed] [Google Scholar]
- Knudson D. L., Harrap K. A. Replication of nuclear polyhedrosis virus in a continuous cell culture of Spodoptera frugiperda: microscopy study of the sequence of events of the virus infection. J Virol. 1975 Jan;17(1):254–268. doi: 10.1128/jvi.17.1.254-268.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson D. L., Tinsley T. W. Replication of a nuclear polyhedrosis virus in a continuous cell culture of Spodoptera frugiperda: purification, assay of infectivity, and growth characteristics of the virus. J Virol. 1974 Oct;14(4):934–944. doi: 10.1128/jvi.14.4.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacKinnon E. A., Henderson J. F., Stoltz D. B., Faulkner P. Morphogenesis of nuclear polyhedrosis virus under conditions of prolonged passage in vitro. J Ultrastruct Res. 1974 Dec;49(3):419–435. doi: 10.1016/s0022-5320(74)90055-0. [DOI] [PubMed] [Google Scholar]
- POSTLETHWAITE R., MAITLAND H. B. The eclipse phase of vaccinia virus growing in chick embryo cell monolayers and some technical procedures which affect its demonstration. J Hyg (Lond) 1960 Jun;58:133–145. doi: 10.1017/s0022172400038213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Grace T. D. Studies on a nuclear polyhedrosis virus in Bombyx mori cells in vitro. 1. Multiplication kinetics and ultrastructural studies. J Ultrastruct Res. 1974 Jun;47(3):384–399. doi: 10.1016/s0022-5320(74)90016-1. [DOI] [PubMed] [Google Scholar]
- SMITH K. O., SHARP D. G. Interaction of virus with cells in tissue cultures. I. Adsorption on and growth of vaccinia virus in L cells. Virology. 1960 Jul;11:519–532. doi: 10.1016/0042-6822(60)90097-0. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of nuclear polyhedrosis virus DNAs. J Virol. 1973 Dec;12(6):1336–1346. doi: 10.1128/jvi.12.6.1336-1346.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Trichoplusia ni granulosis virus granulin: a phenol-soluble, phosphorylated protein. J Virol. 1975 Nov;16(5):1108–1116. doi: 10.1128/jvi.16.5.1108-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Volkman L. E. Comparison of biophysical and morphological properties of occluded and extracellular nonoccluded baculovirus from in vivo and in vitro host systems. J Virol. 1976 Mar;17(3):962–972. doi: 10.1128/jvi.17.3.962-972.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHN J. L., FAULKNER P. SUSCEPTIBILITY OF AN INSECT TISSUE CULTURE TO INFECTION BY VIRUS PREPARATIONS OF THE NUCLEAR POLYHEDROSIS OF THE SILKWORM (BOMBYX MORI L.). Virology. 1963 Jul;20:484–489. doi: 10.1016/0042-6822(63)90098-9. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D. Nuclear polyhedrosis virus detection: relative capabilities of clones developed from Trichoplusia ni ovarian cell line TN-368 to serve as indicator cells in a plaque assay. J Virol. 1975 Dec;16(6):1630–1637. doi: 10.1128/jvi.16.6.1630-1637.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zherebtsova E. N., Strokovskaya L. I., Gudz-Gorban A. P. Subviral infectivity in nuclear polyhedrosis of the great wax moth (Galleria mellonella L.). Acta Virol. 1972 Sep;16(5):427–431. [PubMed] [Google Scholar]



