Abstract
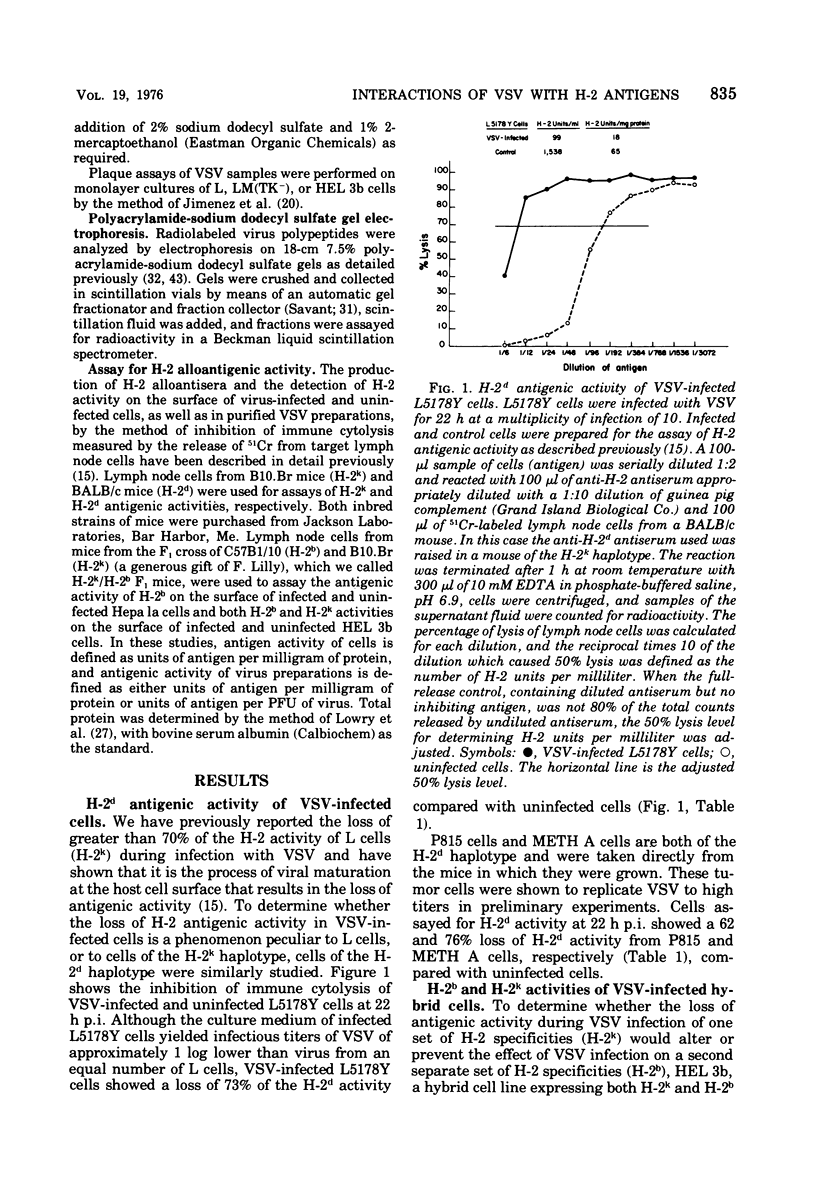
The process of maturation of vesicular stomatitis virus (VSV) results in the loss of 70% of the H-2k antigenic activity from L-cell plasma membranes. This phenomenon is also demonstrated during VSV infection of cells of the H-2d haplotype. Using the method of inhibition of immune cytolysis, VSV-infected L5178Y tissue culture cells and VSV-infected METH A fibrosarcoma cells grown in vivo show a loss of H-2d activity of 73 and 76%, respectively. Using monospecific antisera, it is seen that VSV infection results in a significant loss of antigenic activity of the gene products of both the H-2D and H-2K regions in cells of the H-2d and H-2k haplotypes. In hybrid cells expressing H-2k as well as H-2b, VSV infection results in the decrease of both H-2 antigenic activities to the same extent. VSV purified from L cells shows considerable H-2k activity, but the reaction of this virus with anti-H-2k serum does not prevent a normal subsequent infection with this virus. VSV may associate with H-2 antigen in the culture medium, but the results of mixing VSV with uninfected H-2-containing homogenates suggest that this association occurs only when the host cell and the cell homogenate share the same H-2 haplotype. Velocity sedimentation of VSV, which would remove contaminating cellular membrane fragments, does not separate H-2 activity from VSV. H-2 activity is also stably associated with VSV throughout sequential sucrose gradient centrifugation steps. It is possible that H-2 antigen is a structural component of VSV grown in murine cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Takahashi T. Viral and cellular surface antigens of murine leukemias and myelomas. Serological analysis by immunoelectron microscopy. J Exp Med. 1972 Mar 1;135(3):443–457. doi: 10.1084/jem.135.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Phenotypic mixing between group A arboviruses. Nature. 1966 Jun 25;210(5043):1397–1399. doi: 10.1038/2101397a0. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Compans R. W. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J Virol. 1970 May;5(5):609–616. doi: 10.1128/jvi.5.5.609-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Progressive loss of H-2 antigens with concomitant increase of cell-surface antigen(s) determined by Moloney leukemia virus in cultured murine lymphomas. J Natl Cancer Inst. 1973 Feb;50(2):347–362. doi: 10.1093/jnci/50.2.347. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Atkinson P. H., Summers D. F. Interactions of vesicular stomatitis virus structural proteins with HeLa plasma membranes. Nat New Biol. 1971 May 26;231(21):121–123. doi: 10.1038/newbio231121a0. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Nathenson S. G. Distribution of H-2 alloantigenic specificities on radiolabeled papain-solubilized antigen fragments. J Immunol. 1971 Aug;107(2):563–570. [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D., Nathenson S. G., Cherry M. The molecular basis of codominant expression of the histocompatibility-2 genetic region. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1394–1397. doi: 10.1073/pnas.69.6.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D., Nathenson S. G. The distribution of alloantigenic specificities of native H-2 products. J Immunol. 1972 Mar;108(3):596–600. [PubMed] [Google Scholar]
- Darlington G. J., Bernard H. P., Ruddle F. H. Human serum albumin phenotype activation in mouse hepatoma--human leukocyte cell hybrids. Science. 1974 Sep 6;185(4154):859–862. doi: 10.1126/science.185.4154.859. [DOI] [PubMed] [Google Scholar]
- FISCHER G. A. Studies of the culture of leukemic cells in vitro. Ann N Y Acad Sci. 1958 Dec 5;76(3):673–680. doi: 10.1111/j.1749-6632.1958.tb54884.x. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Edidin M. Cell surface antigens of a mouse testicular teratoma. Identification of an antigen physically associated with H-2 antigens on tumor cells. J Exp Med. 1974 Jul 1;140(1):61–78. doi: 10.1084/jem.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWATSON A. F., WHITMORE G. F. The development and structure of vesicular stomatitis virus. Virology. 1962 Apr;16:466–478. doi: 10.1016/0042-6822(62)90228-3. [DOI] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Effect of vesicular stomatitis virus infection on the histocompatibility antigen of L cells. J Virol. 1972 Oct;10(4):578–585. doi: 10.1128/jvi.10.4.578-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Newcastle disease virus infection of L cells. J Virol. 1974 Jul;14(1):162–169. doi: 10.1128/jvi.14.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Influenza virus effects on cell membrane proteins. Science. 1970 Jan 9;167(3915):202–205. doi: 10.1126/science.167.3915.202. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez L., Bloom B. R., Blume M. R., Oettgen H. F. On the number and nature of antigen-sensitive lymphocytes in the blood of delayed-hypersensitive human donors. J Exp Med. 1971 Apr 1;133(4):740–751. doi: 10.1084/jem.133.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycolipid content of vesicular stomatitis virus grown in baby hamster kidney cells. J Virol. 1971 Mar;7(3):416–417. doi: 10.1128/jvi.7.3.416-417.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. doi: 10.1128/jvi.7.1.59-70.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Nathenson S. G. Studies on the carbohydrate portion of membrane-located mouse H-2 alloantigens. Biochemistry. 1970 Dec 8;9(25):4875–4883. doi: 10.1021/bi00827a008. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G. Biochemical properties of histocompatibility antigens. Annu Rev Genet. 1970;4(0):69–90. doi: 10.1146/annurev.ge.04.120170.000441. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Davies D. A. Solubilization and partial purification of mouse histocompatibility antigens from a membranous lipoprotein fraction. Proc Natl Acad Sci U S A. 1966 Aug;56(2):476–483. doi: 10.1073/pnas.56.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L. Enzymatic and immunologic alterations in mice infected with lactic dehydrogenase virus. Am J Pathol. 1971 Sep;64(3):733–746. [PMC free article] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Ricciuti F. C., Ruddle F. H. Assignment of three gene loci (PGK, HGPRT, G6PD) to the long arm of the human X chromosome by somatic cell genetics. Genetics. 1973 Aug;74(4):661–678. doi: 10.1093/genetics/74.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of an infectious virus-antibody complex with Rous sarcoma virus and antibodies directed against the major virus glycoprotein. J Virol. 1976 Mar;17(3):1063–1067. doi: 10.1128/jvi.17.3.1063-1067.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Regeneration of transplantation antigens on mouse cells. Transplant Proc. 1971 Mar;3(1):180–182. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shimada A., Nathenson S. G. Removal of neuraminic acid from H-2 alloantigens without effect of antigenic reactivity. J Immunol. 1971 Oct;107(4):1197–1199. [PubMed] [Google Scholar]
- Shimada A., Yamane K., Nathenson S. G. Comparison of the peptide composition of two histocompatibility-2 alloantigens. Proc Natl Acad Sci U S A. 1970 Mar;65(3):691–696. doi: 10.1073/pnas.65.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell G. D., Cherry M., Démant P. Evidence that H-2 private specificities can be arranged in two mutually exclusive systems possibly homologous with two subsystems of HL-A. Transplant Proc. 1971 Mar;3(1):183–186. [PubMed] [Google Scholar]
- Ting C. C., Herberman R. B. Inverse relationship of polyoma tumour specific cell surface antigen to H-2 histocompatibility antigens. Nat New Biol. 1971 Jul 28;232(30):118–120. doi: 10.1038/newbio232118a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Kiley M. P., Snyder R. M., Schnaitman C. A. Cytoplasmic compartmentalization of the protein and ribonucleic acid species of vesicular stomatitis virus. J Virol. 1972 Apr;9(4):672–683. doi: 10.1128/jvi.9.4.672-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Stabilization of enveloped viruses by dimethyl sulfoxide. J Virol. 1968 Sep;2(9):953–954. doi: 10.1128/jvi.2.9.953-954.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee Y. C., Hackett A. J., Talens L. Vesicular stomatitis virus maturation sites in six different host cells. J Gen Virol. 1970;7(2):95–102. doi: 10.1099/0022-1317-7-2-95. [DOI] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]
- Závada J., Rosenbergová M. Phenotypic mixing of vesicular stomatitis virus with fowl plague virus. Acta Virol. 1972 Mar;16(2):103–114. [PubMed] [Google Scholar]
- Závada J. VSV pseudotype particles with the coat of avian myeloblastosis virus. Nat New Biol. 1972 Nov 22;240(99):122–124. doi: 10.1038/newbio240122a0. [DOI] [PubMed] [Google Scholar]
- Závada J., Zázadová Z., Malír A., Koćent A. VSV pseudotype produced in cell line derived from human mammary carcinoma. Nat New Biol. 1972 Nov 22;240(99):124–125. doi: 10.1038/newbio240124a0. [DOI] [PubMed] [Google Scholar]



