Abstract
Purpose
This is a review of literature on the indications, technique, and outcome of portal vein embolization (PVE).
Methods
A systematic literature search on outcome of PVE from 1990 to 2011 was performed in Medline, Cochrane, and Embase databases.
Results
Forty-four articles were selected, including 1,791 patients with a mean age of 61 ± 4.1 years. Overall technical success rate was 99.3 %. The mean hypertrophy rate of the FRL after PVE was 37.9 ± 0.1 %. In 70 patients (3.9 %), surgery was not performed because of failure of PVE (clinical success rate 96.1 %). In 51 patients (2.8 %), the hypertrophy response was insufficient to perform liver resection. In the other 17 cases, 12 did not technically succeed (0.7 %) and 7 caused a complication leading to unresectability (0.4 %). In 6.1 %, resection was cancelled because of local tumor progression after PVE. Major complications were seen in 2.5 %, and the mortality rate was 0.1 %. A head-to-head comparison shows a negative effect of liver cirrhosis on hypertrophy response. The use of n-butyl cyanoacrylate seems to have a greater effect on hypertrophy, but the difference with other embolization materials did not reach statistical significance. No difference in regeneration is seen in patients with cholestasis or chemotherapy.
Conclusions
Preoperative PVE has a high technical and clinical success rate. Liver cirrhosis has a negative effect on regeneration, but cholestasis and chemotherapy do not seem to have an influence on the hypertrophy response. The use of n-butyl cyanoacrylate may result in a greater hypertrophy response compared with other embolization materials used.
Keywords: Liver, Resection, Portal vein, Embolization, Regeneration, Future remnant, Colorectal metastasis
Introduction
Liver resection is in many cases the only option for long-term survival for patients with primary or secondary liver malignancies. Unfortunately, only 10–20 % of patients with colorectal liver metastases are candidates for liver resection. The resectability rate for hepatocellular carcinoma is approximately 20–30 % in patients with normal livers but is reduced in patients with cirrhotic livers [1]. In literature, the postoperative liver failure rate ranges from 0 to 30 % and is still the major cause of death following major liver resection. When patients prove unresectable because of insufficient remnant liver volume, portal vein embolization (PVE) is one of the methods to stimulate growth of the future remnant liver (FRL), thereby sustaining the possibility of extensive liver resection.
The first to demonstrate the regenerative capacity of the liver following portal vein occlusion were Rous and Larimore in the 1920s. In a rabbit model, they showed atrophy of the hepatic lobe ipsilateral to the ligated portal branches, while compensatory hypertrophy was observed in the contralateral lobe [2]. In 1961, portal vein ligation was reported in humans as part of a two-stage extended hepatectomy [3]. Kinoshita et al. [4] reported the first preoperative PVE in a human being in 1986. Since then, numerous reports have shown the efficacy of inducing compensatory hypertrophy of the FRL after PVE in preparation for surgery to resect primary or metastatic cancers in the liver [5–7].
Several techniques for portal vein occlusion have been reported, including intraoperative portal branch ligation [8–10], transileocolic PVE [11–13], and the percutaneous transhepatic ipsilateral [14, 15] or contralateral [16, 17] PVE technique. The underlying principle is to block the portal venous blood flow to the liver segments that are planned to be resected. This induces atrophy of the ipsilateral liver segments and compensatory hypertrophy of the contralateral liver segments, resulting in increase of the size of the FRL. In addition to the different techniques, different embolization materials are used clinically, e.g., polyvinyl alcohol particles (PVA), coils, gelatin sponge, n-butyl cyanoacrylate and lipiodol, or fibrin glue.
Many clinical studies have been published on the effects of PVE on hypertrophy of the FRL in small and larger patient cohorts. However, only few data have been published on the difference between the use of different embolization materials and the effect of chemotherapy or preexisting liver cirrhosis on the growth of the FRL after PVE.
In 2008, a meta-analysis was published by Abulkhir et al. which reviewed all publications on PVE between 1990 and 2005. They focused especially on the differences between various access techniques (transhepatic vs. transileocolic) regarding the ensuing hypertrophy response and surgical outcome [18]. However, with the growing availability of radiological intervention suites, during the past decades, the percutaneous transhepatic technique has become the standard technique for PVE. In addition, many new articles on PVE have been published since Abulkhir’s report.
In this review, we systematically evaluated all publications on PVE in the past 20 years to assess the technical and clinical results of PVE, with special interest in the influence of chemotherapy, preexisting liver cirrhosis, cholestasis, and the use of different embolization materials on the hypertrophy response.
Materials and Methods
Search Strategy
A systematic literature search was performed in Medline, Cochrane, and Embase from January 1, 1990 to May 1, 2011. The applied search heading was: “portal vein embolization” limited to clinical studies, including at least 10 cases, published in the English language. Titles and abstracts were screened to identify potentially relevant articles. Referred and related articles also were checked. Articles were selected following the selection criteria and were independently evaluated by two of the authors (vL, vdE), using a scoring list. The final selection was made in consensus.
Selection Criteria
All clinical studies on PVE were included for further analysis. Full-text articles were retrieved and were included if they contained information on patient characteristics, indication for PVE, pre- and post-PVE liver volumes or percentages of the FRL, the technique that was used, time between PVE and CT/surgery, results, and complications of PVE, as well as results of liver surgery.
After the initial search, articles were excluded because they were written in a non-English language, were reports about portal vein ligation, were animal studies, were articles concerning chemoembolization, or were review articles. Furthermore, articles were excluded when patient characteristics, indications, methods, and results were not adequately described or when the FRL data were not sufficient and could not be extracted from the published data. Articles that overlapped with previously published data, that were published by the same author, or overlap with patient cohorts from the same study group or combined study groups was suspected were excluded.
Study Quality and Data Extraction
All included studies were evaluated for study quality characteristics by two reviewers (vL, vdE) independently. Study quality was assessed using an adapted version of a checklist of the Dutch Cochrane Centre [19].
The main points of interest included: (1) patient characteristics (number of patients, age, sex, type of liver tumor, liver fibrosis, chemotherapy); (2) indication for PVE (minimal percentage FRL based on CT volumetry data or indocyanine green (ICG) clearance); (3) embolization technique (transileocolic, transhepatic ipsilateral, transhepatic contralateral) and embolization material used (polyvinyl alcohol particles (PVAc), gelatin sponge, n-butyl cyanoacrylate, fibrin glue, ethanol, coils, vascular plug, or a combination); (4) data on CT volumetry; (5) follow-up (PVE success rate (successful occlusion of the portal vein), clinical success rate, post-PVE complications and morbidity); and (6) surgical outcome (percentage and type of resection, postoperative complications and mortality). Articles were valid and used for data extraction if the above-mentioned points were described clearly.
Results
The broad initial search using the search heading “portal vein embolization” resulted in 961 publications. Primary survey of the abstracts and articles excluded 684 articles dealing with subjects other than PVE, experimental animal studies or articles in a non-English language.
After critical evaluation of the remaining full text articles, 84 articles remained for the final scoring, using an item-list with the minimum requirements for final inclusion. Finally, 44 publications [5, 9, 11, 12, 14–17, 20–55] were included for meta-analysis (Fig. 1), consisting of 1,791 patients, including 1,139 men (63.6 %) and 617 women (34.5 %). The sex of the remaining 35 patients could not be extracted from the articles. The mean age was 61 ± 4.1 years. The underlying pathology is summarized in Table 1.
Fig. 1.
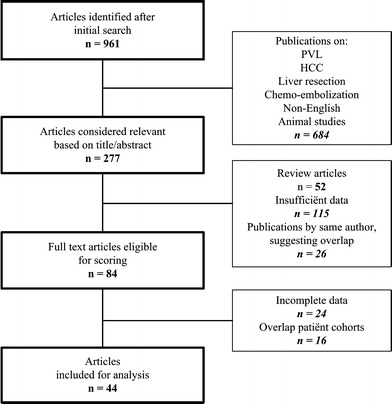
Flowchart showing selection of papers for analysis
Table 1.
Underlying pathology
| No. of patients (%) | |
|---|---|
| Colorectal metastasis | 709 (39.6) |
| Cholangiocarcinoma | 518 (28.9) |
| Hepatocellular carcinoma | 365 (20.4) |
| Gallbladder carcinoma | 164 (9.2) |
| Other (NET, angiosarcoma, cystadenocarcinoma) | 32 (1.8) |
| Benign (adenoma, hemangioma) | 5 (0.3) |
Indications for PVE
The indication for PVE varied in literature, but the percentage of the FRL was mainly used as the criterion for PVE. A resection of more than 70–75 % of the total liver volume in normal livers and more than 60–65 % in compromised livers (i.e., cirrhosis, fibrosis) was mainly the threshold for performing preoperative PVE in most studies. Three studies [20–22] used the ICG plasma disappearance rate or retention rate at 15 min [56]. A 15-min retention rate of more than 15–20 % in combination with a large liver resection constituted an indication for PVE.
In the pre-procedural workup computed tomography (CT) scans were performed to measure the volumes of the total liver, the part planned to be resected, total tumor volume, and the FRL. In most studies (30/44, 68.2 %), the absolute volumes were used to calculate the percentage FRL [5, 9, 11, 12, 14–17, 23–45, 57].
In the other studies (14/44, 31.8 %), the total estimated volume (TELV) was calculated using CT volumetry in combination with the body surface area, in the equation:
PVE Technique
PVE is performed by using a transileocolic or transhepatic approach. The transileocolic approach requires a minilaparotomy or can be performed as part of a two-stage resection. Using the transhepatic approach, the procedure can be performed by ipsilateral or contralateral puncture (Table 2).
Table 2.
Technique of PVE
| No. of procedures (%) | |
|---|---|
| Procedural approach | |
| Transileocolic | 223 (12.4) |
| Transhepatic ipsilateral | 963 (53.8) |
| Contralateral | 605 (33.8) |
| Embolized branches | |
| Segment 5–8 | 1,430 (79.9) |
| Segment 4–8 | 209 (11.7) |
| Segment 1–4 | 41 (2.3) |
| Other/unknown | 111 (6.2) |
The embolization materials mainly used for PVE were PVA, gelatin sponge, fibrin glue, n-butyl cyanoacrylate with lipiodol, polidocanol-foam, or combinations of these materials with coils or Amplatzer vascular plugs (Table 3). Gelatin sponge and n-butyl cyanoacrylate, as the primary embolization-material, were used the most in the evaluated studies (59.5 %), often in combination with other materials.
Table 3.
Embolization materials used
| Embolization materials | No. of patients | (%) |
|---|---|---|
| PVA particles + coils [14, 27, 30, 47, 48, 51] | 250 | 14.7 |
| PVA + alcohol [25] | 3 | |
| PVA + Amplatzer vascular plug [40] | 10 | |
| Gelatin sponge + lipiodol [11, 35–37, 49, 52] | 130 | 26.3 |
| Gelatin sponge + coils [44, 50, 70] | 71 | |
| Gelatin sponge + thrombin + urografine [12, 33] | 102 | |
| Gelatin sponge + urografine [20, 22] | 120 | |
| Gelatin sponge + polidocanol [36] | 8 | |
| Gelatin sponge + amplatzer [45] | 41 | |
| Fibrin glue/Beriplast + lipiodol [15, 36, 39, 54] | 177 | 9.9 |
| N-butyl cyanoacrylate + lipiodol [5, 9, 16, 17, 24–27, 29, 31, 36, 41, 42, 47, 53, 57] | 554 | 32.5 |
| N-butyl cyanoacrylate + gelatin sponge [23] | 11 | |
| N-butyl cyanoacrylate + Amplatzer vascular plug [26, 42] | 18 | |
| Embol-78 [38] | 51 | 2.8 |
| Ethanol + lipiodol [15, 34] | 159 | 10.2 |
| Ethanol + gelfoam + lipiodol [43] | 24 | |
| Ethoxysclerol/air-foam [28, 32] | 30 | 1.8 |
| Ethibloc + lipiodol [46, 48] | 33 | 1.8 |
Success Rate of PVE Procedure and its Effect on the Hypertrophy Response
The mean time interval between PVE and the follow-up CT scan was 25.9 ± 10.1 (range, 14–42) days.
The mean technical success rate of the PVE procedures was 99.3 % (range, 86.6–100 %). Reasons for failure were the impossibility of cannulating the portal system [17, 34, 42, 46] because of altered portal anatomy caused by the tumor mass or unexpected thrombosis of the portal system due to tumor progression/invasion [17, 25, 46, 47]. The clinical success rate (successful PVE procedure, inducing enough hypertrophy of the FRL to allow resection) however was 96.1 %.
In 70 patients (3.9 %), surgery was not performed. In 51 patients (2.8 %), the hypertrophy response was insufficient to perform the resection, although the embolization procedure was successful. In the other 19 cases, 12 did not technically succeed (0.7 %) and 7 caused a complication leading to nonresectability (0.4 %). These complications consisted of severe cholangitis, large abscesses and sepsis, and portal venous or mesentericoportal venous thrombosis.
Hypertrophy Response
The growth of the FRL as a result of PVE can be calculated or expressed in two ways:
The difference in FRL volume before and after embolization in relation to the FRL volume before embolization (percentage volume increase):
The difference between the percentage FRL before and after embolization (in literature referred to degree of hypertrophy [DH]):
when available, the percentage FRL volume increase was extracted from the article; otherwise, it was calculated from the available data. The mean increase of the FRL volume was 37.9 ± 0.1 % (20.5–69.4 %).
Atrophy of the Embolized Lobe
Embolization of the liver not only causes hypertrophy of the nonembolized lobe but also atrophy of the embolized lobe. Only 10 studies, including 593 patients, contained all data on total liver volumes, FRL volumes, and the volumes of the embolized lobe before and after PVE [16, 24, 25, 34, 36, 45–49]. From these studies, we could calculate the percentage of atrophy of the embolized liver (EL), using the following equation:
In these studies, the influence of the tumor volume was not taken into account. The mean percentage of atrophy of the embolized liver in these studies was −12.3 % (range, −24.5 to 0.0 %), measured 29 days after PVE (range, 14–42).
Influence of Different Variables on the Hypertrophy Response
A meta-analysis on the variables influencing the hypertrophy response was not possible because of inhomogeneity of the studies and a limited number of articles within the subgroups. Insufficient data were available to make a strong statistical comparison between the effect of right PVE and additional embolization of segment 4 branches on the hypertrophy response. The same applies to the effect of cholestasis. For evaluation of the effect of chemotherapy and cirrhosis/fibrosis on the hypertrophy response, enough studies are available for a head-to-head comparison (Tables 4, 5). Comparing the data, chemotherapy seems to have no influence on the hypertrophy response; however, patients with preexisting chronic liver disease (cirrhosis or fibrosis) show less hypertrophy response than patients with a normal liver. Statistical significance is not given in these studies.
Table 4.
Influence of chemotherapy on the hypertrophy response
| Article | No. of patients | Chemo/non-chemo | %FRL chemo | %FRL non-chemo | Significance |
|---|---|---|---|---|---|
| Covey [14] | 100 | 43/57 | 22 | 26 | Not known |
| Nafidi [42] | 20 | 13/7 | 45.8 | 41.2 | NS |
| Ribero [51] | 112 | 28/80 | 9.0 (DH) | 8.5 (DH) | NS |
| De Baere [16] | 107 | 97/10 | 56.6–71.2 | 83.6 | NSa |
NS not significant in the studies, DH degree of hypertrophy
aSignificant difference in hypertrophy response was seen in patients who received chemotherapy with platin agents
Table 5.
Influence of cirrhosis/fibrosis on the hypertrophy response
Table 6 shows only the studies that used a single embolization material. There seems to be a trend that the use of the permanent occluding n-butyl cyanoacrylate results in a greater % FRL volume increase compared with gelatin sponge, fibrin glue, and PVA.
Table 6.
Influence of embolization material on the hypertrophy response
| Embolization material | Article | No. of patients | % Increase FRL |
|---|---|---|---|
| PVA + coils/vascular plug | Esschert [30] | 10 | 26.1 |
| Libicher [40] | 10 | 26.4 | |
| Covey [14] | 100 | 24.3 | |
| Gelatin sponge | Fujii [11] | 30 | 17.8 |
| Imamura [33] | 84 | 30.7 | |
| Kakizawa [35] | 14 | 23.8 | |
| Kim [37] | 17 | 27.0 | |
| Kusaka [12] | 18 | 21.2 | |
| Makuuchi [20] | 54 | 37.9 | |
| Nanashima [49] | 30 | 29.4 | |
| Sugawara [22] | 66 | 35.8 | |
| N-butyl cyanoacrylate | Baere [16] | 107 | 57.8 |
| Barbaro [24] | 26 | 53 | |
| Capussotti [9] | 31 | 48.5 | |
| Elias [29] | 68 | 59.1 | |
| Giraudo [17] | 146 | 41.7 | |
| Sirichindakul [53] | 29 | 27.5 | |
| Broering [57] | 17 | 69.4 | |
| Fibrin glue | Liem [54] | 15 | 31.4 |
| Nagino [15] | 105 | 27.4 |
Complications After PVE
Fifteen articles lacked a detailed description of complications encountered after embolization [9, 11, 12, 16, 24, 31, 40–43, 49–53]. From the other 29 studies (1,179/1,248 patients), the complication rates are summarized in Table 7.
Table 7.
Complications after PVE
| % of total patients | |
|---|---|
| Minor complications | |
| Fever | 36.9 |
| Elevation of transaminase | 34.8 |
| Abdominal discomfort/pain | 22.9 |
| Nausea and vomiting | 2.0 |
| Ileus | 1.2 |
| Major complications | |
| Portal thrombosis | 0.8 |
| Embolization of nontarget vessels | 0.6 |
| Liver hematoma | 0.4 |
| Infection/abscess | 0.4 |
| Intra-abdominal bile leakage | 0.3 |
In 0.4 %, major complications after PVE led to nonresectability of the patient. These complications consisted of severe cholangitis, large abscesses and sepsis, and portal venous or mesentericoportal venous thrombosis.
The only study to describe PVE-related mortality was published by Giraudo et al. [17]. In a group of 146 patients, 1 patient died 20 days after PVE due to lethal pulmonary embolism. No embolization material was detected in the lung. A second patient developed cholangitis and died of septic shock 39 days after PVE. All other studies reported no PVE-related mortality, resulting in an overall mortality rate of 0.1 %.
Liver Resection
In total, 20 % (358/1,791) of the originally planned liver resections after PVE were cancelled. Seven studies (327 patients) lacked a detailed description of the cause of cancellation. In the other 37 studies (1,464 patients), 18.7 % of the planned resections were cancelled: in 6.1 % because of local intrahepatic tumor progression or newly developed metastases in the FRL, making resection impossible; in 8.1 % because of extrahepatic tumor spread (peritoneal metastases, mesenteric lymph node metastases, or lung metastases); and in 4.5 % by other causes (insufficient hypertrophy of FRL despite PVE, complications of PVE leading to nonresectability, patients refusing further treatment, preoperative mortality).
The mean period between PVE and liver surgery was 36.9 (range, 21–84) days. The types of operative procedures are summarized in Table 8. In more than 70 %, a right hemihepatectomy or extended hemihepatectomy was performed.
Table 8.
Surgical procedures
| No. of patients | % | |
|---|---|---|
| Right hemihepatectomy | 774 | 43.2 |
| Extended right hemihepatectomy | 516 | 28.8 |
| Left hemihepatectomy | 21 | 1.2 |
| Extended left hemihepatectomy | 45 | 2.5 |
| Trisegmentectomy right | 36 | 2.0 |
| Other (central resection, segmentectomy) | 41 | 2.3 |
| No resection | 358 | 20.0 |
Complications after surgery can be divided into major and minor complications. Major complications are defined as complications that required surgical treatment and/or lead to prolonged hospital stay. Minor complications are defined as complications that could be treated conservatively, not leading to prolonged hospital stay.
In 11 publications, a detailed description of the postoperative complications after resection was lacking [9, 12, 14, 15, 25, 38–41, 46, 54]. In the other 33 articles (1,210 patients), the overall morbidity was 21.7 %. Major and minor complications are given in Table 9. The overall mortality after liver resection was 3.3 %. Primary liver failure (0.4 %) or liver failure in combination with multiple organ failure (1.2 %) caused death in 50 % of the cases. Other causes were myocardial infarction (0.1 %), sepsis (0.2 %), abdominal/liver bleeding (0.2 %), multiple organ failure (0.4 %), cholangitis (0.1 %), or unknown cause (0.4 %).
Table 9.
Complications after surgery
| Major complications | 10.4 % |
| Liver failure | 5.5 % |
| Portal thrombosis | 0.1 % |
| Bile leakage | 3.1 % |
| Abdominal/liver bleeding | 1.0 % |
| Cholangitis | 0.2 % |
| Myocardial infarction | 0.1 % |
| Multiple organ failure | 0.4 % |
| Minor complications | 11.3 % |
| Ascites | 2.6 % |
| Pleural effusion | 2.9 % |
| Abscesses | 1.8 % |
| Urine tract infection | 0.9 % |
| Wound infection | 2.0 % |
| Pneumonia | 1.1 % |
Discussion
Since the first publication on clinical PVE by Kinoshita in 1986 [4], many articles have been published on this subject. The exact mechanisms leading to atrophy of the embolized lobe and hypertrophy of the FRL are still unknown. Recent studies have shown that in addition to the redistribution of portal blood flow PVE induces an increase in hepatic growth factor (HGF) and transforming growth factor (TGF)-α and -β, which contribute to the hypertrophy of the non-embolized lobe [60, 61].
New techniques have been developed, and new embolization materials have been used and tested. The results of PVE and its role in the management of liver malignancies is mainly based on small or larger case series; No randomized, controlled trials on the efficacy of PVE have been conducted. Only one meta-analysis has been published on PVE [18]. This meta-analysis mainly focused on the differences between the surgical transileocolic (TIPE) and the percutaneous transhepatic (PTPE) technique, demonstrating a significantly higher increase in FRL in PTPE than in TIPE. There were no differences in major complications [18]. However, with the increasing availability of radiological intervention suites, the percutaneous transhepatic technique has become the standard technique for PVE. Percutaneous PVE can be performed by an ipsilateral or contralateral approach. Using the ipsilateral approach (53.8 % of the cases in this review) has the advantage of not puncturing the healthy FRL tissue, thereby reducing the risk of complications like portal vein thrombosis, dissection, or subcapsular hematoma of the FRL. However, reverse-curved catheters or multiple lumen balloon occlusion catheters are usually necessary depending on the embolization material used. Additional embolization of the segment 4 branches often is easier when using the ipsilateral approach. The contralateral approach (33.8 %) is easier in catheterization of the right portal branches and delivering the embolization material in the direction of the portal flow. This reduces the chance of migration of embolization material in the portal branches of the FRL. This review could not extract enough data to evaluate the differences in complications of the ipsilateral or contralateral approach. However, studies by Ribero et al. [51] and Di Stefano et al. [62], which evaluated complications of the ipsilateral and contralateral approach, respectively, showed almost the same types of complications and no significant difference in complication rates.
The selection of patients for PVE is based traditionally on CT volumetry. Most studies use a FRL volume of 25–30 % of the original liver volume as threshold to select patients for PVE when no compromised liver function is expected. In patients with a compromised liver function, such as in postchemotherapy liver damage, liver cirrhosis/fibrosis, and long-lasting cholestasis, a threshold of 35–40 % is preferred as minimum FRL volume. Worldwide there is consensus on these indications. Functional information can be obtained by the ICG plasma disappearance or retention rate test at 15 min. This technique, introduced in 1980, can accurately estimate postresection remnant liver function [56]. According to the literature, only few authors, mainly Japanese, have used this method to select patients for preoperative PVE. More recently developed quantitative liver function tests, such as 99Tc-labelled mebrofenin hepatobiliary scintigraphy HBS [63] and 99Tc-galactosyl-human serum albumin (GSA) scintigraphy, could play an important role in a more accurate selection of patients for PVE.
It is important to calculate the percentage of FRL volume following PVE to ensure that enough functional liver tissue is left after resection. The importance of the size of the FRL is stressed by Ribero et al. [51]. They showed that both a small FRL and limited degree of hypertrophy (DH) are strongly associated with postoperative hepatic dysfunction. The percentage of FRL volume can be calculated by using the absolute volumes by CT volumetry or by relating FRL volume (measured by CT volumetry) to a standardized liver volume based on BSA [58, 59]. Monitoring FRL function after PVE is difficult, because only a few liver function tests can measure the specific increase of the FRL. 99Tc-labelled mebrofenin HBS with single photon emission tomography (SPECT) [63] and 99Tc-GSA scintigraphy can be used for this purpose [50, 64, 65]. De Graaf et al. showed that the increase of FRL function exceeded the increase of FRL volume, suggesting that the necessary waiting time until resection could be shorter than indicated by volumetric parameters only.
There is no consensus regarding the optimal waiting time between PVE and liver resection. We found a wide range of time intervals between PVE and the follow-up CT scan: 14–42 days (mean, 25.9 ± 10.1 days). A longer time interval allows extra growth of the FRL. However, volumetric data presented by Ribero et al. [51] show that after the initial hypertrophy in the first 3 weeks, a plateau phase is reached. This is confirmed in the study by Nagino et al. [15].
Additionally, there is the issue of induction of tumor growth by PVE. Clinical studies demonstrate that tumor progression after PVE is possible in both the embolized and nonembolized liver segments. However, so far, accurate data regarding the risk of tumor progression after PVE are currently not available [66]. In this study 6.1 % of planned liver resections are cancelled because of local intrahepatic tumor progression after PVE. This can be regarded as complication of the treatment, causing irresectability. A direct causality seems obvious and is described in literature but is not yet proven. An additional 8.1 % of the resections are cancelled because of extrahepatic tumor spread (peritoneal metastases and distant metastases). To restrict tumor growth, the time between PVE and liver resection should be limited. Furthermore, sequential transarterial chemoembolization and PVE can be performed, particularly in patients with HCC [67] to limit tumor growth.
Post-PVE chemotherapy is another option in patients with CRM. Beal et al. reported a reduction in tumor size in six of the ten patients who had chemotherapy compared with tumor growth in four of the five patients without chemotherapy. However, they also observed less hypertrophy of the FRL in patients who received chemotherapy in the weeks between PVE and resection [25]. Other studies showed no significant difference in hypertrophy response or in postoperative complications when chemotherapy was continued [68]. A few large studies evaluated in this review show no significant difference in increase of the FRL volume after PVE in patients who previously did or did not receive chemotherapy [14, 16, 42, 51]. However, de Baere et al. described a significant lower hypertrophy response in patients who received chemotherapy with platin agents. Restricted by the limited number of articles and their inhomogeneity, evaluation was only possible by head-to-head comparison.
The same applies to the effect of preexisting liver damage (liver cirrhosis and fibrosis) on hypertrophy response after PVE. Comparison of relevant studies show an impaired hypertrophy response compared with normal livers; however, statistical significance has not been demonstrated. Farges et al. [31] stated that patients with cirrhotic livers and a normal hypertrophy response had less postoperative complications. On the other hand, failure of increase of the FRL could be considered an indicator of inability of regeneration of liver parenchyma and liver resection should be avoided.
Many different embolization materials have been applied for PVE. The combination of n-butyl cyanoacrylate and lipiodol and the combination of PVA particles with coils are mostly used. Both are nonabsorbable materials, which lead to persistent occlusion of the portal branches, preventing peripheral recanalization. Because gelatin sponge is absorbable, portal recanalization is frequently seen, sometimes 2 weeks after PVE [6, 69]. PVA particles are easy to use and provide permanent occlusion in the periphery of the portal venous system. Little inflammatory reaction of the liver tissue is seen when using PVA. The use of n-butyl cyanoacrylate requires more experience of the radiologist, because delivery must be very precise to prevent embolization of nontargeted branches. Using the appropriate delivering catheters, procedure time can be decreased. N-butyl cyanoacrylate induces a strong inflammatory reaction, rendering surgical resection sometimes technically more difficult [6]. Large clinical studies that compare the effect of different embolization materials on the hypertrophy response are lacking. The data in this review suggest that the use of n-butyl cyanoacrylate results in a higher % FRL volume increase.
Finally, both the overall technical success of PVE (99.3 %) and clinical success rate (96.1 %) of PVE are very high. Only 2.8 % of the patients could not undergo a liver resection because of insufficient hypertrophy. Suggested reasons for insufficient hypertrophy after successful PVE are recanalization of the embolized portal branches, activation of underlying liver disease, and the presence of major portal hypertension with portosystemic shunting [31]. Only 0.4 % of patients appear unresectable because of PVE-related complications, such as a large subcapsular hematoma, portal thrombosis, or biliary or infectious complications in the FRL after a contralateral procedure. Overall complication rates are higher, but these complications rarely need treatment and they rarely lead to unresectability.
Conclusions
Preoperative PVE is an effective method to increase FRL volume with a high technical and clinical success rate. The complication rate is low, but local tumor progression after PVE is an imminent cause of unresectablilty. Preexisting liver damage due to cirrhosis seems to have a negative effect on the hypertrophy response. Chemotherapy however does not seem to have any influence on the hypertrophy response, except for platin agents. The use of n-butyl cyanoacrylate may result in a greater hypertrophy response compared with the other embolization materials used.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Garcea G, Ong SL, Maddern GJ. Predicting liver failure following major hepatectomy. Dig Liver Dis. 2009;41:798–806. doi: 10.1016/j.dld.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Rous P, Larimore LD. Relation of the portal blood to liver maintenance: a demonstration of liver atrophy conditional on compensation. J Exp Med. 1920;31:609–632. doi: 10.1084/jem.31.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honjo I, Kozaka S. Extensive resection of the liver in two stages. Rev Int Hepatol. 1965;15:309–319. [PubMed] [Google Scholar]
- 4.Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–808. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Baere T, Roche A, Elias D, et al. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–1391. doi: 10.1002/hep.510240612. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki S, Makuuchi M, Kakazu T, et al. Resection for multiple metastatic liver tumors after portal embolization. Surgery. 1994;115:674–677. [PubMed] [Google Scholar]
- 8.Honjo I, Suzuki T, Ozawa K, et al. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg. 1975;130:296–302. doi: 10.1016/0002-9610(75)90389-X. [DOI] [PubMed] [Google Scholar]
- 9.Capussotti L, Muratore A, Baracchi F, et al. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg. 2008;143:978–982. doi: 10.1001/archsurg.143.10.978. [DOI] [PubMed] [Google Scholar]
- 10.Are C, Iacovitti S, Prete F, et al. Feasibility of laparoscopic portal vein ligation prior to major hepatectomy. HPB (Oxford) 2008;10:229–233. doi: 10.1080/13651820802175261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii Y, Shimada H, Endo I, et al. Changes in clinicopathological findings after portal vein embolization. Hepatogastroenterology. 2000;47:1560–1563. [PubMed] [Google Scholar]
- 12.Kusaka K, Imamura H, Tomiya T, et al. Factors affecting liver regeneration after right portal vein embolization. Hepatogastroenterology. 2004;51:532–535. [PubMed] [Google Scholar]
- 13.Shimura T, Suehiro T, Suzuki H, et al. Trans-ileocecal portal vein embolization as a preoperative treatment for right trisegmentectomy with caudate lobectomy. J Surg Oncol. 2007;96:438–441. doi: 10.1002/jso.20829. [DOI] [PubMed] [Google Scholar]
- 14.Covey AM, Brown KT, Jarnagin WR, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451–455. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 15.Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Baere T, Teriitehau C, Deschamps F, et al. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17:2081–2089. doi: 10.1245/s10434-010-0979-2. [DOI] [PubMed] [Google Scholar]
- 17.Giraudo G, Greget M, Oussoultzoglou E, et al. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 19.www.dcc.cochrane.org
- 20.Makuuchi M, Kosuge T, Lygidakis NJ. New possibilities for major liver surgery in patients with Klatskin tumors or primary hepatocellular carcinoma—an old problem revisited. Hepatogastroenterology. 1991;38:329–336. [PubMed] [Google Scholar]
- 21.Nanashima A, Yamaguchi H, Shibasaki S, et al. Relationship between CT volumetry and functional liver volume using technetium-99 m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization before major hepatectomy: a preliminary study. Dig Dis Sci. 2006;51:1190–1195. doi: 10.1007/s10620-006-8031-x. [DOI] [PubMed] [Google Scholar]
- 22.Sugawara Y, Yamamoto J, Higashi H, et al. Preoperative portal embolization in patients with hepatocellular carcinoma. World J Surg. 2002;26:105–110. doi: 10.1007/s00268-001-0189-y. [DOI] [PubMed] [Google Scholar]
- 23.Bae JH, Kim KC, Ryeom HK. Percutaneous preoperative portal vein embolization using a combination of gelatin sponge and Histoacryl glue. Acta Radiol. 2009;50:1119–1125. doi: 10.3109/02841850903258041. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro B, Caputo F, Tebala C, et al. Preoperative right portal vein embolisation: indications and results. Radiol Med. 2009;114:553–570. doi: 10.1007/s11547-009-0383-9. [DOI] [PubMed] [Google Scholar]
- 25.Beal IK, Anthony S, Papadopoulou A, et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol. 2006;79:473–478. doi: 10.1259/bjr/29855825. [DOI] [PubMed] [Google Scholar]
- 26.Bent CL, Low D, Matson MB, et al. Portal vein embolization using a nitinol plug (Amplatzer vascular plug) in combination with Histoacryl glue and iodinized oil: adequate hypertrophy with a reduced risk of nontarget embolization. Cardiovasc Intervent Radiol. 2009;32:471–477. doi: 10.1007/s00270-009-9515-9. [DOI] [PubMed] [Google Scholar]
- 27.Cotroneo AR, Innocenti P, Marano G, et al. Pre-hepatectomy portal vein embolization: single center experience. Eur J Surg Oncol. 2009;35:71–78. doi: 10.1016/j.ejso.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Chung SH, Lee MS, Kim KS, et al. Foam sclerotherapy using polidocanol (Aethoxysclerol) for preoperative portal vein embolization in 16 patients. Cardiovasc Intervent Radiol. 2011;34(6):1236–1243. doi: 10.1007/s00270-010-0079-5. [DOI] [PubMed] [Google Scholar]
- 29.Elias D, Ouellet JF, de Baere T, et al. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294–299. doi: 10.1067/msy.2002.120234. [DOI] [PubMed] [Google Scholar]
- 30.van den Esschert JW, de Graaf W, van Lienden KP, et al. Volumetric and functional recovery of the remnant liver after major liver resection with prior portal vein embolization: recovery after PVE and liver resection. J Gastrointest Surg. 2009;13:1464–1469. doi: 10.1007/s11605-009-0929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong YK, Choi SB, Lee KH, et al. The efficacy of portal vein embolization prior to right extended hemihepatectomy for hilar cholangiocellular carcinoma: a retrospective cohort study. Eur J Surg Oncol. 2011;37:237–244. doi: 10.1016/j.ejso.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1991;29:1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 34.Ji W, Li JS, Li LT, et al. Role of preoperative selective portal vein embolization in two-step curative hepatectomy for hepatocellular carcinoma. World J Gastroenterol. 2003;9:1702–1706. doi: 10.3748/wjg.v9.i8.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakizawa H, Toyota N, Arihiro K, et al. Preoperative portal vein embolization with a mixture of gelatin sponge and iodized oil: efficacy and safety. Acta Radiol. 2006;47:1022–1028. doi: 10.1080/02841850600979063. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko T, Nakao A, Takagi H. Clinical studies of new material for portal vein embolization: comparison of embolic effect with different agents. Hepatogastroenterology. 2002;49:472–477. [PubMed] [Google Scholar]
- 37.Kim MJ, Choo SW, Do YS, et al. Use of double-occlusion balloon catheter: preoperative portal vein embolization for induction of future remnant liver hypertrophy. Cardiovasc Intervent Radiol. 2004;27:16–20. doi: 10.1007/s00270-003-0505-z. [DOI] [PubMed] [Google Scholar]
- 38.Ko GY, Sung KB, Yoon HK, et al. Preoperative portal vein embolization with a new liquid embolic agent. Radiology. 2003;227:407–413. doi: 10.1148/radiol.2272011702. [DOI] [PubMed] [Google Scholar]
- 39.Lee KC, Kinoshita H, Hirohashi K, et al. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109–115. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- 40.Libicher M, Herbrik M, Stippel D, et al. Portal vein embolization using the Amplatzer vascular plug II: preliminary results. Rofo. 2010;182:501–506. doi: 10.1055/s-0028-1110019. [DOI] [PubMed] [Google Scholar]
- 41.Lindner P, Cahlin C, Friman S, et al. Extended right-sided liver resection for colorectal liver metastases–impact of percutaneous portal venous embolisation. Eur J Surg Oncol. 2006;32:292–296. doi: 10.1016/j.ejso.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Nafidi O, Desy D, Letourneau R, et al. Hypertrophy of the non-embolized liver after chemotherapy. HPB (Oxford) 2009;11:103–107. doi: 10.1111/j.1477-2574.2009.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarazov PG, Granov DA, Sergeev VI, et al. Preoperative portal vein embolization for liver malignancies. Hepatogastroenterology. 2006;53:566–570. [PubMed] [Google Scholar]
- 44.Tsuda M, Kurihara N, Saito H, et al. Ipsilateral percutaneous transhepatic portal vein embolization with gelatin sponge particles and coils in preparation for extended right hepatectomy for hilar cholangiocarcinoma. J Vasc Interv Radiol. 2006;17:989–994. doi: 10.1097/01.RVI.0000223716.61444.E4. [DOI] [PubMed] [Google Scholar]
- 45.Yoo H, Ko GY, Gwon DI, et al. Preoperative portal vein embolization using an Amplatzer vascular plug. Eur Radiol. 2009;19:1054–1061. doi: 10.1007/s00330-008-1240-2. [DOI] [PubMed] [Google Scholar]
- 46.Radeleff B, Schawo S, Hoffmann K, et al. Efficacy and safety of percutaneous transhepatic portal embolization before right liver resection using an Ethibloc/lipiodol mixture: a single-center experience. Dig Surg. 2008;25:52–59. doi: 10.1159/000118795. [DOI] [PubMed] [Google Scholar]
- 47.Pamecha V, Glantzounis G, Davies N, et al. Long-term survival and disease recurrence following portal vein embolization prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16:1202–1207. doi: 10.1245/s10434-008-0269-4. [DOI] [PubMed] [Google Scholar]
- 48.Ladurner R, Brandacher G, Riedl-Huter C, et al. Percutaneous portal vein embolization in preparation for extended hepatic resection of primary nonresectable liver tumours. Dig Liver Dis. 2003;35:716–721. doi: 10.1016/S1590-8658(03)00415-8. [DOI] [PubMed] [Google Scholar]
- 49.Nanashima A, Sumida Y, Abo T, et al. Clinical significance of portal vein embolization before right hepatectomy. Hepatogastroenterology. 2009;56:773–777. [PubMed] [Google Scholar]
- 50.Hirai I, Kimura W, Fuse A, et al. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99 mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495–506. doi: 10.1067/msy.2003.138. [DOI] [PubMed] [Google Scholar]
- 51.Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 52.Shin JH, Yoon HK, Kwon J, et al. Volumetric analysis of the liver after right portal vein embolization: mid-term follow-up based on embolization score. Clin Radiol. 2010;65:288–296. doi: 10.1016/j.crad.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Sirichindakul B, Nonthasoot B, Taesombat W, et al. Role of portal vein embolization in hepatobiliary malignancy. Hepatogastroenterology. 2007;54:2297–2300. [PubMed] [Google Scholar]
- 54.Liem MS, Liu CL, Tso WK, et al. Portal vein embolisation prior to extended right-sided hepatic resection. Hong Kong Med J. 2005;11:366–372. [PubMed] [Google Scholar]
- 55.Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 56.Takasaki T, Kobayashi S, Suzuki S, et al. Predetermining postoperative hepatic function for hepatectomies. Int Surg. 1980;65:309–313. [PubMed] [Google Scholar]
- 57.Broering DC, Hillert C, Krupski G, et al. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2002;6:905–913. doi: 10.1016/S1091-255X(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 58.Urata K, Hashikura Y, Ikegami T, et al. Standard liver volume in adults. Transplant Proc. 2000;32:2093–2094. doi: 10.1016/S0041-1345(00)01583-9. [DOI] [PubMed] [Google Scholar]
- 59.Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi H, Beppu T, Sugita H, et al. Serum HGF and TGF-beta1 levels after right portal vein embolization. Hepatol Res. 2010;40:311–317. doi: 10.1111/j.1872-034X.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- 61.Kusaka K, Imamura H, Tomiya T, et al. Expression of transforming growth factor-alpha and -beta in hepatic lobes after hemihepatic portal vein embolization. Dig Dis Sci. 2006;51:1404–1412. doi: 10.1007/s10620-006-9105-5. [DOI] [PubMed] [Google Scholar]
- 62.Di Stefano DR, de Baere T, Denys A, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–630. doi: 10.1148/radiol.2342031996. [DOI] [PubMed] [Google Scholar]
- 63.de Graaf W, van Lienden KP, van den Esschert JW, et al. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825–834. doi: 10.1002/bjs.7456. [DOI] [PubMed] [Google Scholar]
- 64.Nishiyama Y, Yamamoto Y, Hino I, et al. 99 mTc galactosyl human serum albumin liver dynamic SPET for pre-operative assessment of hepatectomy in relation to percutaneous transhepatic portal embolization. Nucl Med Commun. 2003;24:809–817. doi: 10.1097/00006231-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Yumoto Y, Yagi T, Sato S, et al. Preoperative estimation of remnant hepatic function using fusion images obtained by (99 m)Tc-labelled galactosyl-human serum albumin liver scintigraphy and computed tomography. Br J Surg. 2010;97:934–944. doi: 10.1002/bjs.7025. [DOI] [PubMed] [Google Scholar]
- 66.de Graaf W, van den Esschert JW, van Lienden KP, et al. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423–430. doi: 10.1245/s10434-008-0222-6. [DOI] [PubMed] [Google Scholar]
- 67.Aoki T, Imamura H, Hasegawa K, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 68.Goere D, Farges O, Leporrier J, et al. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10:365–370. doi: 10.1016/j.gassur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 70.van den Esschert JW, van Lienden KP, de Graaf W, et al. Portal vein embolization induces more liver regeneration than portal vein ligation in a standardized rabbit model. Surgery. 2011;149:378–385. doi: 10.1016/j.surg.2010.07.045. [DOI] [PubMed] [Google Scholar]


