Abstract
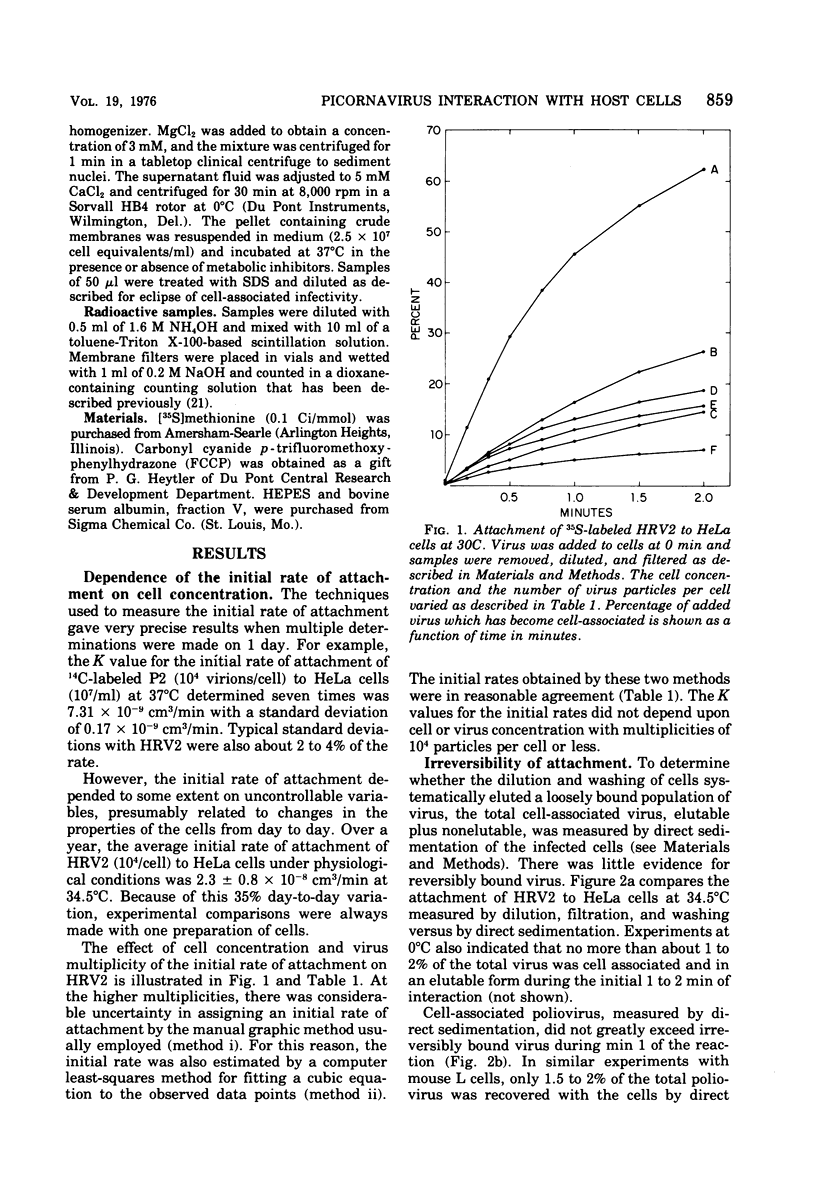
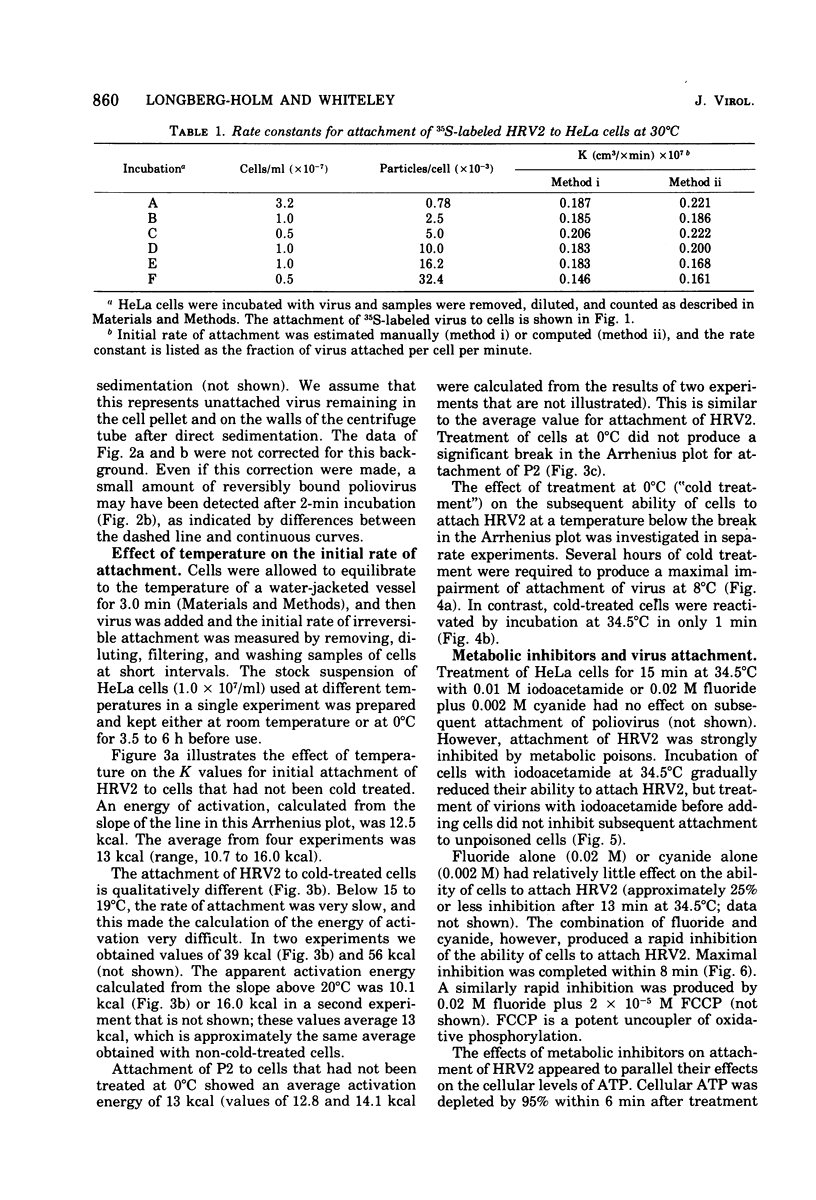
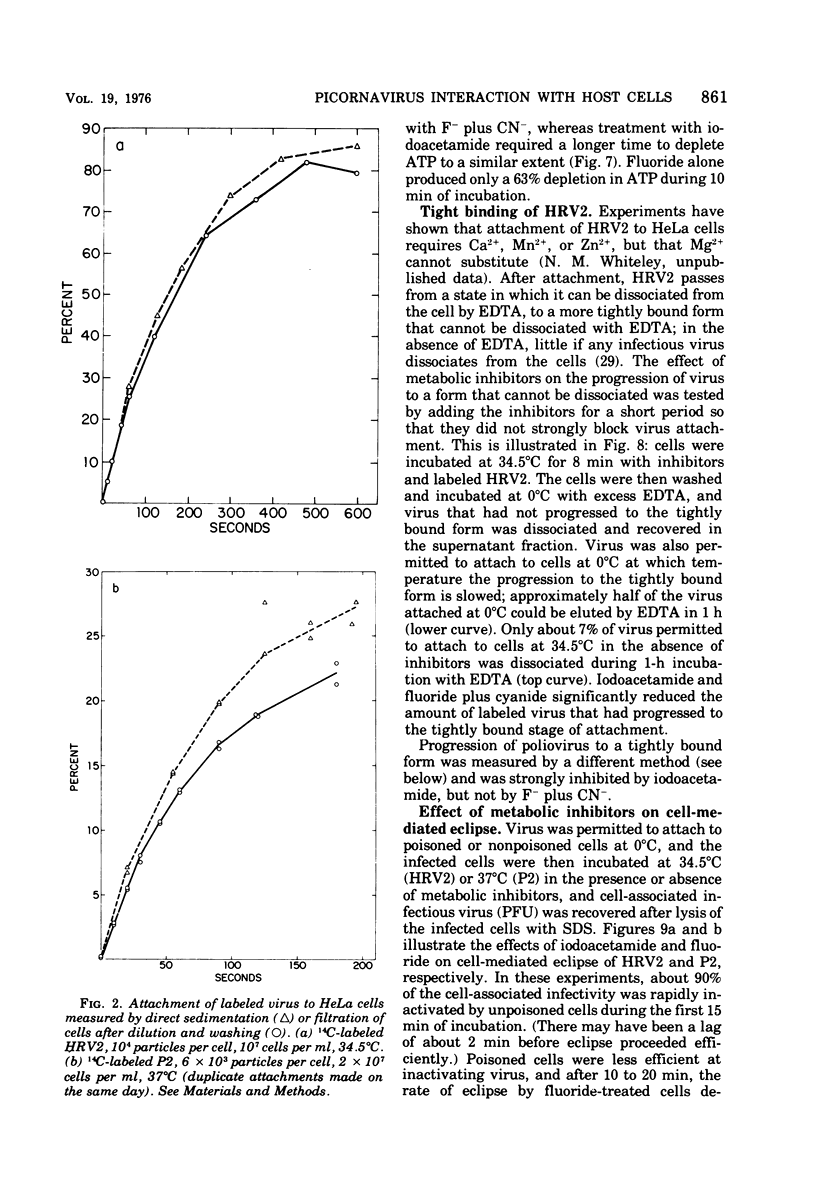
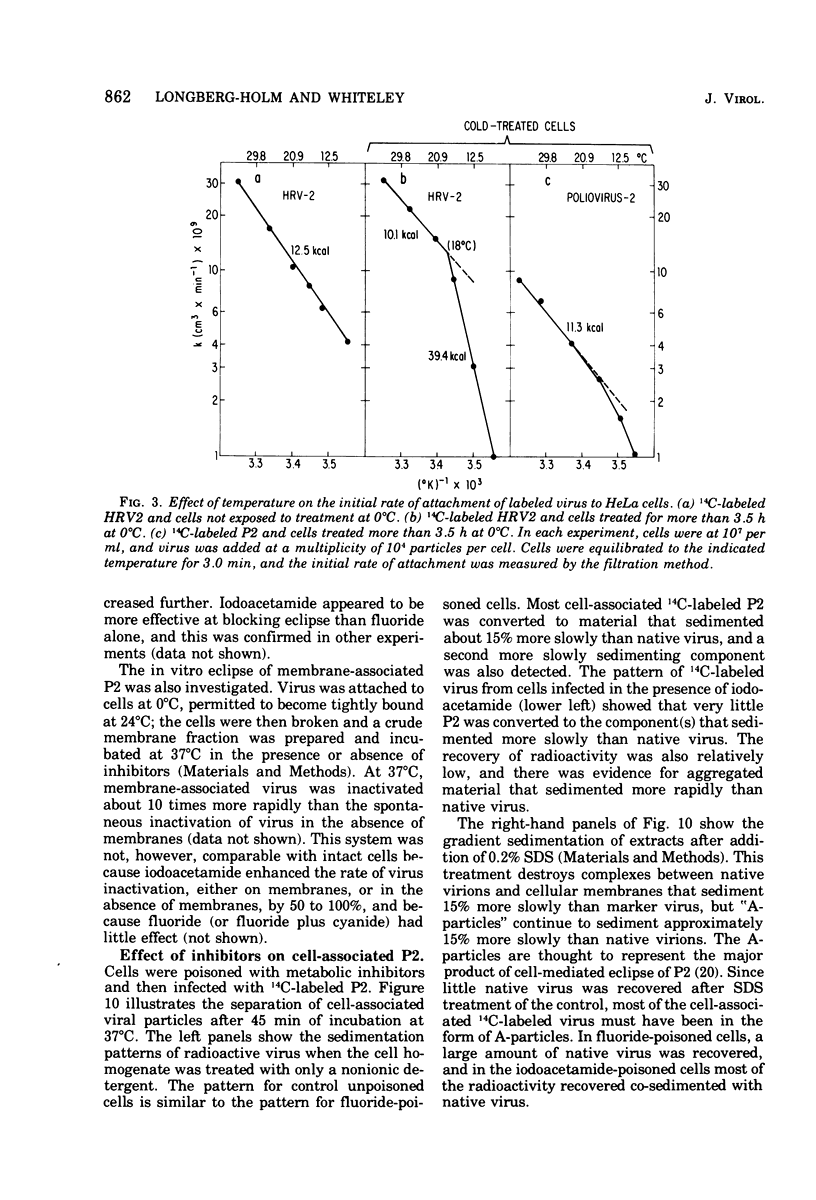
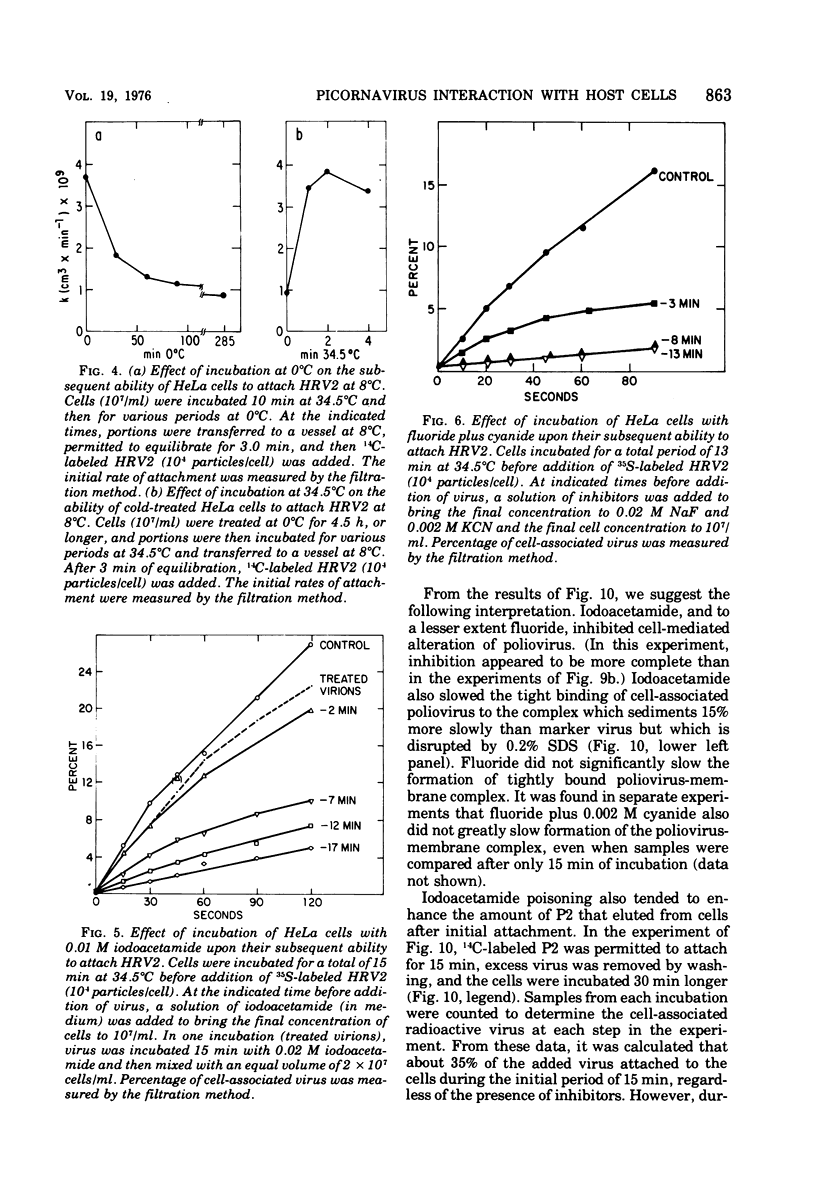
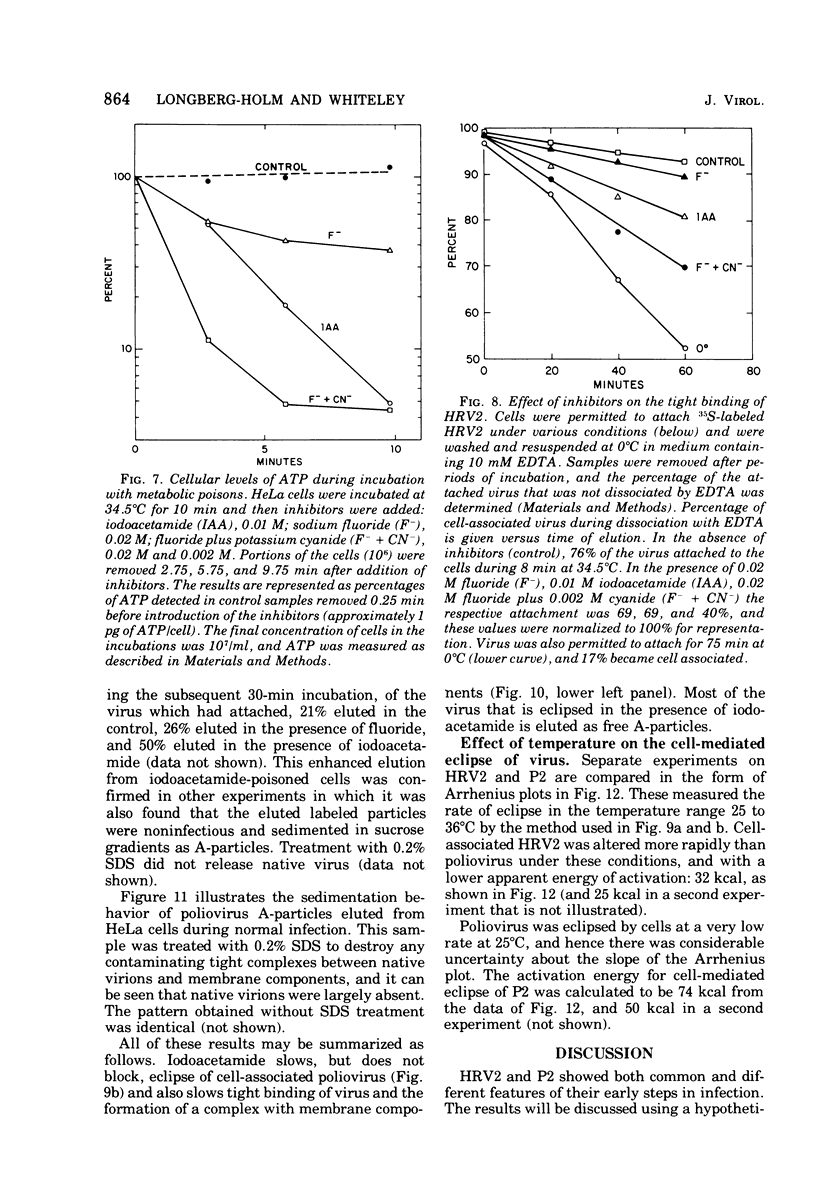
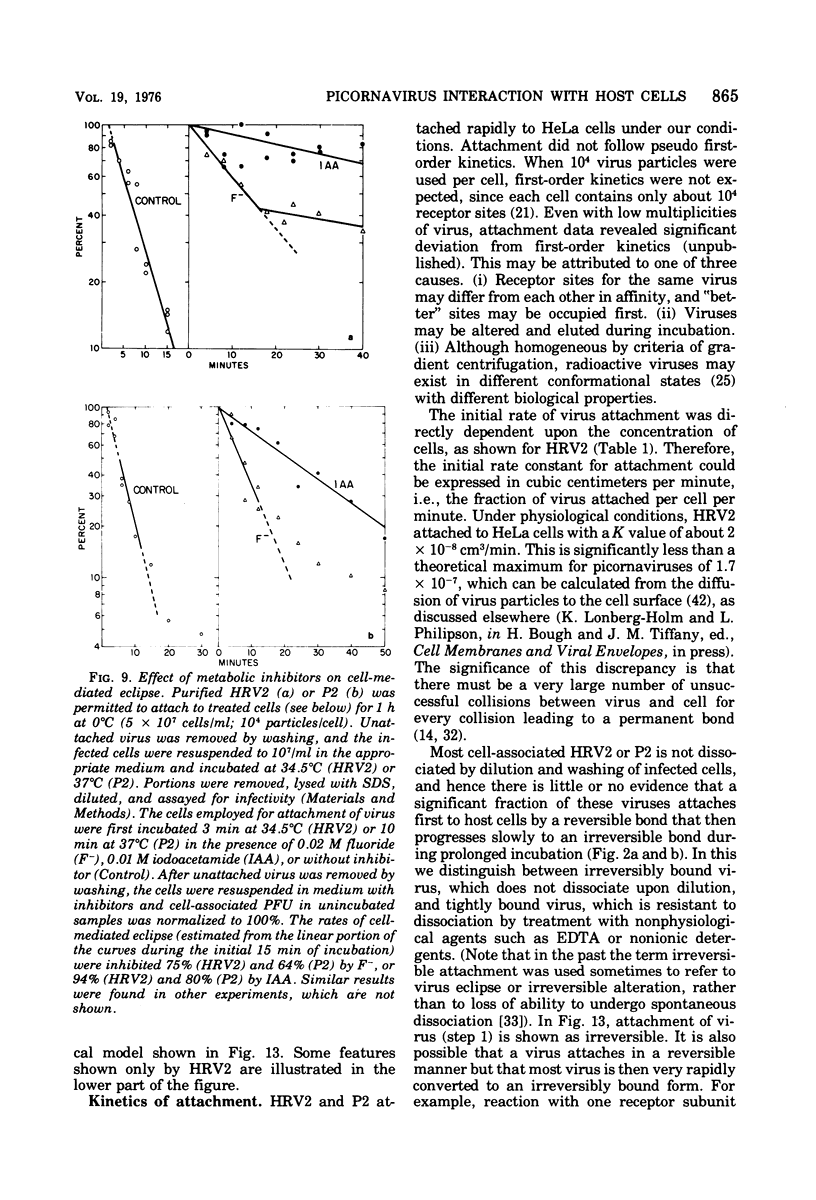
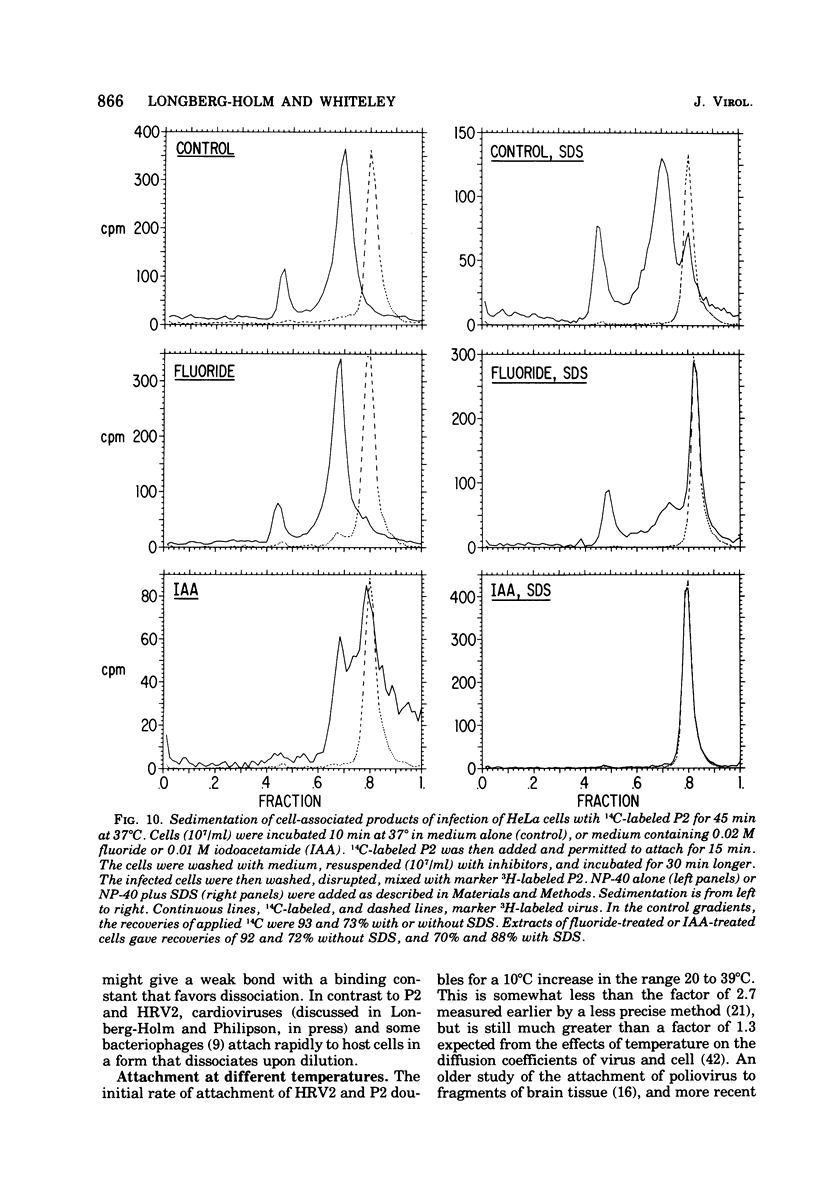
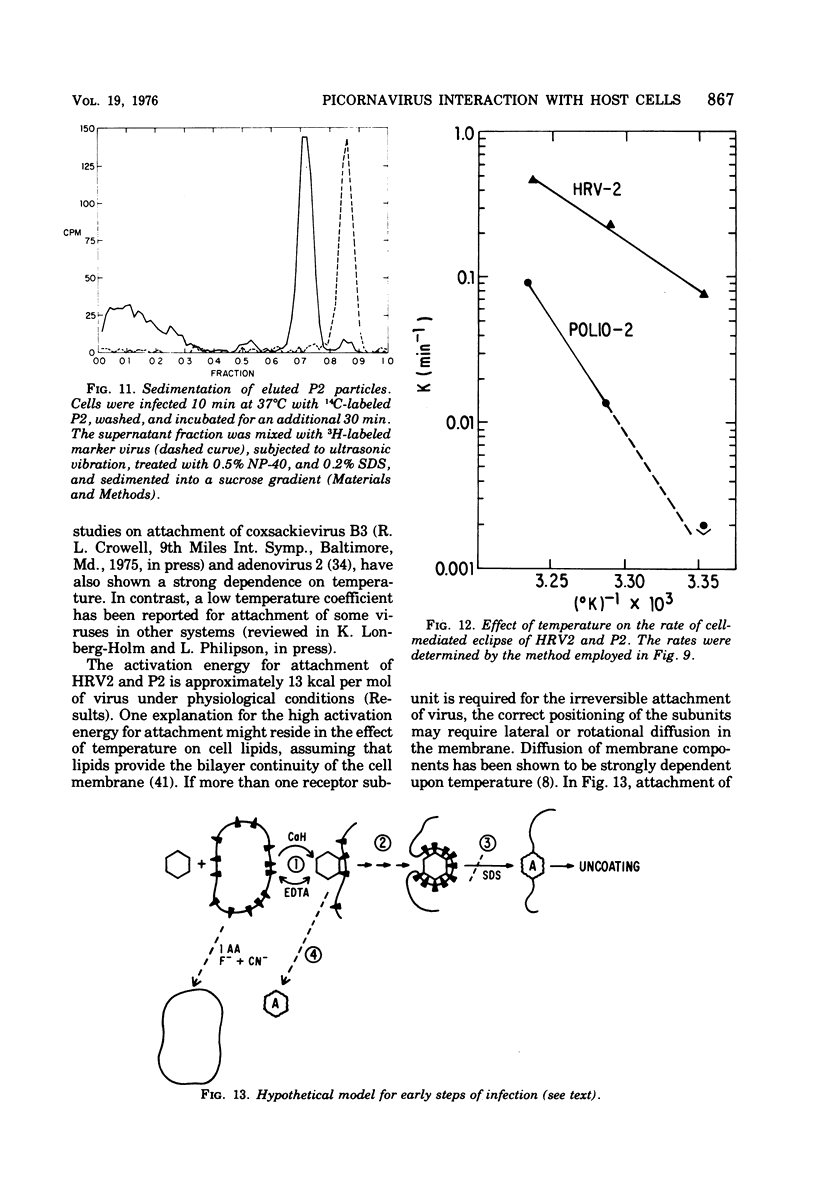
Attachment, ""tight binding'' and eclipse of radioactive poliovirus 2 (P2) and human rhinovirus 2 (HRV 2) were investigated. The activation energy for attachment of both HRV2 and P2 was about 13 kcal/mol. HRV2 differed from P2 in two respects: the Arrhenius plot for attachment of HRV2 showed a break at 15 to 19 degrees C when the cells were first treated several hours at 0 degrees C, and attachment of HRV2 was inhibited by treatment of cells with metabolic poisons able to reduce cellular ATP by more than 90%. Tight binding was determined by isolation of a specific P2-membrane complex or by loss of EDTA dissociability of HRV2. Tight binding of both viruses was slowed by 0.01 M iodoacetamide but not by 0.02 M F-; F- plus 0.002 M CN- slowed tight binding of HRV2 but not of P2. Eclipse, the irreversible alteration of parental virions, was detected by isolation of cell-associated subviral particles or by loss of cell-associated infectious virus. Eclipse of both viruses is slowed by iodoacetamide or F-. It seems likely that the early steps of infection with picornaviruses may be sensitive to alterations in the cell membrane produced by metabolic inhibitors or by treatment at low temperature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN F., CARTWRIGHT B., STEWART D. L. Further studies on the infection of pig-kidney cells by foot-and-mouth disease virus. Biochim Biophys Acta. 1962 May 14;55:768–774. doi: 10.1016/0006-3002(62)90855-7. [DOI] [PubMed] [Google Scholar]
- Chan V. F., Black F. L. Uncoating of poliovirus by isolated plasma membranes. J Virol. 1970 Mar;5(3):309–312. doi: 10.1128/jvi.5.3.309-312.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Differences between the thermal inactivation of picornaviruses at "high" and "low" temperatures. Virology. 1967 Feb;31(2):338–353. doi: 10.1016/0042-6822(67)90179-1. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- GAREN A., PUCK T. T. The first two steps of the invasion of host cells by bacterial viruses. II. J Exp Med. 1951 Sep;94(3):177–189. doi: 10.1084/jem.94.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- KUNIN C. M. Virus-tissue union and the pathogenesis of enterovirus infections. J Immunol. 1962 May;88:556–569. [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Li J. K., Williams R. E., Fox C. F. Effects of temperature and host lipid composition on the infection of cells by Newcastle disease virus. Biochem Biophys Res Commun. 1975 Jan 20;62(2):470–477. doi: 10.1016/s0006-291x(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Lin P. S., Wallach D. F., Tsai S. Temperature-induced variations in the surface topology of cultured lymphocytes are revealed by scanning electron microscopy. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2492–2496. doi: 10.1073/pnas.70.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Kauer J. C. Early alteration of poliovirus in infected cells and its specific inhibition. J Gen Virol. 1975 Jun;27(3):329–342. doi: 10.1099/0022-1317-27-3-329. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Shimshick E. J. Interaction of liposomes with subviral particles of poliovirus type 2 and rhinovirus type 2. J Virol. 1976 Aug;19(2):746–749. doi: 10.1128/jvi.19.2.746-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Noble-Harvey J. Comparison of in vitro and cell-mediated alteration of a human Rhinovirus and its inhibition by sodium dodecyl sulfate. J Virol. 1973 Oct;12(4):819–826. doi: 10.1128/jvi.12.4.819-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K. The effects of concanavalin A on the early events of infection by rhinovirus type 2 and poliovirus type 2. J Gen Virol. 1975 Sep;28(3):313–327. doi: 10.1099/0022-1317-28-3-313. [DOI] [PubMed] [Google Scholar]
- MANDEL B. The use of sodium dodecyl sulfate in studies on the interaction of poliovirus and HeLa cells. Virology. 1962 Jun;17:288–294. doi: 10.1016/0042-6822(62)90119-8. [DOI] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- Marco R., Jazwinski S. M., Kornberg A. Binding, eclipse, and penetration of the filamentous bacteriophage M13 in intact and disrupted cells. Virology. 1974 Nov;62(1):209–223. doi: 10.1016/0042-6822(74)90316-x. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren L. C., Scaletti J. V., James C. G. Isolation and properties of enterovirus receptors. Wistar Inst Symp Monogr. 1968;8:123–135. [PubMed] [Google Scholar]
- Noble-Harvey J., Lonberg-Holm K. Sequential steps in attachment of human rhinovirus type 2 to HeLa cells. J Gen Virol. 1974 Oct;25(1):83–91. doi: 10.1099/0022-1317-25-1-83. [DOI] [PubMed] [Google Scholar]
- Novotny C. P., Fives-Taylor P. Retraction of F pili. J Bacteriol. 1974 Mar;117(3):1306–1311. doi: 10.1128/jb.117.3.1306-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan R. J., Bundy L., Bradley R., Paranchych W. Unusual arsenate poisoning of the F pili of Escherichia coli. J Bacteriol. 1973 Jul;115(1):76–81. doi: 10.1128/jb.115.1.76-81.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G. On uncertainties inherent in the determination of the efficiency of collision between virus particles and cells. Biochim Biophys Acta. 1963 Mar 19;66:279–281. doi: 10.1016/0006-3002(63)91196-x. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L., LIND M. ENTEROVIRUS ECLIPSE IN A CELL-FREE SYSTEM. Virology. 1964 Jul;23:322–332. doi: 10.1016/0042-6822(64)90254-5. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- Philipson L. Attachment and eclipse of adenovirus. J Virol. 1967 Oct;1(5):868–875. doi: 10.1128/jvi.1.5.868-875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse H. G., Fox C. F. Concanavalin A mediated hemagglutination and binding properties of LM cells. Biochem Biophys Res Commun. 1974 Mar 15;57(1):323–331. doi: 10.1016/s0006-291x(74)80393-1. [DOI] [PubMed] [Google Scholar]
- Rittenhouse H. G., Williams R. E., Wisnieski B., Fox C. F. Alterations of characteristic temperatures for lectin interactions in LM cells with altered lipid composition. Biochem Biophys Res Commun. 1974 May 7;58(1):222–228. doi: 10.1016/0006-291x(74)90915-2. [DOI] [PubMed] [Google Scholar]
- Roesing T. G., Toselli P. A., Crowell R. L. Elution and uncoating of Coxsackievirus B3 by isolated HeLa cell plasma membranes. J Virol. 1975 Mar;15(3):654–657. doi: 10.1128/jvi.15.3.654-657.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., ALLISON A. C. Virus particle adsorption. I. Theory of adsorption and experiments on the attachment of particles to non-biological surfaces. Biochim Biophys Acta. 1959 Jul;34:10–23. doi: 10.1016/0006-3002(59)90228-8. [DOI] [PubMed] [Google Scholar]



