Abstract
The mucosal immune system of the female reproductive tract is of central importance for protection against sexually transmitted diseases, including HIV; however, this arm of the immune system remains poorly understood. Antiviral CTL responses never have been documented in the genital tract and the role of CTL in this anatomic site is unknown. In this study, CD8+ intraepithelial lymphocytes (IEL) in the vaginas of six simian immunodeficiency virus (SIV)-infected female rhesus macaques were identified by immunohistochemistry to be CD2+ and TCR β-chain+. In addition, the majority of CD8+ IEL contained TIA-1+ cytoplasmic granules that are associated with CTL activity. CD8+ T cells were isolated from the vaginal epithelium and submucosa and amplified by limiting dilution in the presence of feeder cells. SIV p55gag and/or gp16Oenv-specific lysis was detected in cultures of vaginal epithelial but not submucosal CD8+ T lymphocytes. Estimated SIV-specific precursor CTL frequencies were higher in the vaginal CD8+ IEL population of chronically infected monkeys than in the same cells from acutely infected monkeys or a naive control monkey. These results provide the first demonstration that antiviral CTL are present in the vaginal epithelium, and suggest that a vaccine may be able to generate anti-HIV CTL in the genital mucosa.
In female rhesus macaques and women, the tissues of vagina and cervix contain a full complement of immune cells including APCs and effector lymphocytes (1–3). The female reproductive tract has the capacity to mount an Ab response to a variety of pathogens and Ags, including HIV and simian immunodeficiency virus (SIV)4 (4–8). But the role of local antiviral CTL in this anatomic site is unknown. CD8+ MHC class I-restricted CTL have been identified in the blood and organs of HIV-infected people and SIV-infected monkeys (9–11), but there have been no reports of HIV- or SIV-specific CTL in the genital tract. In fact, antiviral CTL responses have never been documented in the genital mucosa of any species. Because HIV and SIV can be transmitted sexually (12–14), any vaccine should attempt to limit the spread of HIV from the reproductive tract to the systemic lymphoid tissues. Thus, it is essential to characterize the local immune response to vaginal pathogens.
There are numerous CD8+ T cells in the epithelium and superficial submucosa of the vagina and cervix in both rhesus macaques and humans (1–3). At other mucosal surfaces that have been examined, namely gut and respiratory mucosa, the CD8+ intraepithelial lymphocyte (IEL) population, at least in mice, contains about 50% TCR αβ+ T cells and 50% γδ T cells (15). In this study, we show that the CD8+ vaginal IEL in the rhesus monkey are CD2+ and TCR β-chain+, consistent with a primary population of αβ+ T lymphocytes in this compartment. Furthermore, most of these cells contain TIA-1+ cytoplasmic granules that have been associated with classical CD8+ CTL activity (16, 17). We therefore sought to determine whether SIV-specific CTLs are present in the vaginal mucosa of SIV-infected rhesus macaques.
Materials and Methods
Animals and SIV infection
Colony-bred adult female rhesus macaques (Macaca mulatta), seronegative for simian type D retroviruses, simian T cell leukemia virus, and SIV, were housed in accordance with the American Association for Accreditation of Laboratory Animal Care. The investigators adhered to the guidelines of the Committee on Care and Use of Laboratory Animals, National Resources Council. Two acutely infected monkeys (2 wk) and four chronically infected monkeys (6 to 10 mo) had been inoculated intravaginally with SIVmac251, an uncloned biologic isolate propagated in PBMC. Two virus stocks were used that had been titrated by intravaginal inoculation of female rhesus macaques to establish a persistent infection (13). The virus used to prepare these stocks was provided by R. C. Desrosiers, New England Regional Primate Research Center (Southborough, MA). The presence of SIV in the PBMC of rhesus macaques was detected by coculture of 5 to 10 × 106 PBMC with 106 CEM × 174 cells, as previously described (18). Cultures were maintained for 8 wk before terminating as negative. Beginning at 2 wk postinfection, SIVp27 Ag was consistently detected in PBMC coculture supernatants from the four monkeys with chronic SIV infection. SIV was isolated from PBMC and peripheral lymph nodes of the monkeys killed at 2 wk postinfection. All the animals were clinically healthy at the time they were killed by i.v. injection of pentobarbital. One uninfected adult female macaque was killed as a mock control for the histologic and immunologic assays described below.
Histology and immunohistochemistry
At necropsy, inguinal, axillary, and genital (iliac or obturator) lymph nodes, spleen. and vagina were obtained and divided three ways. One portion of each tissue sample was collected in sterile medium for lymphocyte isolation, one portion was fixed in formalin for routine histologic examination, and the remaining portion was embedded in O.C.T. compound (Tissue-Tek, Wiles Inc., Elkhart, IN) snap-frozen in liquid nitrogen-cooled Freon, and stored at −70°C. Frozen sections of vagina were examined for specific cell populations using previously described immunohistochemical techniques (19). The following mouse anti-human mAbs were used: anti-CD2 mAb (T11; Coulter Immunology, Hialeah, FL), anti-CD8 mAb (T8; Dako Corp., Carpenteria CA), TIA-1 mAb (Coulter Immunology), and anti-TCR β-chain mAb (bF1; T Cell Diagnostics, Cambridge, MA). Briefly, cryostat sections of approximately 6 µm in thickness were mounted on silane-coated slides and fixed in methanol. Sections from all animals were examined with all mAbs using single-label indirect immuno-flourescence. In the single-labeled sections, the binding of the mAb was detected with a FITC-conjugated horse anti-mouse IgG (Vector Labs., San Francisco, CA) plyclonal sera. Immunofluorescence triple staining of CD2+/CD8+/TIA-1+ T cells in the vaginal epithelium (Fig. 2) utilized a TIA-1/Aminomethylcoumarin(AMCA)-conjugated horse anti-mouse (Vector Labs.) combination followed by a biotinylated-T8/streptavidin-Texas Red (Vector Labs.) combination and a FITC-conjugated anti-CD2 mAb. Immunofluorescence double staining of TCR β-chain+/CD8+ cells in the vaginal epithelium (Fig. 3) utilized the anti-β-chain Ab/FITC-conjugated horse anti-mouse IgG combination followed by the biotinylated-T8/streptavidin-Texas Red combination. Between each staining step, the slides were extensively washed in PBS. As a negative control, an irrelevant mouse mAb was substituted for the primary Ab. The stained slides were examined by epifluorescence microscopy using a Zeiss Axiophot microscope with appropriate filters (Carl Zeiss, Inc. Thornwood, NY). To view double-labeled slides, a combination FITC/Texas Red filter was used (Zeiss) and to photograph the triple-labeled slides, a double exposure of the slide using the FITC/Texas Red filter and the AMCA filter was made.
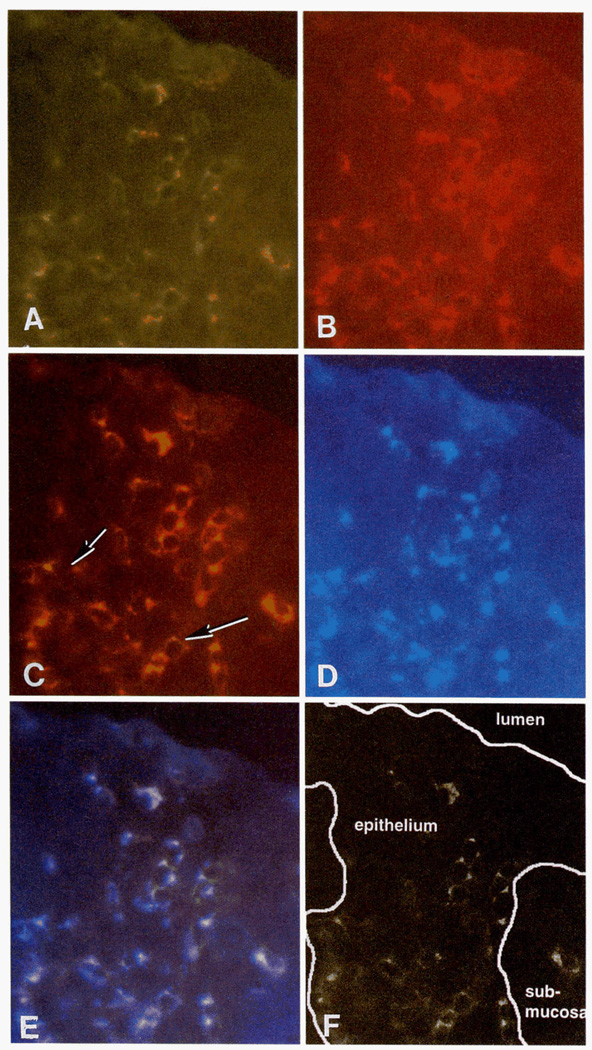
FIGURE 2.
A single, representative frozen section of vaginal mucosa from a chronically SIV-infected monkey was stained with three mAbs. A, CD2+ T cells (green) in vaginal epithelium. B, CD8+ cells (red) in same tissue section. C, Immunofluorescence double staining of CD2+/CD8+ cells in same section of vaginal epithelium. Note that all CD2+ T cells in vaginal epithelium are CD8+. Double-labeled cells are yellow, some cells (arrows) have variably distinct areas of red (CD8 expression) and yellow (colocalization of CD2 and CD8) staining or green (CD2 expression) and yellow (colocalization of CD2 and CD8) staining. D, TIA-1+ cytoplasmic granules (blue) in CD8+ T cells in same section of vaginal epithelium. E, Immunofluorescence triple staining of CD2+/CD8+/TIA-1+ T cells in vaginal epithelium. This figure demonstrates that all CD2+, CD8+ T cells in vaginal epithelium are TIA-1+. F, A computer-labeled image to clarify anatomic components in A-E. Original magnification: ×630.
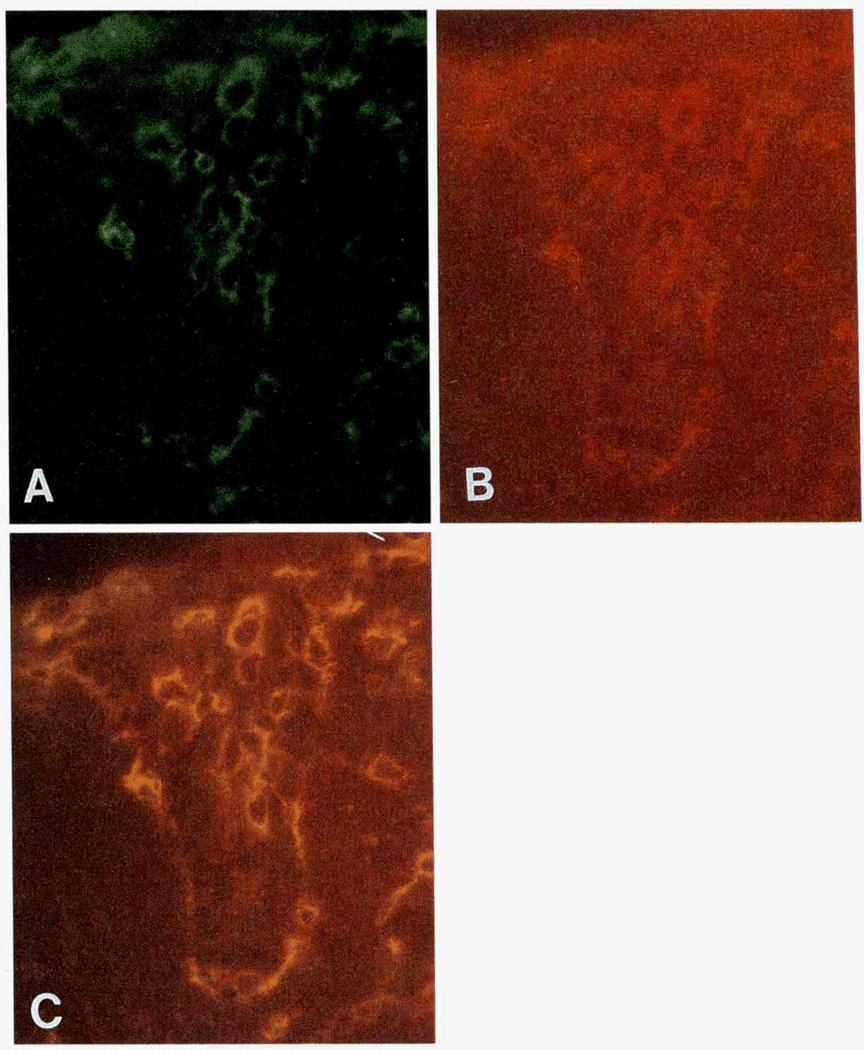
FIGURE 3.
A frozen section of vagina (serial section of slide in Fig. 2). A-C, Photographs of a single representative cryostat section stained with two mAbs. A, Cells expressing TCR β-chain (green) in vaginal mucosa. B, CD8+ cells (red) in same section of vaginal mucosa. C, Coexpression of CD8 and TCR β-chain in vaginal epithelial T cells. Double-labeled cells are yellow. This figure demonstrates expression of TCR β-chain by all CD8+ T cells in vaginal epithelium. Original magnification: ×630.
Isolation of lymphocytes from vaginal mucosa
The vaginal epithelium was manually separated from the submucosa after incubation for 1 h in Dispase II, 1.2 U/ml (Boehringer Mannheim, Indianapolis, IN) at 37°C. The epithelium was disassociated into a cell suspension by incubation in 0.25% trypsin for 1 h followed by repeated pipetting. CD8+ cells were positively selected from the cell suspension using two rounds of immunomagnetic bead isolation (M450 anti-CD8 Dynabeads; Dynal, Great Neck, NY). The anti-CD8+ beads were removed from the cells using Detach-a-Bead (Dynal) according to the manufacturer’s directions. The CD8+ vaginal epithelial cells were washed once in PBS and resuspended in RPMI 1640 medium.
Lymphocytes were isolated from the vaginal submucosa following enzymatic digestion (20). The vaginal submucosa was diced into 1 cm3 pieces and incubated overnight on an orbital shaker at 37°C in RPMI 1640 containing 10% FCS, 25 mM HEPES buffer (Life Technologies, Gaithersburg, MD), 100 U/ml penicillin, 100 µg/ml streptomycin (Life Technologies, Inc.), 2.5 µg/ml amphotericin B (Life Technologies, Inc.), 10−5 M β-mercaptoethanol (Kodak, Rochester, NY), 0.01% collagenase (Boehringer Mannheim), 0.01% deoxyribonuclease (Sigma Chemical Co., St. Louis, MO), and 0.01% soybean trypsin inhibitor (Sigma Chemical Co.). After digestion, the cells were isolated by low speed centrifugation and the cell pellet resuspended in 20 ml complete medium. The cell suspension was overlayed on a 25% isotonic Percol (Pharmacia Biotech, Inc. Pisataway, NJ) gradient and centrifuged at 2000 rpm for 20 min. The cell pellet was recovered and CD8+ lymphocytes were positively selected as described above.
Detection of SIV-specific CTL
The details of the bulk CTL assay have been previously reported (21). Briefly, lymphocytes were stimulated with Con A, 10 µg/ml, (Sigma Chemical Co.) and cultured for 14 days in RPMI 1640 medium supplemented with 10% FCS, antibiotics, and 5% human lymphocyte-conditioned medium (human IL-2; Schiaparelli Biosystems, Columbia, MD) and 20 U/ml recombinant human IL-2 (donated by Cetus Cop, Emeryville, CA). Autologous B lymphocytes were transformed by Herpes pupio (594S × 1055 producer cell line, provided by M. Sharp, Southwest Foundation for Biomedical Research, San Antonio, TX) and infected overnight with wild-type vaccinia virus (vvWR) or recombinant vaccinia viruses expressing the SIV major core protein, p55gag. (vvgag) or envelope glycoprotein, gp160env (vvenv) of SIVmac239 (provided by L. Giavedoni and T. Yilma, University of California, Davis, CA) and then labeled with 50 µCi of 51chromium (Na2CrO4; Amersham, Arlington Heights, IL) per 106 cells. Effector and target cells were added together at multiple E/T ratios in a 4-h chromium-release assay, and percent specific lysis was calculated from supernatant chromium measured in a liquid scintillation counter (Micro-beta 1450, Wallac Biosystems, Gaithersburg, MD). Specific lysis was considered positive if it was greater than twofold (three SDs) above the lysis of vvWR targets, and if it was at least 10%.
Lymphocytes isolated from the vaginal epithelium and submucosa were cultured in a limiting dilution format due to the low number of recovered cells. The relative frequencies of SIV gag and env-specific cytotoxic T lymphocyte precursor (CTLp) were determined using standard methods (22). Isolated CD8+ lymphocytes were diluted threefold serially for three dilutions in complete medium with replicates of 28 to 30 wells per dilution in 96-well round-bottom plates (Fisher Scientific, Santa Clara, CA). The cells were stimulated with Con A (10 µg/ml, Sigma Chemical Co.) and supplemented with human irradiated PBMC as feeder cells at a concentration of 1 × 105/well and 5% human IL-2 (Schiaparelli). On day 7 of culture, 20 U/ml recombinant human IL-2 (provided by Cetus, Inc.) was added. Cytotoxicity was measured on day 14. Individual wells were split three ways and assayed for cytolytic function in a 5-h chromium-release assay against autologous target cells infected with vvWR, vvgag, or vvenv as described above. Positive wells had at least 15% specific lysis based on a bimodal distribution of chromium release (23) and greater than twofold specific lysis above the vvWR target. CTLp frequencies were calculated by χ2 goodness of fit analysis using the method of maximum likelihood (24).
Results
CTL activity in peripheral lymphoid tissues of monkeys after intravaginal inoculation of SIVmac
The four animals that were infected with SIV for 9 to 10 mo were seropositive for SIV Ags by whole-virus ELISA (data not shown) and were clinically healthy at the time they were killed. Before selection for use in this study, all the animals chronically infected with SIV had CTL activity in PBMC directed against the SIV major core protein, p55gag, and envelope glycoprotein, gp160env. The effector activity was associated with a CD8+, MHC-restricted lymphocyte population (data not shown). At the time of necropsy, CTL activity against both SIV gag and env Ags was detected in PBMC and lymphocytes isolated from spleen, genital, and inguinal or axillary lymph nodes of three of four chronically infected monkeys. There was no difference in the strength of the CTL response detected in the genital lymph nodes, inguinal nodes, blood, or spleen (Fig. 1). The fourth animal had low level env-specific lysis in all the tissues. CTL activity against SIV gag was not detected in this animal (data not shown). Of the two acutely infected monkeys, one had low level env-specific CTL activity but no detectable gag-specific CTL activity in the genital lymph nodes, PBMC, and systemic lymphoid tissues. The other acutely infected monkey had both gag- and env-specific CTL activity in the PBMC and spleen and low level env-specific activity in lymph nodes. Thus, in most of the acutely and chronically infected monkeys, CTL activity could be detected in the genital lymph nodes and systemic lymphoid tissues. The uninfected monkey had very low env-specific CTL activity and no detectable gag-specific activity in peripheral blood (data not shown).
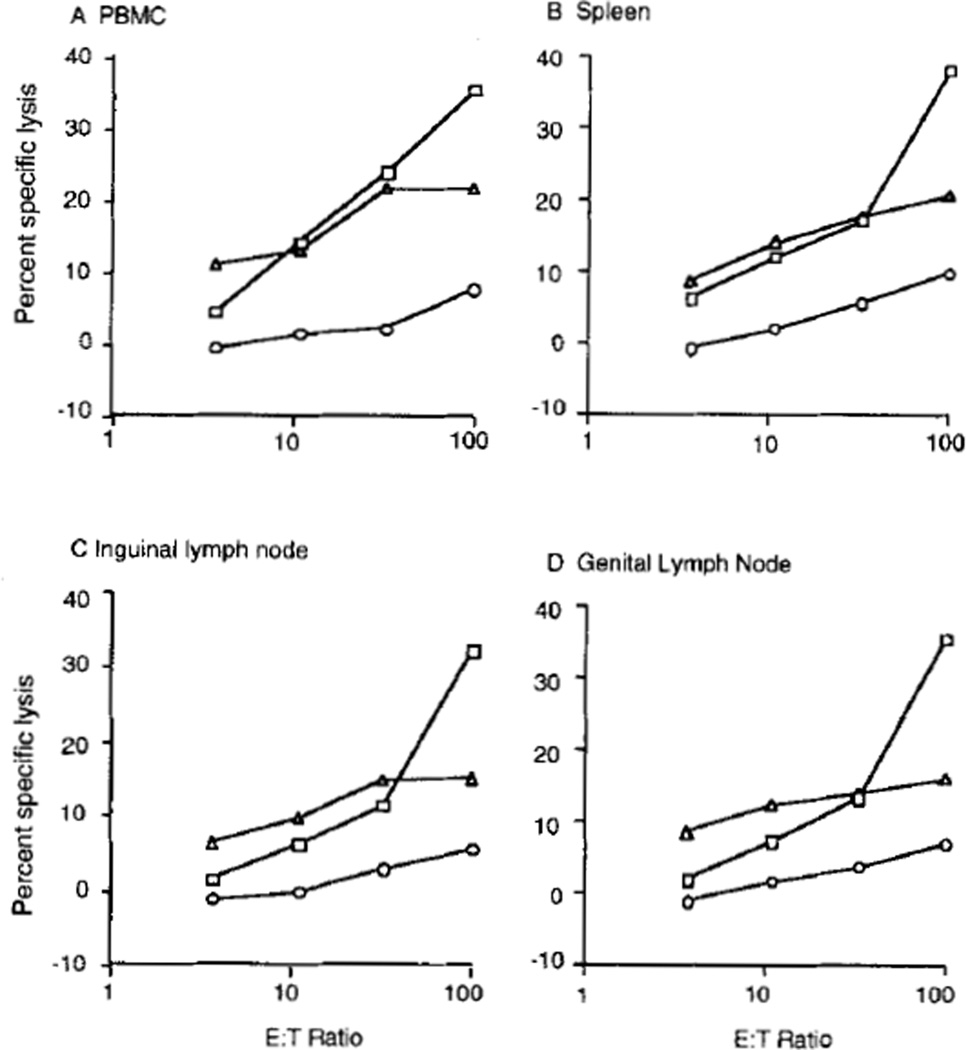
FIGURE 1.
SIV-specific cytolytic activity in lymphoid tissues of chronically infected Monkey 23024. A, PBMC; B, spleen; C, inguinal lymph node; D, genital lymph node. Lymphocytes were stimulated for 14 days, and then cytotoxic activity was measured against autologous lymphoblastoid cells infected with wild-type vaccinia virus (open circles), recombinant vaccinia virus expressing SIVgag (squares) or recombinant vaccinia virus expressing SIVenv (triangles).
CD8+ lymphocytes in vaginal epithelium express αβ TCR and have cytotoxic granules
As reported previously for normal multiparous female rhesus macaques and women (1–3), immunohistochemical staining demonstrated significant numbers of CD2+, CD8+ T cells in the vaginal epithelium and superficial submucosa of all animals, including the control monkey (Fig. 2, A-C). The single-labeled sections demonstrated cells positively labeled for CD8, CD2, and TIA-1 Ags in all sections of vaginal mucosa examined, and these markers seemed to localize to the same population of cells. The majority of CD8+ IEL contained cytoplasmic TIA-1+ granules (Fig. 2D). The triple-labeled sections confirmed that the CD8, CD2, and TIA-1 Ags were coexpressed by a single population of cells (Fig. 2E). The expression of TIA-1 by CD8+ lymphocytes is associated with cytotoxic activity (16, 17, 25). Compared with the control animal and previously published reports (3), the number of CD8+ T cells in the vaginal mucosa of both the acutely and chronically infected monkeys was increased slightly. In addition, in the SIV-infected animals, the CD8+ cells extended into the superficial layers of the stratified squamous vaginal epithelium. These changes were present despite the fact that no significant histopathologic changes in the epithelium were recognized. All of the CD8+ T cells in the vaginal epithelium of SIV-infected animals and the control animal expressed the β-chain of the TCR as determined by immunohistochemistry (Fig. 3, A-C). Thus, these CD8+, TIA-1+ vaginal IEL belong to the subset of T cells expressing the αβ-TCR. We were unable to identify mAbs capable of detecting γδ TCR+ T cells in cryostat sections of rhesus macaques, and we cannot state categorically that γδ T cells do not exist in the vaginal mucosa. However, we were able to demonstrate that all CD8+ cells in the vaginal mucosa express the TCR β-chain (Fig. 2). Thus, by exclusion, it is unlikely that CD8+ γδ T cells are present in the vaginal mucosa of rhesus macaques. As previously reported (3). CD4+ T cells were seen only rarely in the vaginal epithelium, although significant numbers were seen in the submucosa.
Vaginal IEL of SIV-infected monkeys contain antiviral CTL
In this study, all rhesus macaques intravaginally inoculated with SIV had detectable SIV-specific CTLp in the vaginal epithelium (Table I). In contrast, SIV-specific lysis could not be detected in the CD8+ lymphocytes isolated from the vaginal submucosa of these four animals. The estimated frequencies of CTLp in the vaginal IEL population varied among the animals, but there tended to be higher CTLp frequencies in the chronically infected monkeys compared with the monkeys infected for 2 wk, as shown in Figure 4. SIV gag-specific CTL activity was detected in the CD8+ vaginal IEL of all four animals chronically infected with SIV. In the vaginal epithelium of Monkey 24877, CTLp were relatively common with an estimated frequency of 1 in 2425 CD8+ lymphocytes. This was the only chronically infected animal that had detectable env-specific CTL activity in the vaginal epithelium, with a CTLp frequency of 1 in 8566 CD8+ lymphocytes. Monkey 23024 had the lowest level of gag-specific CTLp in the vaginal epithelium: 1 in 11,097 CD8+ lymphocytes.
Table I.
SIV-specific cytotoxic T cells in the vaginal epithelium of rhesus macaques
| Reciprocal of SIV gag and env CTLp Frequencies |
|||
|---|---|---|---|
| Monkey # | Duration of Infection | vv-gag targets | vv-env targets |
| 20035 | Chronic | 4,306 (2,589–12,777)a | >60,000 |
| 22429 | Chronic | 5,l 14 (3,697–8,288) | >182,000 |
| 23024 | Chronic | 11,097 (6,180–54,279) | >64,400 |
| 24877 | Chronic | 2,425 (1,685–4,321) | 8,566 (5,286–22,560) |
| 24803 | Acute | 26,686 (13,307–4,999,507) | 19,654 (9,951–790,556) |
| 25597 | Acute | >294,000 | 18,664 (13,027–32,901) |
| 16947 | Naive | >70,000 | >70,000 |
95% confidence intervals.
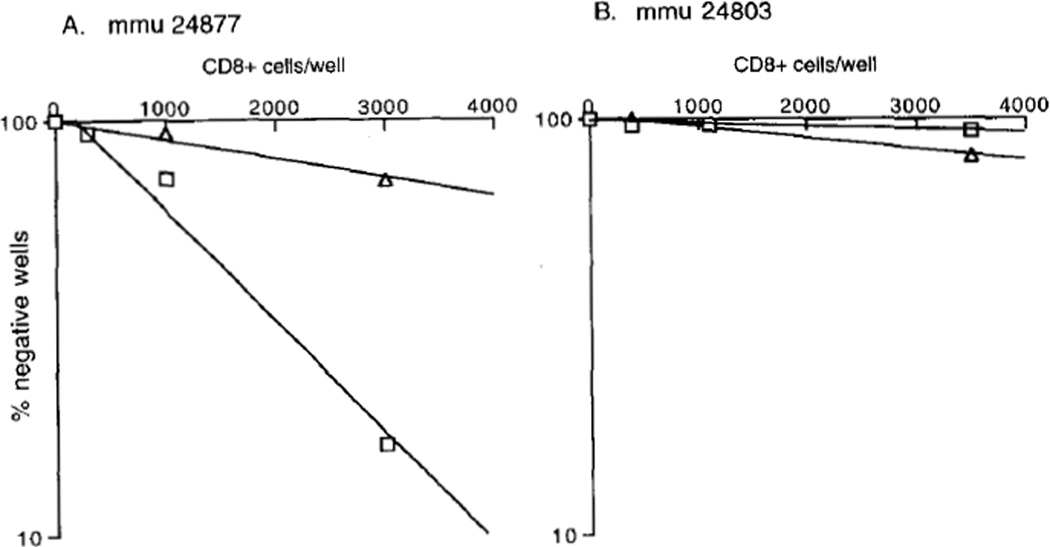
FIGURE 4.
Comparison of SIV-specific pCTL frequencies in vaginal epithelium from a chronically SIV-infected rhesus macaque (A), and an acutely SIV-infected rhesus macaque (B). Vaginal epithelial CD8+ lymphocytes were stimulated in culture for 14 days and then analyzed for cytotoxic activity against autologous lymphoblastoid cells infected with vvWR, vvgag (squares), and vvenv (triangles). Percentage of negative wells is plotted against number of CD8+ T cells per well, and frequency is extrapolated from × intercept of regression line with 37%.
CTLp frequencies were also estimated in the vaginal CD8+ IELs isolated from the monkeys infected with SIV for 2 wk. Monkey 24803 had a gag-specific CTLp frequency of 1 in 26,686 CD8+ lymphocytes and an env-specific frequency of 1 in 19,654 CD8+ lymphocytes. The second monkey, 25597, had no detectable precursor frequency for gag and 1 in 18,664 CD8+ lymphocytes were positive for env-specific lysis. Under identical limiting dilution conditions, we could not establish an estimate for anti-SIV CTLp frequencies in axillary lymph node lymphocyte or vaginal CD8+ IELs from the naive monkey (Table I).
Flow cytometric analysis of the cell populations derived from the epithelial lymphocyte cultures demonstrated that 98% of the cells were CD8+ (data not shown). In some experiments, after the limiting dilution wells were harvested for cytotoxicity assays, selected positive wells were restimulated at 100 cells/well with fresh feeder cells and mitogen. When retested after 14 days, most wells that were initially positive or negative for SIV-specific lysis remained so. Individual wells were subsequently expanded, but we were unable to maintain lytic activity after several months of growth.
Discussion
These results demonstrate that SIV-specific CTL activity is present in the CD8+ T cell population in the vaginal epithelium of SIV-infected animals. Monkeys with a chronic infection had a higher CTLp frequency than the monkeys infected for 2 wk. The frequency estimates of SIV gag-specific CTL in the vaginal lymphocytes were consistent with the previously reported range of CTLp present in PBMC of SIV-infected rhesus macaques, a range of 1 in 7,180 to 1 in 12,490 PBMC (26). In previous reports, we (21) and others (27) have shown that the effector cells from SIV-infected monkeys, generated by secondary in vitro stimulation with Con A, are CD8+ and MHC restricted. The present immunohistochemical results confirm that CD8+ vaginal IEL are also CD2+ and TCR β-chain+. Although we were unable to directly label TCR γδ cells, we were able to indirectly demonstrate that such cells are not present or are very rare in the vaginal mucosa of rhesus macaques. Thus, it is unlikely that the CD8+ vaginal IEL mediating SIV-specific target cell lysis are γδ T cells. But we cannot completely exclude the possibility that the isolated CD8+ vaginal IEL populations contained a few γδ T cells.
CTL have been shown to be the major means by which an immune response eliminates many systemic viral infections (28–32). The generation of antiviral CTL activity in vaginal IEL appears to be part of the normal immune response to intravaginal inoculation of SIV. SIV-infected cells have been detected in the vaginal epithelium of chronically infected rhesus macaques (19), and the CTL in the vaginal epithelium may be recognizing these cells. In fact, CD8+ CTL have been found in the skin rash that occurs in SIV-infected macaques (11), and the CD8+ T cells in the lesion were in contact with degenerating CD4+ Langerhans cells (33), suggesting that the CTL may have been responding to viral Ag in these cells. It is also possible that these SIV-specific CD8+ CTL are homing to the vaginal epithelium as part of a normal pattern of lymphocyte recirculation unrelated to the presence of virus-infected cells. The immune cell population in the ectocervical and vaginal epithelia are immunophenotypically similar (3), and it is likely that similar CTL activity is present in the CD8+ T cells in the ectocervical squamous epithelium.
The route of infection may be important for the generation of mucosal CTL. All the monkeys used in this study were infected by vaginal inoculation of SIV. It will be informative to determine whether i.v. inoculated animals have similar anti-SIV CTL activity in the genital immune system. Virus-specific CTLs can be induced in the gut-associated lymphoid tissues as well as in other mucosa-associated tissues by mucosal viral infections or mucosal contact with viral Ags (34–36). Following intravaginal immunization with live-attenuated HSV type 2, mice are protected against genital transmission of virulent HSV type 2 (37). Protection is transferred to naive, syngeneic mice by adoptive transfer of genital lymph node lymphocytes, and these HSV-stimulated lymphocytes migrate preferentially to the genital tract of challenged mice (38). This result suggests that virus-specific CTL generated in the genital lymph nodes participate in effective genital immune responses against a sexually transmitted viral pathogen.
The induction of an effective CTL response to a virus is usually advantageous to the host. Mucosal immune responses, secreted Ab, and CTL, capable of preventing or eliminating virus infection, comprise the first line of immune defense to prevent viral dissemination and subsequent systemic infection. The success of a vaccine capable of preventing the heterosexual transmission of HIV may rely on the ability of the vaccine to generate specific immune responses in genital tissues and draining lymph nodes. The results reported in this study provide the first direct demonstration that antiviral CTL are present in the vaginal epithelium, and suggest that an appropriate immunization regimen may be able to generate anti-HIV CTL in the mucosal immune system of the genital tract.
Acknowledgments
This work was made possible by the excellent technical support of Jennifer Collins, Steven Joye, Ellen McGowan, Carol Oxford, and Judy Torten. We thank Dr. J. R. McGhee for critical review of the manuscript.
Abbreviations used in this paper
- SIV
simian immunodeficiency virus
- CTLp
cytotoxic T lymphocyte precursor
- IEL
intraepithelial lymphocyte
Footnotes
This work was supported by the Contraceptive Research and Development Program (CONRAD, DPE-3044-A-00-6063-00), National Institutes of Health Grants AI-35545 and RR-00169, and Pediatric AIDS Foundation Grant 50440-15-PG. M.B.M. is supported by a Scholar Award from the American Foundation for AIDS Research. The views expressed by the authors do not necessarily reflect the views of CONRAD.
References
- 1.Edwards JNT, Morris HB. Langerhans cells and lymphocyte subsets in the female genital tract. Br. J. Obstet. Gynecol. 1985;92:974. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 2.Roncalli M, Sideri M, Gie P, Servida E. Immunophenotypic anal ysis of the transformation zone of human cervix. Lab. Invest. 1988;58:141. [PubMed] [Google Scholar]
- 3.Miller CJ, McChesney M, Moore PF. Langerhans cells, macro- phages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab. Invest. 1992;67:628. [PubMed] [Google Scholar]
- 4.Ogra P, Ogra S. Local antibody response to polio vaccine in the human female genital tract. J. Irnmunol. 1973;110:1307. [PubMed] [Google Scholar]
- 5.Merriman H, Woods S, Winter C, Fahnlander A, Corey L. Secretory IgA antibody in cervicovaginal secretions from women with genital infection due to herpes simplex virus. J. Infect. Dis. 1984;149:505. doi: 10.1093/infdis/149.4.505. [DOI] [PubMed] [Google Scholar]
- 6.Belec L, Georges A, Steenman G, Martin P. Antibodies to human immunodeficiency virus in vaginal secretions of heterosexual women. J. Infect. Dis. 1989;160:385. doi: 10.1093/infdis/160.3.385. [DOI] [PubMed] [Google Scholar]
- 7.Miller C, Kang D, Marthas M, Moldoveanu Z, Kiyono H, Marx P, Eldridge J, Mestecky J, McGhee J. Genital secretory immune response to chronic SIV infection: a comparison between intravenously and genitally inoculated rhesus macaques. Clin. Exp. Immunol. 1992;88:520. doi: 10.1111/j.1365-2249.1992.tb06481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu XS, Belec L, Pillot J. Anti-gp 160 IgG and IgA antibodies associated with a large increase in total IgG in cervicovaginal secretions from human immunodeficiency virus type 1-infected women. J. Infect. Dis. 1993;167:1189. doi: 10.1093/infdis/167.5.1189. [DOI] [PubMed] [Google Scholar]
- 9.Autran B, Plata F, Debre P. MHC-restricted cytotoxicity against HIV. J. Acquired Immune Defic. Syndr. 1991;4:361. [PubMed] [Google Scholar]
- 10.Reimann KA, Tenner-Racz K, Racz P, Montefiori DC, Yasutomi Y, Lin W, Ransil BJ, Letvin NL. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 1994;68:2362. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, Ringler DJ, Miller MD, Yasutomi Y, Hasunuma T, Letvin NL. Simian immunodeficiency virus-specific cytotoxic T lymphocytes are present in the AIDS-associated skin rash in rhesus monkeys. J. Immunol. 1992;149:728. [PubMed] [Google Scholar]
- 12.Miller CJ, Alexander NJ, Sutjipto S, Lackner AA, Hendrickx AG, Gettie A, Lowenstine LJ, Jennings M, Marx PA. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 1989;63:4277. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, Hendrickx AG. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 1994;68:6391. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CJ, McGhee JR, Gardner MB. Mucosal immunity, HIV transmission and AIDS. Lab. Invest. 1993;68:129. [PubMed] [Google Scholar]
- 15.Lefrancois L, Puddington L. Extrathymic intestinal T cell development: virtual reality? Immunol. Today. 1995;16:16. doi: 10.1016/0167-5699(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 16.Tian Q, Streuli M, Saito H, Schlossman SF, Anderson PJ. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentatlon in cells. Cell. 1991;67:629. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- 17.Tenner-Racz K, Racz P, Thome C, Meyer CG, Anderson PJ, Schlossman SF, Letvin NL. Cytotoxic effector granules recognized by the monoclonal antibody TIA-1 are present in the CD8+ lymphocytes in lymph nodes of human immunodeficiency virus-1-infected patients. Am. J. Purhol. 1993;142:1750. [PMC free article] [PubMed] [Google Scholar]
- 18.Lohman B, Higgins J, Marthas M, Marx P, Pedersen N. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1991;29:2187. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CJ, Vogel P, Alexander NJ, Sutjipto S, Hendrickx AG, Marx PA. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am. J. Pathol. 1992;141:655. [PMC free article] [PubMed] [Google Scholar]
- 20.James S, Graeff A. Spontaneous and lymphokine-induced cytotoxic activity of monkey intestinal mucosal lymphocytes. Cell. Immunol. 1985;93:387. doi: 10.1016/0008-8749(85)90143-1. [DOI] [PubMed] [Google Scholar]
- 21.Lohman BL, McChesney MB, Miller CJ, McGowan E, Joye SM, Van Rompay KKA, Reay E, Antipa L, Pedersen NC, Marthas ML. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J. Virol. 1994;68:7021. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefkovits I, Waldman H. Limiting Dilution Analysis of Cells in the Immune System. Cambridge, UK: Cambridge University Press; 1979. [Google Scholar]
- 23.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL response to HIV-1 and Epstein Barr virus in late disease. J. Exp. Med. 1993;177:249. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 1981;126:1614. [PubMed] [Google Scholar]
- 25.Anderson PJ, Nagler-Anderson C, O’Brien C, Levine H, Watkons S, Slayter HS, Blue ML, Schlossman SF. A monoclonal antibody reactive with a 15 kDa cytoplasmic granule associated protein defines a subpopulation of CD8+ lymphocytes. J. Immunol. 1990;144:574. [PubMed] [Google Scholar]
- 26.Venet A, Bourgault I, Aubertin A-M, Kieny M-P, Levy J-P. Cytotoxic T lymphocyte response against multiple simian immunodeficiency virus (SIV) proteins m SIV-infected macaques. J. Immunol. 1992;148:2899. [PubMed] [Google Scholar]
- 27.Miller MD, Lord CI, Stallard V, Mazzara GP, Letvin NL. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J. Immunol. 1990;144:122. [PubMed] [Google Scholar]
- 28.Yap KL, Ada GL, McKenzie IFC. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 29.Quinnan GVJ, Kirmani N, Rook AH, Manischewitz JF, Jackson L, Moreschi G, Santos GW, Saral R, Burns W. Cytotoxic T cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone marrow transplant recipients. N. Engl. J. Med. 1982;307:7. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 30.Byme JA, Oldstone MBA. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J. Virol. 1984;51:682. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J. Exp. Med. 1984;160:814. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cannon MJ, Openshaw PJM, Askonas BA. Cytotoxic T cells clear virus hut augment lung pathology in mice infected with respiratory syncitial virus. J. Exp. Med. 1988;168:1163. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringler DJ, Hancock WW, King NW, Letvin NL, Daniel MD, Desrosiers RC, Murphy GF. Immunophenotypic characterization of the cutaneous exanthem of SIV-infected rhesus monkeys: apposition of degenerative Langerhans cells and cytotoxic lymphocytes during the development of acquired immunodeficiency syndrome. Am. J. Pathol. 1987;126:199. [PMC free article] [PubMed] [Google Scholar]
- 34.Issekutz TB. The response of gut-associated T lymphocytes to intestinal viral immunization. J. Immrmol. 1984;133:2955. [PubMed] [Google Scholar]
- 35.London SD, Rubin DH, Cebra JJ. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer’s patches. J. Exp. Med. 1987;165:830. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Offit PA, Dudzik KI. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J. Viral. 1989;63:3507. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott MR, Smiley JR, Leslie P, Brais I, Rudzroga HE, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 1984;51:747. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott MR, Goldsmith CH, Rosenthal KL, Brais LJ. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 1989;159:460. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]