Abstract
BACKGROUND
Resistance to therapy with BRAF kinase inhibitors is associated with reactivation of the mitogen-activated protein kinase (MAPK) pathway. To address this problem, we conducted a phase 1 and 2 trial of combined treatment with dabrafenib, a selective BRAF inhibitor, and trametinib, a selective MAPK kinase (MEK) inhibitor.
METHODS
In this open-label study involving 247 patients with metastatic melanoma and BRAF V600 mutations, we evaluated the pharmacokinetic activity and safety of oral dabrafenib (75 or 150 mg twice daily) and trametinib (1, 1.5, or 2 mg daily) in 85 patients and then randomly assigned 162 patients to receive combination therapy with dabrafenib (150 mg) plus trametinib (1 or 2 mg) or dabrafenib monotherapy. The primary end points were the incidence of cutaneous squamous-cell carcinoma, survival free of melanoma progression, and response. Secondary end points were overall survival and pharmacokinetic activity.
RESULTS
Dose-limiting toxic effects were infrequently observed in patients receiving combination therapy with 150 mg of dabrafenib and 2 mg of trametinib (combination 150/2). Cutaneous squamous-cell carcinoma was seen in 7% of patients receiving combination 150/2 and in 19% receiving monotherapy (P = 0.09), whereas pyrexia was more common in the combination 150/2 group than in the monotherapy group (71% vs. 26%). Median progression-free survival in the combination 150/2 group was 9.4 months, as compared with 5.8 months in the monotherapy group (hazard ratio for progression or death, 0.39; 95% confidence interval, 0.25 to 0.62; P<0.001). The rate of complete or partial response with combination 150/2 therapy was 76%, as compared with 54% with monotherapy (P = 0.03).
CONCLUSIONS
Dabrafenib and trametinib were safely combined at full monotherapy doses. The rate of pyrexia was increased with combination therapy, whereas the rate of proliferative skin lesions was nonsignificantly reduced. Progression-free survival was significantly improved. (Funded by GlaxoSmithKline; ClinicalTrials.gov number, NCT01072175.)
Pharmacologic inhibition of the mitogen-activated protein kinase (MAPK) pathway has proved to be a major advance in the treatment of metastatic melanoma. The use of vemurafenib and dabrafenib, agents that block MAPK signaling in patients with melanoma and the BRAF V600E mutation, has been associated with prolonged survival and progression-free survival, respectively, in randomized phase 3 trials involving patients with previously untreated melanoma.1–6 Trametinib mediates blockade of MAPK kinase (MEK), which is downstream of BRAF in the MAPK pathway and has been associated with improved progression-free and overall survival in BRAF V600 melanoma (comprising both V600E and V600K mutations).7,8
In spite of these advances, 50% of patients who are treated with BRAF or MEK inhibitors have disease progression within 6 to 7 months after the initiation of treatment.3,6 Several mechanisms mediating resistance to BRAF inhibitors through MAPK reactivation have been described, including the up-regulation of bypass pathways mediated by cancer Osaka thyroid kinase (COT),9 development of de novo NRAS or MEK mutations,10,11 and dimerization or variant splicing of mutant BRAF V600.12 In addition, MAPK-independent signaling through receptor tyrosine kinases, such as platelet-derived growth factor receptor β,10 insulin-like growth factor 1 receptor,13 and hepatocyte growth factor receptor, have been associated with resistance.14 New therapeutic strategies are needed to address these resistance mechanisms.
In preclinical models, rapid recovery of MAPK pathway signaling has been associated with BRAF-inhibitor resistance, and complete inhibition of the MAPK pathway is needed to induce cell death in BRAF V600 melanoma.15,16 This can be achieved by combining a BRAF inhibitor with a MEK inhibitor.15,16 The emergence of cutaneous squamous-cell carcinoma early in the course of BRAF-inhibitor therapy has been associated with paradoxical MAPK pathway activation during BRAF inhibition.17 In an experimental model of squamous-cell carcinoma, the addition of a MEK inhibitor to a BRAF inhibitor reduced this effect.17
In an attempt to delay resistance to BRAF inhibition and explore the safety of combination therapy with BRAF and MEK inhibition, we conducted a phase 1 and 2 study to investigate the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib in patients with metastatic BRAF V600 melanoma.
METHODS
PATIENTS
From March 26, 2010, to July 7, 2011, we screened 443 patients at 16 centers for participation in the study. Patients 18 years of age or older who had histologically confirmed metastatic melanoma with either BRAF V600E or BRAF V600K mutations were eligible for inclusion. BRAF mutation status was determined locally. Eligible patients had measurable disease, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (with 0 indicating asymptomatic and 1 ambulatory but restricted in strenuous activity), and adequate organ function. Patients with treated brain metastases and at least a 3-month history of stable disease were allowed to enroll. Additional eligibility criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org. All patients provided written informed consent.
STUDY DESIGN AND TREATMENT
This was an open-label study designed to assess the safety, pharmacokinetic activity, and clinical activity of combination therapy with dabrafenib plus trametinib. The study was conducted in four parts (Fig. 1 in the Supplementary Appendix), three of which are reported here. Part A confirmed the absence of a drug–drug interaction between repeated doses of trametinib and a single dose of dabrafenib (see the Pharmacokinetics Section in Methods in the Supplementary Appendix). Part B evaluated the side-effect profile, safety, and pharmacokinetic activity of escalating doses of dabrafenib (75 and 150 mg twice daily) in combination with trametinib (1, 1.5, and 2 mg once daily). Part B also included two additional cohorts: patients with metastatic melanoma and a BRAF V600 mutation who had disease progression during previous treatment with a BRAF inhibitor and patients with colorectal cancer and a BRAF V600 mutation. (Results for the latter two cohorts are not reported here.)
Part C was a phase 2 study in which patients were randomly assigned in a 1:1:1 ratio to receive 150 mg of dabrafenib twice daily plus once-daily trametinib, at a dose of either 1 mg (combination 150/1) or 2 mg (combination 150/2), or 150 mg of dabrafenib monotherapy twice daily. Patients who had disease progression while receiving dabrafenib monotherapy could cross over to receive combination 150/2. Primary end points for this portion of the study were the incidence of cutaneous squamous-cell carcinoma, progression-free survival, response rate, duration of response, and safety. Secondary end points were pharmacokinetic activity and overall survival. In part C only, patients could have undergone no more than one previous chemotherapy regimen for advanced or metastatic melanoma, but those who had previously received BRAF or MEK inhibitors were not eligible.
STUDY OVERSIGHT
The protocol was approved by the institutional review board at each participating center and complied with country-specific regulatory requirements. The study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was designed by the academic authors in conjunction with representatives of the sponsor, GlaxoSmithKline. The data were collected by the sponsor and analyzed in collaboration with the authors. The authors vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol, which is available at NEJM.org. The manuscript was prepared by the first and last authors, but all the authors contributed to subsequent drafts. All authors made the decision to submit the manuscript for publication. Editorial support that did not involve writing was provided by MediTech Media, which was funded by the sponsor.
STATISTICAL ANALYSIS
For parts A and B, we did not test any formal hypotheses; all analyses were descriptive. The sample size for part C was based on the demonstration of a reduction in the rate of cutaneous squamous-cell carcinoma from 20% with dabrafenib monotherapy to 3% with combination therapy, with a power of 82% and a type I error rate of 5%. We based the choice of this primary end point on a preclinical scientific hypothesis that this drug combination would attenuate the development of cutaneous squamous-cell carcinoma.
Efficacy analyses were performed in the intention-to-treat population in part C. The prespecified rate of progression events required for the final analysis in part C was 70% across the three study groups, which was reached on May 31, 2012. We used Kaplan–Meier methods to estimate progression-free survival and compared the results among the three study groups with a log-rank test. The statistical analysis plan is available at NEJM.org.
RESULTS
PATIENTS
We enrolled 247 patients with metastatic melanoma who had not received previous BRAF-inhibitor treatment (Table 1, and Table S3 in the Supplementary Appendix): 8 patients in part A, 77 patients in part B (including 24 receiving combination 150/2), and 162 in part C (with 54 per study group). Rates of poor prognostic features, such as M1c disease, brain metastases, and elevated lactate dehydrogenase levels, were similar in the cohort in part B and each of the study groups in part C. One patient had a BRAF V600R mutation, and all others had a V600E or V600K mutation. After a median follow-up of 14.1 months in part C, 49 of the 162 patients continued to receive treatment (23 patients in each of the two combination-therapy groups and 3 patients in the monotherapy group).
Table 1.
Characteristics of the Patients at Baseline in Part C (Intention-to-Treat Population).*
| Characteristic | Dabrafenib Monotherapy (N = 54) | Combination 150/1 (N = 54) | Combination 150/2 (N = 54) |
|---|---|---|---|
| Median age (range) — yr | 50 (18–82) | 49 (23–85) | 58 (27–79) |
| Male sex — no. (%) | 29 (54) | 30 (56) | 34 (63) |
| ECOG performance status — no. (%) | |||
| 0 | 34 (63) | 38 (70) | 35 (65) |
| 1 | 20 (37) | 16 (30) | 19 (35) |
| Metastatic status — no. (%)† | |||
| M0 | 1 (2) | 1 (2) | 0 |
| M1a | 11 (20) | 9 (17) | 6 (11) |
| M1b | 5 (9) | 11 (20) | 10 (19) |
| M1c | 37 (69) | 33 (61) | 38 (70) |
| History of brain metastases — no. (%) | 4 (7) | 7 (13) | 2 (4) |
| Elevated lactate dehydrogenase level — no. (%)‡ | 27 (50) | 25 (46) | 22 (41) |
| BRAF mutation — no. (%) | |||
| V600E | 45 (83) | 45 (83) | 47 (87) |
| V600K | 9 (17) | 9 (17) | 7 (13) |
| Previous chemotherapy — no. (%) | 12 (22) | 15 (28) | 7 (13) |
| Previous immunotherapy — no. (%) | 8 (15) | 16 (30) | 13 (24) |
Part C was a phase 2 study in which patients were randomly assigned to receive 150 mg of dabrafenib twice daily plus once-daily trametinib at a dose of either 1 mg (combination 150/1) or 2 mg (combination 150/2) or 150 mg of dabrafenib monotherapy twice daily. There were no significant differences among groups except that patients in the combination 150/2 group were older than those in the monotherapy group (P = 0.04). ECOG denotes Eastern Cooperative Oncology Group.
The criteria of the American Joint Committee on Cancer for distant metastasis are as follows: M0, no detectable evidence of distant metastases; M1a, metastases to skin, subcutaneous tissue, or distant lymph nodes; M1b, metastases to lung; and M1c, metastases to all other visceral sites or distant metastases to any site combined with an elevated serum lactate dehydrogenase level.
The lactate dehydrogenase level was considered to be elevated if it was more than the upper limit of the normal range.
DOSE ESCALATION IN PART B
Dose escalation began with half the recommended monotherapy dose for both dabrafenib and trametinib, with no dose-limiting toxic effects at the first three dose levels: 75 mg of dabrafenib twice daily plus 1 mg of trametinib once daily, 150 mg of dabrafenib twice daily plus 1 mg of trametinib once daily, and 150 mg of dabrafenib twice daily plus 1.5 mg of trametinib once daily. Among the 24 patients who were treated at the highest dose level — 150 mg of dabrafenib twice daily plus 2 mg of trametinib once daily (combination 150/2) — there was one dose-limiting toxic effect: recurrent neutrophilic panniculitis, which was treated with glucocorticoids and an eventual dose reduction. The maximum tolerated dose combination was not reached in this study. The recommended phase 2 dose was combination 150/2, which combines the recommended monotherapy dose for each agent. All toxic effects in part B are summarized in Table S5 in the Supplementary Appendix.
SAFETY AND SIDE-EFFECT PROFILE
In part C, the incidence of cutaneous squamous-cell carcinoma (including keratoacanthoma) in patients receiving dabrafenib monotherapy was 19%, as compared with 2% for combination 150/1 and 7% for combination 150/2 (P = 0.004 and P = 0.09, respectively) (Table 2). The rates of rash in the combination 150/1 and 150/2 groups were lower than the rate in the monotherapy group (20% and 27%, respectively, vs. 36%), but MEK inhibitor–associated acneiform dermatitis was more prevalent in the combination-therapy groups (11% and 16%, respectively, vs. 4%).
Table 2.
Adverse Events Reported in Part C.*
| Adverse Event | Dabrafenib Monotherapy (N = 5)† | Combination 150/1 (N = 54) | Combination 150/2 (N = 55)† | |||
|---|---|---|---|---|---|---|
| Grade 3 or 4 | All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | All Grades | |
| number of patients (percent) | ||||||
|
| ||||||
| Any event | 23 (43) | 53 (100) | 26 (48) | 53 (98) | 32 (58) | 55 (100) |
|
| ||||||
| Pyrexia | 0 | 14 (26) | 5 (9) | 37 (69) | 3 (5) | 39 (71) |
|
| ||||||
| Chills | 0 | 9 (17) | 1 (2) | 27 (50) | 1 (2) | 32 (58) |
|
| ||||||
| Fatigue | 3 (6) | 21 (40) | 1 (2) | 31 (57) | 2 (4) | 29 (53) |
|
| ||||||
| Nausea | 0 | 11 (21) | 3 (6) | 25 (46) | 1 (2) | 24 (44) |
|
| ||||||
| Vomiting | 0 | 8 (15) | 2 (4) | 23 (43) | 1 (2) | 22 (40) |
|
| ||||||
| Diarrhea | 0 | 15 (28) | 0 | 14 (26) | 1 (2) | 20 (36) |
|
| ||||||
| Headache | 0 | 15 (28) | 1 (2) | 20 (37) | 0 | 16 (29) |
|
| ||||||
| Peripheral edema | 0 | 9 (17) | 0 | 13 (24) | 0 | 16 (29) |
|
| ||||||
| Cough | 0 | 11 (21) | 0 | 6 (11) | 0 | 16 (29) |
|
| ||||||
| Arthralgia | 0 | 18 (34) | 0 | 24 (44) | 0 | 15 (27) |
|
| ||||||
| Rash | 0 | 19 (36) | 0 | 11 (20) | 0 | 15 (27) |
|
| ||||||
| Night sweats | 0 | 3 (6) | 0 | 8 (15) | 0 | 13 (24) |
|
| ||||||
| Decreased appetite | 0 | 10 (19) | 0 | 16 (30) | 0 | 12 (22) |
|
| ||||||
| Myalgia | 1 (2) | 12 (23) | 0 | 13 (24) | 1 (2) | 12 (22) |
|
| ||||||
| Constipation | 0 | 6 (11) | 1 (2) | 9 (17) | 0 | 12 (22) |
|
| ||||||
| Elevated blood alkaline phosphatase | 0 | 1 (2) | 3 (6) | 12 (22) | 0 | 5 (9) |
|
| ||||||
| Hyperkeratosis | 0 | 16 (30) | 0 | 3 (6) | 0 | 5 (9) |
|
| ||||||
| Alopecia | 0 | 18 (34) | 0 | 5 (9) | 0 | 3 (5) |
|
| ||||||
| Grade 3‡ | All Grades | Grade 3‡ | All Grades | Grade 3‡ | All Grades | |
|
| ||||||
| Cutaneous squamous-cell carcinoma§ | 9 (17) | 10 (19) | 1 (2) | 1 (2) | 3 (5) | 4 (7) |
|
| ||||||
| Skin papilloma | 0 | 8 (15) | 0 | 4 (7) | 0 | 2 (4) |
|
| ||||||
| Hyperkeratosis | 0 | 16 (30) | 0 | 3 (6) | 0 | 5 (9) |
|
| ||||||
| Decreased ejection fraction | 0 | 0 | 1 (2) | 2 (4) | 0 | 5 (9) |
|
| ||||||
| Cardiac failure | 0 | 0 | 1 (2) | 1 (2) | 0 | 0 |
|
| ||||||
| Hypertension | 0 | 2 (4) | 0 | 2 (4) | 1 (2) | 5 (9) |
|
| ||||||
| Chorioretinopathy | 0 | 0 | 0 | 0 | 1 (2) | 1 (2) |
Listed are all adverse events that were reported in more than 20% of patients in any group, regardless of whether a causal relationship was likely. In addition to these events, there was one death from sepsis in the combination 150/1 group and there were three deaths in the combination 150/2 group (two from brain hemorrhage and one from pulmonary embolism). None of these events were considered to be related to a study drug. Neutropenia (grade 3 or 4) occurred in 11% of patients in the combination 150/2 group, with one case of febrile neutropenia. Acneiform dermatitis occurred in 11% of patients in the combination 150/1 group, 16% in the combination 150/2 group, and 4% in the monotherapy group, with no grade 3 or 4 events reported.
One patient who was assigned to the monotherapy group received combination 150/2 and so was included in the combination 150/2 safety analyses.
For these categories, no grade 4 events were reported.
Keratoacanthoma was classified as cutaneous squamous-cell carcinoma.
Known MEK inhibitor–associated toxic effects, including peripheral edema, hypertension, decreased cardiac ejection fraction, and ocular events, occurred more frequently in the combination-therapy groups than in the monotherapy group. Conversely, known BRAF inhibitor–induced hyperproliferative skin lesions, such as cutaneous squamous-cell carcinoma, papilloma, and hyperkeratosis, were observed less frequently in the combination-therapy groups than in the monotherapy group.
The most frequent adverse events observed in the combination 150/2 group were pyrexia (all grades, 71%; grade 3, 5%) and chills (all grades, 58%; grade 3, 2%). Pyrexia that was associated with severe chills or hypotension or that required hospitalization was more frequent in the combination 150/1 and combination 150/2 groups than in the monotherapy group (19% and 25%, respectively, vs. 2%). Two patients in the combination 150/1 group and one patient in the combination 150/2 group had drug-related pyrexia that was associated with hyponatremia or renal insufficiency.
Other adverse events, in addition to pyrexia and chills, that were more common in the combination 150/2 group than in the monotherapy group included fatigue (in 53% of patients), nausea (44%), vomiting (40%), and diarrhea (36%), although the symptoms were rarely grade 3 or 4. The most frequently occurring grade 3 or 4 toxic effect in the combination 150/2 group was neutropenia (in 11% of patients), with one case of febrile neutropenia.
Dose interruptions and delays were frequent in all three study groups (Table S1 in the Supplementary Appendix). In the combination 150/2 group, 32 of 55 patients included in the safety analyses (58%) required dose reductions because of adverse events, the majority of which were associated with pyrexia and were attributed to dabrafenib. Re-escalation of the dabrafenib dose was common, occurring in 28 of 30 patients (93%). Despite the need for dose modifications, the median treatment duration was 10.5 months in the combination 150/1 group, 11.0 months in the combination 150/2 group, and 6.1 months in the monotherapy group.
PHARMACOKINETIC ACTIVITY
Repeat-dose trametinib had no effect on the pharmacokinetic activity of single-dose dabrafenib, with ratios of 0.94 to 1.03 for the comparison between the area under the time–concentration curve and the maximum concentration for dabrafenib before and after trametinib exposure (Table S2 in the Supplementary Appendix). Pharmacokinetic analyses that were performed during dose escalation in part B suggested that there was a modest increase in exposure to dabrafenib, as compared with previously reported data, and no apparent effect on trametinib exposure5,7 (Fig. S2A and S2B in the Supplementary Appendix). These data suggest that trametinib may have a minor inhibitory effect on dabrafenib clearance.
EFFICACY
Part C
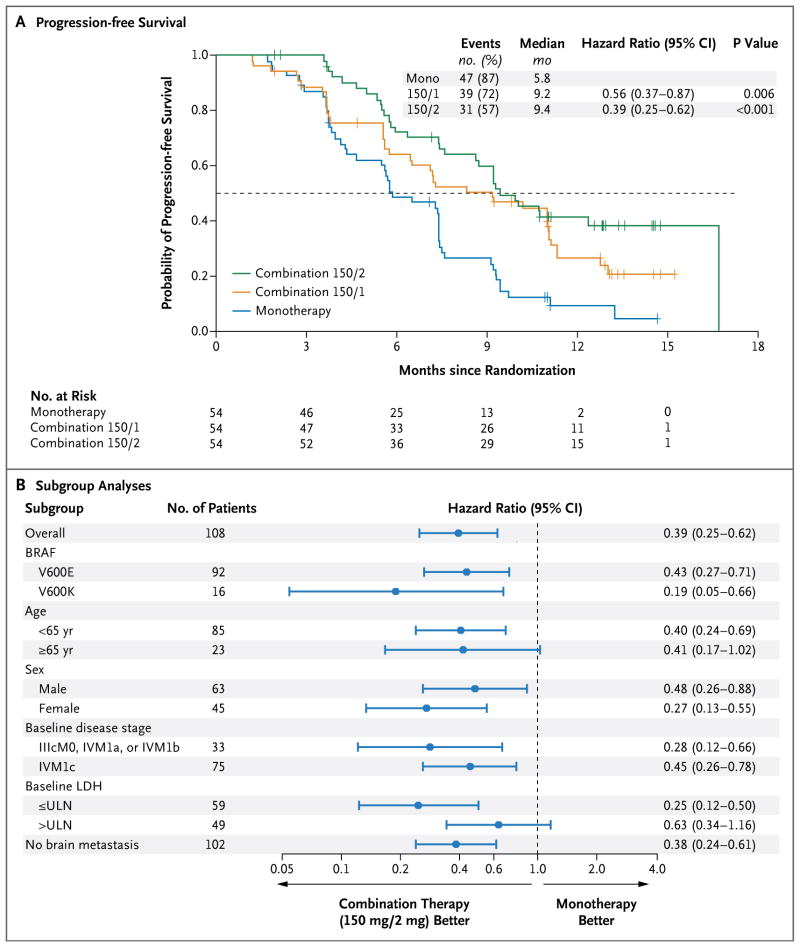
At the time of the prespecified efficacy analysis, the median follow-up for patients in part C was 14.1 months (range, 10.8 to 17.6). The median progression-free survival for patients in the combination 150/2 group was 9.4 months, as compared with 5.8 months with dabrafenib monotherapy (hazard ratio for progression or death, 0.39; 95% confidence interval [CI], 0.25 to 0.62; P<0.001) (Table 3 and Fig. 1A). The improvement in progression-free survival as assessed by an independent review committee was less pronounced (hazard ratio, 0.55; 95% CI, 0.33 to 0.93; P = 0.02) (Fig. S4 in the Supplementary Appendix). This difference in the hazard ratio is largely attributable to an imbalance in informative censoring, most commonly in cases in which new lesions were identified at the study center but were not considered to constitute definitive progression on central review. Less censoring occurred in the combination 150/2 group, in part because of additional follow-up beyond investigator-assessed progression. Such follow-up did not occur for patients who had disease progression while receiving monotherapy. These patients crossed over to receive combination therapy and could no longer be evaluated for disease progression while receiving their originally assigned therapy.
Table 3.
Efficacy End Points in Part C, as Assessed by the Site Investigators (Intention-to-Treat Population).*
| End Point | Dabrafenib Monotherapy (N = 54) | Combination 150/1 (N = 54) | Combination 150/2 (N = 54) |
|---|---|---|---|
| Progression-free survival — mo | |||
| Median (95% CI) | 5.8 (4.6–7.4) | 9.2 (6.4–11.0) | 9.4 (8.6–16.7) |
| Hazard ratio for death or progression (95% CI) | Reference | 0.56 (0.37–0.87) | 0.39 (0.25–0.62) |
| P value | Reference | 0.006 | <0.001 |
| Progression-free survival at 12 mo (95% CI) — % | 9 (3–20) | 26 (15–39) | 41 (27–54) |
| Best response — no. (%) | |||
| Complete response | 2 (4) | 3 (6) | 5 (9) |
| Partial response | 27 (50) | 24 (44) | 36 (67) |
| Stable disease | 22 (41) | 24 (44) | 13 (24) |
| Progressive disease | 3 (6) | 2 (4) | 0 |
| Could not be evaluated | 0 | 1 (2) | 0 |
| Complete or partial response | |||
| No. of patients | 29 | 27 | 41 |
| Percent of patients (95% CI) | 54 (40–67) | 50 (36–64) | 76 (62–86) |
| P value | Reference | 0.77 | 0.03 |
| Duration of response — mo | |||
| Median | 5.6 | 9.5 | 10.5 |
| 95% CI | 4.5–7.4 | 7.4–NA | 7.4–14.9 |
Hazard ratios and P values are for the comparison between each combination-therapy group and the monotherapy group. NA denotes not achieved.
Figure 1. Progression-free Survival and Subgroup Analyses.
Panel A shows Kaplan–Meier curves for progression-free survival, with progression assessed by the site investigators, in an analysis comparing two doses of combination therapy — 150 mg of dabrafenib twice daily plus once-daily trametinib at a dose of either 1 mg (combination 150/1) or 2 mg (combination 150/2) — with dabrafenib monotherapy (mono). Panel B shows subgroup analyses for patients receiving either combination 150/2 or monotherapy. Both these analyses were performed in part C of the study. The melanoma staging criteria of the American Joint Committee on Cancer are defined as follows: stage IIIc, metastases to 4 or more nodes (or in-transit metastasis); and stage IV, metastases beyond nodes. The criteria for distant metastasis are defined as follows: M0, no detectable evidence of distant metastases; M1a, metastases to skin, subcutaneous tissue, or distant lymph nodes; M1b, metastases to lung; and M1c, metastases to all other visceral sites or distant metastases to any site combined with an elevated serum lactate dehydrogenase (LDH) level. ULN denotes upper limit of the normal range.
At 1 year, 41% of patients in the combination 150/2 group were alive and progression-free, as compared with 9% in the monotherapy group (P<0.001). Progression-free survival was consistently improved in all prognostic subgroups of the combination 150/2 group, as compared with monotherapy (Fig. 1B). Notably, both patients with the BRAF V600E mutation and those with the BRAF V600K mutation had significant improvement in progression-free survival.
An improvement in the other efficacy end points was observed in the combination 150/2 group, as compared with the monotherapy group: 76% versus 54% for the rate of complete or partial response (P = 0.03), and 10.5 months (95% CI, 7.4 to 14.9) versus 5.6 months (95% CI, 4.5 to 7.4) for the median duration of response. Median overall survival was not reached at the time of this analysis. The percentage of patients who were alive at 12 months was 79% in the combination 150/2 group and 70% in the monotherapy group, even though 80% of patients in the monotherapy group crossed over to the combination 150/2 group at the time of disease progression (Fig. S3 in the Supplementary Appendix).
Part B
For all patients with a BRAF V600 mutation who participated in the dose-escalation phase, efficacy end points according to dose cohort are listed in Table S4 in the Supplementary Appendix. Among the patients in part B who were treated with combination 150/2, the response rate was 63%, and 2 of 15 responses (13%) were complete radiographic responses. The median duration of response was 11.3 months, and the median progression-free survival was 10.8 months.
DISCUSSION
BRAF-targeted therapy has been established as a treatment standard for patients who have metastatic melanoma with activating BRAF mutations, on the basis of improvement in the rate of survival, as compared with conventional chemotherapy.4 However, clinical evidence of resistance appears on average 6 to 7 months after the initiation of therapy.2–4,6 Several mechanisms of MAPK-dependent resistance to BRAF inhibitors have been described in vitro and corroborated in tumor specimens obtained from patients.9,10,12,13,18 Inhibition of the MAPK pathway downstream of BRAF was hypothesized to suppress mechanisms of resistance. MEK inhibition has been validated as a therapeutic approach in the same patient population,8 providing an opportunity to investigate a regimen combining a BRAF inhibitor with a MEK inhibitor.
We showed that dabrafenib and trametinib could be safely combined when each agent was administered at its full single-agent dose. In comparison with patients receiving dabrafenib monotherapy, patients receiving combination therapy had more frequent and more severe pyrexia and chills; they also had more frequent gastrointestinal toxic effects (e.g., nausea and vomiting), but most of these events were grade 1 or 2. Pyrexia was generally manageable with antipyretic agents. However, recurrent fevers required the use of low-dose oral glucocorticoids. The definition of dose-limiting toxic effects in this protocol pertained only to toxic effects observed during the 21 days of treatment. The combination 150/2 dose was chosen on the basis of the median duration of therapy (11 months). It is possible that higher doses of either agent could be administered and will be considered in other cancers with activating BRAF mutations.
The incidence of acneiform dermatitis, the most common and dose-limiting toxic effect of trametinib, is reduced when dabrafenib and trametinib are coadministered. In a phase 3 trial,8 grade 3 or 4 acneiform dermatitis occurred in 8% of trametinib-treated patients, whereas no patient in the combination 150/2 group had grade 3 or 4 acneiform dermatitis. Proliferative skin lesions, including cutaneous squamous-cell carcinomas, papillomas, and hyperkeratosis, which are commonly seen with dabrafenib monotherapy, were less frequently observed with dabrafenib–trametinib combination therapy. In light of the evidence that BRAF and MEK inhibitors have different effects on the MAPK pathway in BRAF wild-type cells,19–21 it appears likely that trametinib attenuates dabrafenib-induced activation of the MAPK pathway. The mechanism underlying this interaction has been described in a mouse model of squamous-cell carcinoma.17
We hypothesized that progression-free survival would be an important measure of the ability of a MEK inhibitor to overcome acquired or de novo resistance to BRAF inhibition. Indeed, the combination 150/2 (full-dose) group had significantly longer progression-free survival than did the monotherapy group (hazard ratio, 0.39; 95% CI, 0.25 to 0.62; P<0.001). The percentage of patients who were alive and progression-free at 1 year was also substantially higher (41% vs. 9%, P<0.001). The extent of tumor regression was also greater in the combination 150/2 group, with an objective response rate of 76%, as compared with 54% with monotherapy (P = 0.03). In addition, the median duration of response was substantially improved with combination therapy, as compared with dabrafenib monotherapy (10.5 months vs. 5.6 months). Although we did not evaluate trametinib monotherapy in this trial, progression-free survival with trametinib in patients with BRAF V600 melanoma was similar to the outcome with dabrafenib or vemurafenib monotherapy observed in our trial and several other trials.3,4,6,8 Together, these data corroborate previous reports that resistance to BRAF-inhibitor therapy is dependent on the MAPK pathway and that the addition of a MEK inhibitor to a BRAF inhibitor represents one strategy for delaying the emergence of this resistance mechanism.
Currently, we have very little insight into the mechanisms of resistance for this combination regimen. It is critical to determine whether resistance is mediated by reactivation of the MAPK pathway or by MAPK-independent compensatory signaling pathways that have been described previously in preclinical models of melanoma with BRAF mutations. Our trial provides evidence supporting the efficacy of a combination regimen of BRAF–MEK inhibitors in advanced melanoma. Two randomized, phase 3 trials involving patients with metastatic melanoma have been initiated (ClinicalTrials.gov numbers, NCT01584648 and NCT01597908). Interpretation of the survival data may be confounded by the inclusion of a minority of patients who received immunotherapy before enrollment, and additional patients would be expected to receive such therapy on disease progression.
Despite successful development of oncogene-targeted therapy for chronic myeloid leukemia,22 gastrointestinal stromal tumor,23 and subtypes of breast cancer and non–small-cell lung cancer,24–26 it has not yet been possible to develop combination targeted therapies that circumvent acquired resistance. The combination regimen of BRAF–MEK inhibitors described here represents a successful attempt to combine targeted therapies in an oncogene-defined patient population. Furthermore, as a consequence of unique biochemical effects observed with BRAF inhibitors, this combination appears to be associated with a reduced incidence and severity of some of the toxic effects of monotherapy with either a BRAF or MEK inhibitor. We believe that the combination of dabrafenib and trametinib warrants further evaluation as a potential treatment for metastatic melanoma with BRAF V600 mutations and other cancers with these mutations.
Supplementary Material
Acknowledgments
Supported by GlaxoSmithKline.
We thank the patients and their families for their participation and the GlaxoSmithKline BRF113220 study team, including Anne-Marie Martin, Vicki Goodman, Ohad Amit, Michael Streit, Laurie Sherman, Amy Kline, Karen Davis, Jennifer Clark, and Mary Richardson, for their discussions.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600–mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 7.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 9.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS up-regulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106:20411–6. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–90. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paraiso KH, Fedorenko IV, Cantini LP, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturm OE, Orton R, Grindlay J, et al. The mammalian MAPK/ERK pathway exhibits properties of a negative feedback amplifier. Sci Signal. 2010;3:ra90. doi: 10.1126/scisignal.2001212. [DOI] [PubMed] [Google Scholar]
- 17.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, Moriceau G, Kong X, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 22.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 24.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 25.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 26.Kwak EL, Bang Y-J, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [Erratum, N Engl J Med 2011;364:588.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.