Abstract
BACKGROUND
An improvement in overall survival among patients with metastatic melanoma has been an elusive goal. In this phase 3 study, ipilimumab — which blocks cytotoxic T-lymphocyte–associated antigen 4 to potentiate an antitumor T-cell response — administered with or without a glycoprotein 100 (gp100) peptide vaccine was compared with gp100 alone in patients with previously treated metastatic melanoma.
METHODS
A total of 676 HLA-A⋆0201–positive patients with unresectable stage III or IV melanoma, whose disease had progressed while they were receiving therapy for metastatic disease, were randomly assigned, in a 3:1:1 ratio, to receive ipilimumab plus gp100 (403 patients), ipilimumab alone (137), or gp100 alone (136). Ipilimumab, at a dose of 3 mg per kilogram of body weight, was administered with or without gp100 every 3 weeks for up to four treatments (induction). Eligible patients could receive reinduction therapy. The primary end point was overall survival.
RESULTS
The median overall survival was 10.0 months among patients receiving ipilimumab plus gp100, as compared with 6.4 months among patients receiving gp100 alone (hazard ratio for death, 0.68; P<0.001). The median overall survival with ipilimumab alone was 10.1 months (hazard ratio for death in the comparison with gp100 alone, 0.66; P = 0.003). No difference in overall survival was detected between the ipilimumab groups (hazard ratio with ipilimumab plus gp100, 1.04; P = 0.76). Grade 3 or 4 immune-related adverse events occurred in 10 to 15% of patients treated with ipilimumab and in 3% treated with gp100 alone. There were 14 deaths related to the study drugs (2.1%), and 7 were associated with immune-related adverse events.
CONCLUSIONS
Ipilimumab, with or without a gp100 peptide vaccine, as compared with gp100 alone, improved overall survival in patients with previously treated metastatic melanoma. Adverse events can be severe, long-lasting, or both, but most are reversible with appropriate treatment. (Funded by Medarex and Bristol-Myers Squibb; ClinicalTrials.gov number, NCT00094653.)
The incidence of metastatic melanoma has increased over the past three decades,1,2 and the death rate continues to rise faster than the rate with most cancers.3 The World Health Organization (WHO) estimates that worldwide there are 66,000 deaths annually from skin cancer, with approximately 80% due to melanoma.4 In the United States alone, an estimated 8600 persons died from melanoma in 2009.1 The median survival of patients with melanoma who have distant metastases (American Joint Committee on Cancer stage IV) is less than 1 year.5,6 No therapy is approved beyond the first-line therapy for metastatic melanoma, and enrollment in a clinical trial is the standard of care. No therapy has been shown in a phase 3, randomized, controlled trial to improve overall survival in patients with metastatic melanoma.6-9
Regulatory pathways that limit the immune response to cancer are becoming increasingly well characterized. Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is an immune checkpoint molecule that down-regulates pathways of T-cell activation.10 Ipilimumab, a fully human monoclonal antibody (IgG1) that blocks CTLA-4 to promote antitumor immunity,11-14 has shown activity in patients with metastatic melanoma when it has been used as monotherapy in phase 2 studies.15-17 Ipilimumab has also shown activity when combined with other agents,18,19 including cancer vaccines.20,21 One well-studied cancer vaccine comprises HLA-A⋆0201–restricted peptides derived from the melanosomal protein, glycoprotein 100 (gp100). Monotherapy with this vaccine induces immune responses but has limited antitumor activity.22 However, the results of a recent study suggest that gp100 may improve the efficacy of high-dose interleukin-2 in patients with metastatic melanoma.23 With no accepted standard of care, gp100 was used as an active control for our phase 3 study, which evaluated whether ipilimumab with or without gp100 improves overall survival, as compared with gp100 alone, among patients with metastatic melanoma who had undergone previous treatment.
METHODS
PATIENTS
Patients were eligible for inclusion in the study if they had a diagnosis of unresectable stage III or IV melanoma and had received a previous therapeutic regimen containing one or more of the following: dacarbazine, temozolomide, fotemustine, carboplatin, or interleukin-2. Other inclusion criteria were age of at least 18 years; life expectancy of at least 4 months; Eastern Cooperative Oncology Group (ECOG) performance status of 0 (fully active, able to carry on all predisease performance without restriction) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, such as light housework or office work)24; positive status for HLA-A⋆0201; normal hematologic, hepatic, and renal function; and no systemic treatment in the previous 28 days. Exclusion criteria were any other cancer from which the patient had been disease-free for less than 5 years (except treated and cured basal-cell or squamous-cell skin cancer, superficial bladder cancer, or treated carcinoma in situ of the cervix, breast, or bladder); primary ocular melanoma; previous receipt of anti–CTLA-4 antibody or cancer vaccine; autoimmune disease; active, untreated metastases in the central nervous system; pregnancy or lactation; concomitant treatment with any nonstudy anticancer therapy or immunosuppressive agent; or long-term use of systemic corticosteroids.
The protocol was approved by the institutional review board at each participating institution and was conducted in accordance with the ethical principles originating from the Declaration of Helsinki and with Good Clinical Practice as defined by the International Conference on Harmonization. All patients (or their legal representatives) gave written informed consent before enrollment.
STUDY DESIGN AND TREATMENT
In this randomized, double-blind, phase 3 study, we enrolled patients at 125 centers in 13 countries in North America, South America, Europe, and Africa. Between September 2004 and August 2008, patients were randomly assigned to one of three study groups, with stratification according to baseline metastasis stage (M0, M1a, or M1b vs. M1c, classified according to the tumor–node–metastasis [TNM] categorization for melanoma of the American Joint Committee on Cancer), and receipt or nonreceipt of previous interleukin-2 therapy. The full original protocol, a list of amendments, and the final protocol, as well as the statistical analysis plan, are available with the full text of this article at NEJM.org.
Patients were randomly assigned, in a 3:1:1 ratio, to treatment with an induction course of ipilimumab, at a dose of 3 mg per kilogram of body weight, plus a gp100 peptide vaccine; ipilimumab plus gp100 placebo; or gp100 plus ipilimumab placebo — all administered once every 3 weeks for four treatments. In the vaccine groups, patients received two modified HLA-A⋆0201–restricted peptides, injected subcutaneously as an emulsion with incomplete Freund’s adjuvant (Montanide ISA-51): a gp100:209-217(210M) peptide, 1 mg injected in the right anterior thigh, and a gp100:280-288(288V) peptide, 1 mg injected in the left anterior thigh. Peptide injections were given immediately after a 90-minute intravenous infusion of ipilimumab or placebo. Treatment began on day 1 of week 1, and if there were no toxic effects that could not be tolerated, no rapidly progressive disease, and no significant decline in performance status, patients received an additional treatment during weeks 4, 7, and 10. Patients in whom new lesions developed or baseline lesions grew were allowed to receive additional treatments to complete induction. Patients with stable disease for 3 months’ duration after week 12 or a confirmed partial or complete response were offered additional courses of therapy (reinduction) with their assigned treatment regimen if they had disease progression.
The original primary end point was the best overall response rate (i.e., the proportion of patients with a partial or complete response). The primary end point was amended to overall survival (with the amendment formally approved on January 15, 2009) in the ongoing blinded study, on the basis of phase 2 data and in alignment with another ongoing phase 3 trial of ipilimumab involving patients with metastatic melanoma.25 The primary comparison in overall survival was between the ipilimumab-plus-gp100 group and the gp100-alone group. Prespecified secondary end points included a comparison of overall survival between the ipilimumab-alone and the gp100-alone groups and between the two ipilimumab groups, the best overall response rate, the duration of response, and progression-free survival. Subgroup comparisons of overall survival were performed across five prespecified categories: metastasis stage (M0, M1a, or M1b vs. M1c), receipt or nonreceipt of previous interleukin-2 therapy, baseline levels of serum lactate dehydrogenase (less than or equal to the upper limit of the normal range vs. higher than the upper limit of the normal range), age (<65 years vs. ≥65 years), and sex.
The trial was designed jointly by the senior academic authors and the sponsors, Medarex and Bristol-Myers Squibb. Data were collected by the sponsors and analyzed in collaboration with the senior academic authors, who vouch for the completeness and accuracy of the data and analyses and for the conformance of this report to the protocol, as amended. An initial draft of the manuscript was prepared by six of the academic authors in collaboration with the sponsor and a professional medical writer paid by the sponsor. All the authors contributed to subsequent drafts and made the decision to submit the manuscript for publication. All the authors signed a confidentiality disclosure agreement with the sponsor.
ASSESSMENTS
For the assessment of a patient’s eligibility, each patient’s HLA-A⋆0201 status was determined at a central laboratory. Patients who met the study criteria were assigned to receive treatment within 35 days after HLA typing and within 28 days after diagnostic imaging. Computed tomography with contrast material or magnetic resonance imaging of the brain, chest, abdomen, pelvis, and other anatomical regions, as clinically indicated, was performed. Cutaneous lesions were photographed. Tumor assessments were performed at baseline, and all patients who did not have documented early disease progression and who had stable disease or better at week 12 had confirmatory scans at weeks 16 and 24 and every 3 months thereafter. Tumor responses were determined by the investigators with the use of modified WHO criteria to evaluate bidimensionally measurable lesions.26
Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. An immune-related adverse event was defined as an adverse event that was associated with exposure to the study drug and that was consistent with an immune phenomenon. Protocol guidelines for the management of immune-related adverse events included the administration of corticosteroids (orally or intravenously), a delay in a scheduled dose, or discontinuation of therapy.15-17 Assigned doses were delayed in the case of nondermatologic immune-related adverse events of grade 2 or higher until the event improved to grade 1 or lower; if the event did not improve to grade 1 or lower, treatment was discontinued permanently. Monitoring of adverse events continued for at least 70 days after the last dose of study drugs had been administered or until any ongoing event resolved or stabilized. All patients, including those with low-grade changes in bowel frequency or stool consistency, were followed closely. A data and safety monitoring committee provided independent over-sight of safety and the risk–benefit ratio.
During the study enrollment, the following stopping rule was in place: if 10% or more of the patients in any study treatment group, evaluated cumulatively every 3 months, had a nondermatologic-related toxic adverse event of grade 3 or higher that was attributable to the investigational agents and that could not be alleviated or controlled by appropriate care or corticosteroid therapy within 14 days after the initiation of supportive care or corticosteroid therapy, assignment of patients to that study group would be suspended until the sponsor and the data and safety monitoring committee had reviewed the events and determined the appropriate course of action.
STATISTICAL ANALYSIS
The original study sample size of 750 patients was determined on the basis of the primary end point of best overall response rate but was revised with the new primary end point of overall survival. We estimated that with 385 events (deaths) among a total of 500 patients randomly assigned to the ipilimumab-plus-gp100 and the gp100-alone groups, the study would have at least 90% power to detect a difference in overall survival, at a two-sided alpha level of 0.05, with the use of a log-rank test. A total of 481 events were required in all three groups (assuming that the events were distributed in a 3:1:1 ratio in the ipilimumab-plus-gp100, ipilimumab-alone, and gp100-alone groups, respectively). Therefore, all patients who were randomly assigned in the study were to be followed until at least 481 events had occurred in the study. Enrollment was completed on July 25, 2008, when more than 650 patients had been enrolled. A post hoc power analysis showed that the 219 events observed among a total of 273 patients randomly assigned to the ipilimumab-alone and gp100-alone groups provided at least 80% power to detect a difference in overall survival between the two groups, at a two-sided alpha level of 0.05, with the assumption that ipilimumab alone has the same treatment effect as the combination regimen of ipilimumab plus gp100.
Survival was defined as the time from randomization to death from any cause, and progression-free survival as the time from randomization to documented disease progression or death. Event-time distributions were estimated with the use of the Kaplan–Meier method. Cox proportional-hazards models, stratified according to metastasis status and receipt or nonreceipt of previous interleukin therapy, were used to estimate hazard ratios and to test for significance of the timing of events. All reported P values are two-sided, and confidence intervals are at the 95% level. Survival rates were based on Kaplan–Meier estimation, and confidence intervals were calculated with the use of the bootstrap method. Descriptive statistics were used for adverse events.
RESULTS
PATIENTS AND TREATMENT
Among 676 patients enrolled in the study, 403 were randomly assigned to receive ipilimumab plus gp100, 137 to receive ipilimumab alone, and 136 to receive gp100 alone (control group) (Fig. 1 in the Supplementary Appendix, available at NEJM.org). Included among these patients were 82 patients who had metastases in the central nervous system at baseline, of whom 77 received the study drug. The baseline characteristics of the patients are shown in Table 1. Efficacy analyses were performed on the intention-to-treat population, which included all patients who had undergone randomization (676 patients). The safety population included all patients who had undergone randomization and who had received any amount of study drug (643 patients). A total of 242 of 403 patients in the ipilimumab-plus-gp100 group (60.0%), 88 of 137 in the ipilimumab-alone group (64.2%), and 78 of 136 in the gp100-alone group (57.4%) received all four ipilimumab doses or placebo infusions. The most frequent reason for discontinuation of therapy was disease progression.
Table 1. Baseline Characteristics of the Patients✫.
| Variable | Ipilimumab plus gp100 (N = 403) |
Ipilimumab Alone (N = 137) |
gp100 Alone (N = 136) |
Total (N = 676) |
|---|---|---|---|---|
| Mean age — yr | 55.6 | 56.8 | 57.4 | 56.2 |
| Sex — no. (%) | ||||
| Male | 247 (61.3) | 81 (59.1) | 73 (53.7) | 401 (59.3) |
| Female | 156 (38.7) | 56 (40.9) | 63 (46.3) | 275 (40.7) |
| ECOG performance status — no. (%)† | ||||
| 0 | 232 (57.6) | 72 (52.6) | 70 (51.5) | 374 (55.3) |
| 1 | 166 (41.2) | 64 (46.7) | 61 (44.9) | 291 (43.0) |
| 2 | 4 (1.0) | 1 (0.7) | 4 (2.9) | 9 (1.3) |
| 3 | 1 (0.2) | 0 | 0 | 1 (0.1) |
| Unknown | 0 | 0 | 1 (0.7) | 1 (0.1) |
| M stage — no. (%)‡ | ||||
| M0 | 5 (1.2) | 1 (0.7) | 4 (2.9) | 10 (1.5) |
| M1a | 37 (9.2) | 14 (10.2) | 11 (8.1) | 62 (9.2) |
| M1b | 76 (18.9) | 22 (16.1) | 23 (16.9) | 121 (17.9) |
| M1c | 285 (70.7) | 100 (73.0) | 98 (72.1) | 483 (71.4) |
| Lactate dehydrogenase level — no. (%) | ||||
| ≤Upper limit of the normal range | 252 (62.5) | 84 (61.3) | 81 (59.6) | 417 (61.7) |
| >Upper limit of the normal range | 149 (37.0) | 53 (38.7) | 52 (38.2) | 254 (37.6) |
| Unknown | 2 (0.5) | 0 | 3 (2.2) | 5 (0.7) |
| CNS metastases at baseline — no. (%) | 46 (11.4) | 15 (10.9) | 21 (15.4) | 82 (12.1) |
| Received study drug | 42 (10.4) | 15 (10.9) | 20 (14.7) | 77 (11.4) |
| Had had previous treatment for CNS metastases |
39 (9.7) | 15 (10.9) | 19 (14.0) | 73 (10.8) |
| Previous systemic therapy for metastatic disease — no. (%) |
403 (100.0) | 137 (100.0) | 136 (100.0) | 676 (100.0) |
| Previous interleukin-2 therapy — no. (%) | 89 (22.1) | 32 (23.4) | 33 (24.3) | 154 (22.8) |
Percentages may not total 100 because of rounding. CNS denotes central nervous system.
The Eastern Cooperative Oncology Group (ECOG) status ranges from 0 to 5, with higher scores indicating greater impairment (5 indicates death).
The metastasis (M) stage was classified according to the tumor–node–metastasis (TNM) categorization for melanoma of the American Joint Committee on Cancer.
EFFICACY
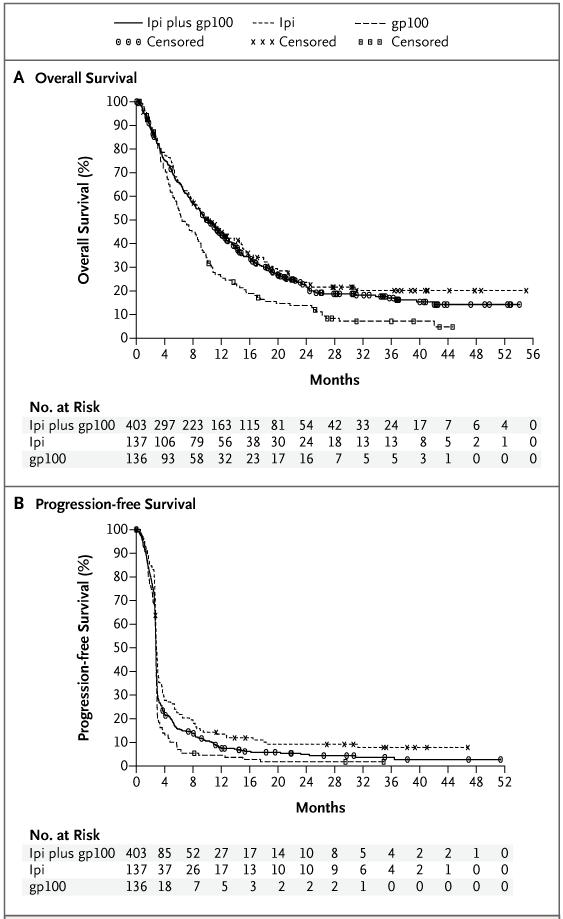
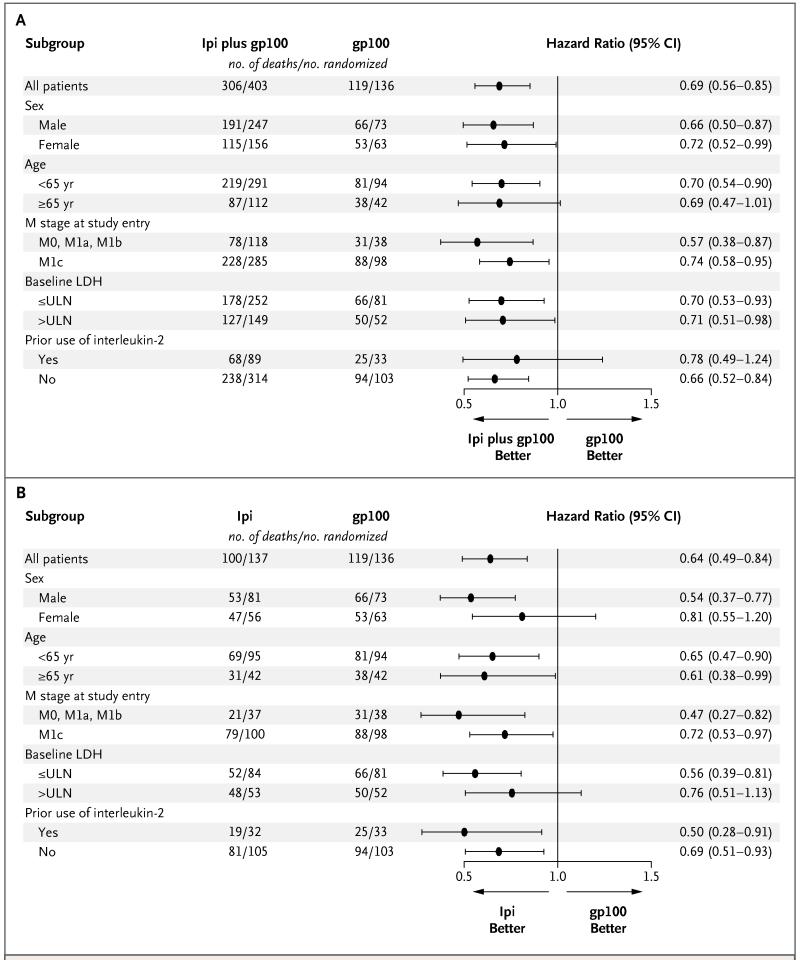
All the analyses of the efficacy end points reported here were prespecified as per protocol. Patients were followed for up to 55 months, with median follow-up times for survival of 21.0 months in the ipilimumab-plus-gp100 group, 27.8 months in the ipilimumab-alone group, and 17.2 months in the gp100-alone group. The median overall survival in the ipilimumab-plus-gp100 group was 10.0 months (95% confidence interval [CI], 8.5 to 11.5), as compared with 6.4 months (95% CI, 5.5 to 8.7) in the gp100-alone group (hazard ratio for death, 0.68; P<0.001). The median overall survival in the ipilimumab-alone group was 10.1 months (95% CI, 8.0 to 13.8) (hazard ratio for death with ipilimumab alone as compared with gp100 alone, 0.66; P=0.003). No difference in overall survival was detected between the two ipilimumab groups (hazard ratio for death with ipilimumab plus gp100, 1.04; P=0.76) (Fig. 1). Analyses of survival showed that the rates of overall survival in the ipilimumab-plus-gp100 group, the ipilimumab-alone group, and the gp100-alone group, respectively, were 43.6%, 45.6%, and 25.3% at 12 months, 30.0%, 33.2%, and 16.3% at 18 months, and 21.6%, 23.5%, and 13.7% at 24 months. The effect of ipilimumab on overall survival was independent of age, sex, baseline serum lactate dehydrogenase levels, metastasis stage of disease, and receipt or nonreceipt of previous interleukin-2 therapy (Fig. 2).
Figure 1. Kaplan–Meier Curves for Overall Survival and Progression-free Survival in the Intention-to-Treat Population.
The median follow-up for overall survival (Panel A) in the ipilimumab (Ipi)-plus-glycoprotein 100 (gp100) group was 21.0 months, and the median overall survival was 10.0 months (95% CI, 8.5 to 11.5); in the ipilimumabalone group, the median follow-up was 27.8 months, and the median overall survival, 10.1 months (95% CI, 8.0 to 13.8); and in the gp100-alone group, the median follow-up was 17.2 months, and the median overall survival, 6.4 months (95% CI, 5.5 to 8.7). The median progression-free survival (Panel B) was 2.76 months (95% CI, 2.73 to 2.79) in the ipilimumab-plus-gp100 group, 2.86 months (95% CI, 2.76 to 3.02) in the ipilimumab-alone group, and 2.76 months (95% CI, 2.73 to 2.83) in the gp100-alone group. The rates of progression-free survival at week 12 were 49.1% (95% CI, 44.1 to 53.9) in the ipilimumab-plus-gp100 group, 57.7% (95% CI, 48.9 to 65.5) in the ipilimumab-alone group, and 48.5% (95% CI, 39.6 to 56.7) in the gp100-alone group.
Figure 2. Subgroup Analyses of Overall Survival.
The prespecified analyses of overall survival among subgroups of patients, as defined by baseline demographic characteristics and stratification factors (metastasis [M] stage, classified according to the tumor–node–metastasis [TNM] categorization for melanoma of the American Joint Committee on Cancer; and receipt or nonreceipt of interleukin-2 therapy), showed that hazard ratios were lower than 1 (indicating a lower risk of death) for each subgroup in the ipilimumab (Ipi)-plus-glycoprotein 100 (gp100) group as compared with the gp100-alone group (Panel A) and for each subgroup in the ipilimumab-alone group as compared with the gp100-alone group (Panel B). Hazard ratios were estimated with the use of unstratified Cox proportional-hazards models. Horizontal lines represent 95% confidence intervals. LDH denotes lactate dehydrogenase, and ULN the upper limit of the normal range.
A 19% reduction in the risk of progression was noted with ipilimumab plus gp100, as compared with gp100 alone (hazard ratio, 0.81; P<0.05), and a 36% reduction in risk of progression was seen with ipilimumab alone as compared with gp100 alone (hazard ratio, 0.64; P<0.001). The reduction in risk with ipilimumab plus gp100 was less than that with ipilimumab alone (hazard ratio with ipilimumab plus gp100, 1.25; P = 0.04). The median values for progression-free survival were similar in all groups at the time of the first assessment of progression (week 12), after which there was a separation between the curves (Fig. 1B).
The highest percentage of patients with an objective response or stable disease was in the ipilimumab-alone group (Table 2); this group had a best overall response rate of 10.9% and a disease control rate (the proportion of patients with a partial or complete response or stable disease) of 28.5%. In the ipilimumab-alone group, 9 of 15 patients (60.0%) maintained an objective response for at least 2 years (26.5 to 44.2 months [ongoing]), and in the ipilimumab-plus-gp100 group, 4 of 23 patients (17.4%) maintained the response for at least 2 years (27.9 to 44.4 months [ongoing]). Neither of the two patients in the gp100-alone group who had a partial response maintained the response for 2 years. Responses to ipilimumab continued to improve beyond week 24: in the ipilimumab-plus-gp100 group, 3 patients with disease progression improved to stable disease, 3 with stable disease improved to a partial response, and 1 with a partial response improved to a complete response; in the ipilimumab-alone group, 2 patients with stable disease improved to a partial response and 3 with a partial response improved to a complete response. Among 31 patients given reinduction therapy with ipilimumab, a partial or complete response or stable disease was achieved by 21 (Table 2).
Table 2. Best Response to Treatment and Time-to-Event Data✫.
| Response and Time to Event | Ipilimumab plus gp100 (N = 403) |
Ipilimumab Alone (N = 137) |
gp100 Alone (N = 136) |
|---|---|---|---|
| Overall survival | |||
| Total no. of deaths | 306 | 100 | 119 |
| Comparison with gp100 alone | |||
| Hazard ratio (95% CI) | 0.68 (0.55–0.85) | 0.66 (0.51–0.87) | — |
| P value by log-rank test | <0.001 | 0.003 | — |
| Comparison with ipilimumab alone | |||
| Hazard ratio (95% CI) | 1.04 (0.83–1.30) | — | — |
| P value by log-rank test | 0.76 | — | — |
| Evaluation of therapy | |||
| Induction | |||
| Best overall response — no. (%) | |||
| Complete response | 1 (0.2) | 2 (1.5) | 0 |
| Partial response | 22 (5.5) | 13 (9.5) | 2 (1.5) |
| Stable disease | 58 (14.4) | 24 (17.5) | 13 (9.6) |
| Progressive disease | 239 (59.3) | 70 (51.1) | 89 (65.4) |
| Not evaluated | 83 (20.6) | 28 (20.4) | 32 (23.5) |
| Best overall response rate — % (95% CI) | 5.7 (3.7–8.4) | 10.9 (6.3–17.4) | 1.5 (0.2–5.2) |
| P value for comparison with gp100 alone | 0.04 | 0.001 | — |
| P value for comparison with ipilimumab alone | 0.04 | — | — |
| Disease control rate — % (95% CI)† | 20.1 (16.3–24.3) | 28.5 (21.1–36.8) | 11.0 (6.3–17.5) |
| P value for comparison with gp100 alone | 0.02 | <0.001 | — |
| P value for comparison with ipilimumab alone | 0.04 | — | — |
| Time to event — mo | |||
| Time to progression — median (95% CI) | 2.76 (2.73–2.79) | 2.86 (2.76–3.02) | 2.76 (2.73–2.83) |
| Time to response — mean (95% CI) | 3.32 (2.91–3.74) | 3.18 (2.75–3.60) | 2.74 (2.12–3.37) |
| Duration of response — median (95% CI) | 11.5 (5.4–NR) | NR (28.1–NR) | NR (2.0–NR) |
| Reinduction‡ | |||
| Best overall response — no./total no. (%) | |||
| Complete response | 0 | 1/8 (12.5) | 0 |
| Partial response | 3/23 (13.0) | 2/8 (25.0) | 0 |
| Stable disease | 12/23 (52.2) | 3/8 (37.5) | 0 |
| Progressive disease | 8/23 (34.8) | 2/8 (25.0) | 1/1 (100.0) |
Of the 143 patients who could not be evaluated for a response, 33 patients did not receive any study drug and 110 patients did not have baseline or week-12 tumor assessments (or both). Percentages may not total 100 because of rounding. NR denotes not reached.
The disease control rate is the percentage of patients with a partial or complete response or stable disease.
A total of 40 patients (29 in the ipilimumab-plus-gp100 group; 9 in the ipilimumab-alone group, and 2 in the gp100-alone group) were given reinduction therapy, but 8 were not included in the efficacy analyses: 3 had major protocol violations and 5 were not eligible owing to the fact that they had had a best overall response of progressive disease during induction and were given reinduction therapy inadvertently.
ADVERSE EVENTS
The adverse events reported in the safety population are listed in Table 3. The most common adverse events related to the study drugs were immune-related events, which occurred in approximately 60% of the patients treated with ipilimumab and 32% of the patients treated with gp100. The frequency of grade 3 or 4 immune-related adverse events was 10 to 15% in the ipilimumab groups and 3.0% in the gp100-alone group. All immune-related events occurred during the induction and reinduction periods; the immune-related adverse events most often affected the skin and gastrointestinal tract. The median time to the resolution of immune-related adverse events of grade 2, 3, or 4 was 6.3 weeks (95% CI, 4.3 to 8.4) in the ipilimumab-plus-gp100 group, 4.9 weeks (95% CI, 3.1 to 6.4) in the ipilimumab-alone group, and 3.1 weeks (95% CI, 1.1 to not reached) in the gp100-alone group.
Table 3. Adverse Events in the Safety Population✫.
| Adverse Event | Ipilimumab plus gp100 (N = 380) | Ipilimumab Alone (N = 131) | gp100 Alone (N = 132) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 | |
| number of patients (percent) | |||||||||
| Any event | 374 (98.4) | 147 (38.7) | 26 (6.8) | 127 (96.9) | 49 (37.4) | 11 (8.4) | 128 (97.0) | 54 (40.9) | 8 (6.1) |
| Any drug-related event | 338 (88.9) | 62 (16.3) | 4 (1.1) | 105 (80.2) | 25 (19.1) | 5 (3.8) | 104 (78.8) | 15 (11.4) | 0 |
| Gastrointestinal disorders | |||||||||
| Diarrhea | 146 (38.4) | 16 (4.2) | 1 (0.3) | 43 (32.8) | 7 (5.3) | 0 | 26 (19.7) | 1 (0.8) | 0 |
| Nausea | 129 (33.9) | 5 (1.3) | 1 (0.3) | 46 (35.1) | 3 (2.3) | 0 | 52 (39.4) | 3 (2.3) | 0 |
| Constipation | 81 (21.3) | 3 (0.8) | 0 | 27 (20.6) | 3 (2.3) | 0 | 34 (25.8) | 1 (0.8) | 0 |
| Vomiting | 75 (19.7) | 6 (1.6) | 1 (0.3) | 31 (23.7) | 3 (2.3) | 0 | 29 (22.0) | 3 (2.3) | 0 |
| Abdominal pain | 67 (17.6) | 6 (1.6) | 0 | 20 (15.3) | 2 (1.5) | 0 | 22 (16.7) | 6 (4.5) | 1 (0.8) |
| Other | |||||||||
| Fatigue | 137 (36.1) | 19 (5.0) | 0 | 55 (42.0) | 9 (6.9) | 0 | 41 (31.1) | 4 (3.0) | 0 |
| Decreased appetite | 88 (23.2) | 5 (1.3) | 1 (0.3) | 35 (26.7) | 2 (1.5) | 0 | 29 (22.0) | 3 (2.3) | 1 (0.8) |
| Pyrexia | 78 (20.5) | 2 (0.5) | 0 | 16 (12.2) | 0 | 0 | 23 (17.4) | 2(1.5) | 0 |
| Headache | 65 (17.1) | 4 (1.1) | 0 | 19 (14.5) | 3 (2.3) | 0 | 19 (14.4) | 3 (2.3) | 0 |
| Cough | 55 (14.5) | 1 (0.3) | 0 | 21 (16.0) | 0 | 0 | 18 (13.6) | 0 | 0 |
| Dyspnea | 46 (12.1) | 12 (3.2) | 2 (0.5) | 19 (14.5) | 4 (3.1) | 1 (0.8) | 25 (18.9) | 6 (4.5) | 0 |
| Anemia | 41 (10.8) | 11 (2.9) | 0 | 15 (11.5) | 4 (3.1) | 0 | 23 (17.4) | 11 (8.3) | 0 |
| Any immune-related event | 221 (58.2) | 37 (9.7) | 2 (0.5) | 80 (61.1) | 16 (12.2) | 3 (2.3) | 42 (31.8) | 4 (3.0) | 0 |
| Dermatologic | 152 (40.0) | 8 (2.1) | 1 (0.3) | 57 (43.5) | 2 (1.5) | 0 | 22 (16.7) | 0 | 0 |
| Pruritus | 67 (17.6) | 1 (0.3) | 0 | 32 (24.4) | 0 | 0 | 14 (10.6) | 0 | 0 |
| Rash | 67 (17.6) | 5 (1.3) | 0 | 25 (19.1) | 1 (0.8) | 0 | 6 (4.5) | 0 | 0 |
| Vitiligo | 14 (3.7) | 0 | 0 | 3 (2.3) | 0 | 0 | 1 (0.8) | 0 | 0 |
| Gastrointestinal | 122 (32.1) | 20 (5.3) | 2 (0.5) | 38 (29.0) | 10 (7.6) | 0 | 19 (14.4) | 1 (0.8) | 0 |
| Diarrhea | 115 (30.3) | 14 (3.7) | 0 | 36 (27.5) | 6 (4.6) | 0 | 18 (13.6) | 1 (0.8) | 0 |
| Colitis | 20 (5.3) | 11 (2.9) | 1 (0.3) | 10 (7.6) | 7 (5.3) | 0 | 1 (0.8) | 0 | 0 |
| Endocrine | 15 (3.9) | 4 (1.1) | 0 | 10 (7.6) | 3 (2.3) | 2 (1.5) | 2 (1.5) | 0 | 0 |
| Hypothyroidism | 6 (1.6) | 1 (0.3) | 0 | 2 (1.5) | 0 | 0 | 2 (1.5) | 0 | 0 |
| Hypopituitarism | 3 (0.8) | 2 (0.5) | 0 | 3 (2.3) | 1 (0.8) | 1 (0.8) | 0 | 0 | 0 |
| Hypophysitis | 2 (0.5) | 2 (0.5) | 0 | 2 (1.5) | 2 (1.5) | 0 | 0 | 0 | 0 |
| Adrenal insufficiency | 3 (0.8) | 2 (0.5) | 0 | 2 (1.5) | 0 | 0 | 0 | 0 | 0 |
| Increase in serum thyrotropin level | 2 (0.5) | 0 | 0 | 1 (0.8) | 0 | 0 | 0 | 0 | 0 |
| Decrease in serum corticotropin level | 0 | 0 | 0 | 2 (1.5) | 0 | 1 (0.8) | 0 | 0 | 0 |
| Hepatic | 8 (2.1) | 4 (1.1) | 0 | 5 (3.8) | 0 | 0 | 6 (4.5) | 3 (2.3) | 0 |
| Increase in alanine aminotransferase | 3 (0.8) | 2 (0.5) | 0 | 2 (1.5) | 0 | 0 | 3 (2.3) | 0 | 0 |
| Increase in aspartate aminotransferase | 4(1.1) | 1 (0.3) | 0 | 1 (0.8) | 0 | 0 | 2 (1.5) | 0 | 0 |
| Hepatitis | 2 (0.5) | 1 (0.3) | 0 | 1 (0.8) | 0 | 0 | 0 | 0 | 0 |
| Other | 12 (3.2) | 5 (1.3) | 0 | 6 (4.6) | 2 (1.5) | 1 (0.8) | 3 (2.3) | 1 (0.8) | 0 |
The adverse events listed here were reported in at least 15% of patients. The most common immune-related adverse events and those of particular clinical relevance are also listed. Patients could have more than one adverse event. Included are all patients who received at least one dose of a study drug (643 patients). A total of 14 deaths (2.2%) were determined by the investigators to be related to the study drug (8 in the ipilimumab-plus-gp100 group, 4 in the ipilimumab-alone group, and 2 in the gp100-alone group). Seven of the 14 deaths related to the study drug were associated with immune-related adverse events: 5 in the ipilimumab-plus-gp100 group (1 patient had grade 3 colitis and septicemia; 3 patients had bowel perforation–inflammatory colitis, bowel perforation, or multiorgan failure–peritonitis; and 1 patient had Guillain–Barré syndrome, which is considered to be consistent with a neurologic immune-related adverse event) and 2 in the ipilimumab-alone group (1 patient had colic bowel perforation and the other had liver failure). Deaths related to the study drug that were not associated with immune-related adverse events included deaths from sepsis, myelofibrosis, and acute respiratory distress syndrome (3 patients in the ipilimumab-plus-gp100 group); severe infection–renal failure–septic shock, and vascular leak syndrome (2 patients in the ipilimumab-alone group), and cachexia and septic shock (2 patients in the gp100-alone group).
The most common immune-related adverse event was diarrhea, which occurred at any grade in 27 to 31% of the patients in the ipilimumab groups. After the administration of corticosteroids, the median time to the resolution of diarrhea of grade 2 or higher was 2.0 weeks for 40 of 44 patients in the ipilimumab-plus-gp100 group and 2.3 weeks for 14 of 15 patients in the ipilimumab-alone group. In addition to corticosteroids, 4 patients received infliximab (anti–tumor necrosis factor α antibody) for diarrhea of grade 3 or higher or colitis. Among the 94 persons who survived for 2 years, residual effects of adverse events included those related to injection-site reactions (16 patients), vitiligo (12), diarrhea or colitis (e.g., proctocolitis with rectal pain) (4), and endocrine immune-related adverse events (e.g., inflammation of the pituitary) that required hormone-replacement therapy (8). Ongoing events in the persons who survived for 2 years included rash, pruritus, diarrhea, anorexia, and fatigue, generally of grade 1 or 2 (in 5 to 15% of the patients) and grade 3 leukocytosis (in one patient). There were 14 deaths related to the study drugs (2.1%), of which 7 were associated with immune-related adverse events.
DISCUSSION
This phase 3 study showed that ipilimumab, either alone or with gp100, improved overall survival as compared with gp100 alone in patients with metastatic melanoma who had undergone previous treatment. More than 70% of the patients had M1c disease (presence of visceral metastases) and more than 36% had elevated lactate dehydrogenase levels, both of which are associated with very poor survival.27,28 The eligibility criteria for patients in this study included HLA-A⋆0201–positive status, on the basis of the mechanism of action of gp100. However, CTLA-4 blockade by ipilimumab is independent of HLA status, as indicated by efficacy and safety outcomes in earlier clinical trials that were similar between HLA-A⋆0201–positive and HLA-A⋆0201–negative patients21 (and unpublished data).
In our study, the efficacy of ipilimumab was not improved by the addition of gp100. It is unlikely that this is due to a lack of gp100 expression in the tumors, because differentiation antigens have been shown to be strongly expressed in more than 90% of melanoma tumors, regardless of stage.29 Some studies of adjuvant therapy for melanoma showed that patients who were administered non–gp100 vaccines had shorter survival than did patients in the control groups.30,31 In contrast, phase 3 trials showed that in subgroups of patients with melanoma, vaccines had clinical activity when used as either adjuvant therapy or therapy for metastatic disease.32,33 Cumulative data show that gp100-based vaccines have immunologic activity, although clinical activity is minimal when gp100 vaccines are administered as monotherapy.22 In a randomized, phase 3 study involving patients with metastatic melanoma, a significant improvement in progression-free survival and response rate, and a nonsignificant improvement in overall survival, were seen with gp100-plus-high-dose interleukin-2, as compared with interleukin-2 alone.23 Although gp100 appeared to attenuate ipilimumab responses in our study, it is important to consider the fact that some radiographic responses of immunotherapeutic agents are not captured by standard response criteria.34 Regardless, such effects of gp100 did not translate into a difference in overall survival between the two ipilimumab groups.
The data in this study are consistent with the results of phase 2 trials of ipilimumab monotherapy in the same patient population.15-17 The data from phase 2 studies suggest that there is a long-term survival effect of ipilimumab monotherapy; ipilimumab monotherapy at a dose of 3 mg per kilogram resulted in 1-year and 2-year survival rates of 39.3% and 24.2%, respectively.16 The long-term effect of ipilimumab in our study is shown by survival analyses at late time points, which showed 1-year and 2-year survival rates of 45.6% and 23.5%, respectively. In recent, randomized, phase 3 trials involving patients with unresectable stage III or IV melanoma who had received previous treatment, 1-year survival rates were reported to be 22% to 38% with various treatment regimens.35,36 The median overall survival in these studies ranged from 5.9 to 9.7 months. Neither these nor other randomized, controlled trials had shown a significant improvement in overall survival.
The adverse-event profile of ipilimumab in this study is consistent with that reported in phase 2 trials,15-17 with the majority of adverse events being immune-related and consistent with the proposed mechanism of action of ipilimumab.11-14 As shown in phase 2 studies, prompt medical attention and early administration of corticosteroids are critical to the management of immune-related adverse events.15-17 Management guidelines (algorithms) for immune-related adverse events involve close patient follow-up and the administration of high-dose systemic corticosteroids — which were used as necessary in our study — for grade 3 or 4 events.37,38
In conclusion, this randomized, controlled trial showed that there was a significant improvement in overall survival among patients with metastatic melanoma. In some patients, side effects can be life-threatening and may be treatment-limiting. Reinduction with ipilimumab at the time of disease progression can result in further clinical benefit. Overall, our findings suggest that the T-cell potentiator ipilimumab may be useful as a treatment for patients with metastatic melanoma whose disease progressed while they were receiving one or more previous therapies.
Supplementary Material
Acknowledgments
Supported by Medarex and Bristol-Myers Squibb.
All study sites and institutions received funding from Medarex or Bristol-Myers Squibb to cover the expenses of the investigators for undertaking this trial.
Dr. Hodi reports receiving consulting fees from Bristol-Myers Squibb–Medarex, Novartis, and Genentech; Dr. O’Day, receiving consulting fees, grants, honoraria, and fees for participation in speakers’ bureaus from Bristol-Myers Squibb; Dr. McDermott, receiving consulting fees from Bristol-Myers Squibb–Medarex; Dr. Gonzalez, receiving honoraria from Bristol-Myers Squibb; Dr. Schadendorf, serving on a board for and receiving consulting fees, fees for expert testimony, and fees for participation in speakers’ bureaus from Bristol-Myers Squibb; Dr. van den Eertwegh, receiving consulting fees from and serving on a board for Bristol-Myers Squibb; Dr. Lutzky, receiving consulting fees and honoraria from Bristol-Myers Squibb; Dr. Lorigan, receiving consulting fees from Bristol-Myers Squibb; Dr. Hogg, serving on a board for Bristol-Myers Squibb (pending); Dr. Ottensmeier, receiving honoraria and grant funding from Bristol-Myers Squibb; Dr. Lebbé, serving on a board for Bristol-Myers Squibb; Dr. Wolchok, serving on a board for Bristol-Myers Squibb; Dr. J.S. Weber, receiving consulting fees from Bristol-Myers Squibb; Drs. Tian, Yellin, and Nichol being former employees of Medarex; Dr. Hoos being currently employed by Bristol-Myers Squibb with stock or stock options; and Dr. Urba, receiving consulting fees from Bristol-Myers Squibb–Medarex. No other potential conflicts of interest relevant to this article were reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org
We thank the patients who volunteered to participate in this study and staff members at the study sites who cared for them; the members of the data and safety monitoring committee; and representatives of the sponsors who were involved in data collection and analyses (in particular, Tai-Tsang Chen, Xiaoping Zhu, Marianne Messina, and Helena Brett-Smith). Editorial and writing assistance was provided by Ward A. Pedersen of StemScientific, funded by Bristol-Myers Squibb.
APPENDIX
The authors’ affiliations are as follows: The Dana-Farber Cancer Institute (F.S.H.) and Beth Israel Deaconess Medical Center (D.F.M.) — both in Boston; the Angeles Clinic and Research Institute, Los Angeles (S.J.O.); St. Mary’s Medical Center, San Francisco (R.W.W.); Vanderbilt University Medical Center, Nashville (J.A.S.); Netherlands Cancer Institute (J.B.H.) and VU University Medical Center (A.J.M.E.) — both in Amsterdam; University of Colorado Cancer Center, Aurora (R.G.); Institut Gustave Roussy, Villejuif, France (C.R.); University Hospital Essen, Essen (D.S., J.M.V.), German Cancer Research Center, University of Mannheim, Mannheim (J.C.H.), and Technical University Munich, Munich (C.P.) — all in Germany; Huntsman Cancer Institute, Salt Lake City (W.A.); Mount Sinai Comprehensive Cancer Center, Miami (J.L.); Christie Hospital NHS Trust, Manchester (P.L.), and Southampton University Hospitals, Southampton (C.H.O.) — both in the United Kingdom; Washington University School of Medicine, St. Louis (G.P.L.); Princess Margaret Hospital, Toronto (D.H., I.Q.); Saint Louis Hospital, Paris (C.L.); Loyola University Medical Center, Maywood, IL (J.I.C.); Memorial Sloan-Kettering Cancer Center, New York (J.D.W.); H. Lee Moffitt Cancer Center, Tampa, FL (J.S.W.); Medarex, Bloomsbury, NJ (J.T., M.J.Y., G.M.N.); Bristol-Myers Squibb, Wallingford, CT (A.H.); and the Earle A. Chiles Research Institute, Portland, OR (W.J.U.).
In addition to the authors, the following investigators (listed by country in alphabetical order) participated in the study: Argentina: M. Chacón, L. Koliren, G.L. Lerzo, R.L. Santos — all in Buenos Aires; Belgium: A. Awada (Brussels), V. Cocquyt (Ghent), J. Kerger (Yvoir), J. Thomas (Leuven), T. Velu (Brussels); Brazil: C. Barrios (Porto Alegre), C. Dzik (São Paulo), M. Federico (São Paulo), J. Hohmann (Barretos), M. Liberrati (Londrina), A. Lima (Santo André), G. Schwartsmann (Porto Alegre), J. Segalla (Jaú); Canada: T. Baetz (Kingston, ON), T. Cheng (Calgary, AB), W. Miller (Montreal), S. Rorke (St. John’s, NL), S. Verma (Ottawa), R. Wong (Winnipeg, MB); Chile: H. Harbst (Santiago), P. Gonzalez-Mella (Viña del Mar), P. Salman (Santiago); France: F. Cambazard (Saint-Etienne), O. Dereure (Montpellier), B. Dreno (Nantes), L. Geoffrois (Vandoeuvre-lès-Nancy), J-J. Grob (Marseille), T. Lesimple (Dunkerque), S. Négrier (Lyon), N. Penel (Lille), A. Thyss (Nice); Germany: J.C. Becker (Würzburg), C. Garbe (Tübingen), S. Grabbe (Molnz), U. Keilholz (Berlin), C. Loquai (Mainz), H. Naeher (Heidelberg), G. Shuler (Erlangen), U. Trefzer (Berlin), J. Welzel (Augsburg); Hungary: Z. Karolyi (Miskolc); Netherlands: R.L.H. Jansen (Maastricht); South Africa: G.L. Cohen (Pretoria), J.I. Raats (Panorama), D.A. Vorobiof (Morningside); Switzerland: R. Dummer (Zurich), O. Michielin (Lausanne); United Kingdom: J. Barber (Cardiff), S. Danson (Sheffield), M. Gore (London), S. Houston (Surrey), C.G. Kelly (Newcastle-upon-Tyne), M. Middleton (Oxford), P.M. Patel (Nottingham), E. Rankin (Dundee, Scotland); United States: M. Adler (Vista, CA), T. Amatruda (Robbinsdale, MN), A. Amin (Charlotte, NC), C. Anderson (Columbia, MO), L. Blakely (Memphis, TN), E. Borden (Cleveland), S. Burdette-Radoux (Burlington, VT), R. Chapman (Detroit), J. Chesney (Louisville, KY), A. Cohn (Denver), F.A. Collichio (Chapel Hill, NC), G. Daniels (La Jolla, CA), J. Drabick (Hershey, PA), J.A. Figueroa (Lubbock, TX), J. Fleagle (Boulder, CO), J. Goydos (New Brunswick, NJ), N. Haas (Philadelphia), E. Hersh (Tucson, AZ), H.L. Kaufman (New York), K.D. Khan (Indianapolis), A. Khurshid (Arlington, TX), J.M. Kirkwood (Pittsburgh), J.J. Kirshner (East Syracuse, NY), H. Kluger (New Haven, CT), D. Lawrence (Boston), D. Lawson (Atlanta), P.D. Leming (Cincinnati), K. Margolin (Seattle), M. Mastrangelo (Philadelphia), B. Mirtsching (Dallas), W. Paroly (San Diego, CA), A.L. Pecora (Hackensack, NJ), D. Pham (Jacksonville, FL), R. Rangineni (St. Joseph, MO), N. Rothschild (West Palm Beach, FL), W.E. Samlowski (Las Vegas), D. Schwartzentruber (Goshen, IN), M. Scola (Morristown, NJ), W.H. Sharfman (Lutherville, MD), J.J. Stephenson (Greenville, SC), N.S. Tchekmedyian (Long Beach, CA), J. Wade (Decatur, IL), M. Wax (Berkeley Heights, NJ), A. Weeks (Collierville, TN), J.L. Zapas (Baltimore).
Footnotes
Drs. Hodi and O’Day contributed equally to this article.
REFERENCES
- 1.American Cancer Society [Accessed June 4, 2010];Cancer facts & figures 2009. at http://www.cancer.org/downloads/STT/500809web.pdf.
- 2.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 3.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–85. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization [Accessed June 4, 2010];Skin cancers. at http://www.who.int/uv/faq/skincancer/en/index1.html. [Google Scholar]
- 5.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. Erratum, N Engl J Med 2004;351:2461. [DOI] [PubMed] [Google Scholar]
- 6.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–95. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM, Kirkwood JM. Reevaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–36. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev. 2007;33:484–96. doi: 10.1016/j.ctrv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Trinh VA. Current management of metastatic melanoma. Am J Health Syst Pharm. 2008;65(Suppl 9):S3–S8. doi: 10.2146/ajhp080460. [DOI] [PubMed] [Google Scholar]
- 10.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 11.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–27. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 12.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14:848–61. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- 14.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–30. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 16.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 17.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with previously treated, advanced melanoma: a multicenter, single-arm phase II study. Ann Oncol. 2010 Feb 10; doi: 10.1093/annonc/mdq013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte–associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwala SS. Novel immunotherapies as potential therapeutic partners for traditional or targeted agents: cytotoxic T-lymphocyte antigen-4 blockade in advanced melanoma. Melanoma Res. 2010;20:1–10. doi: 10.1097/CMR.0b013e328333bbc8. [DOI] [PubMed] [Google Scholar]
- 20.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti–cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartzentruber DJ, Lawson D, Richards J, et al. A phase III multi-institutional randomized study of immunization with the gp100:209–217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27(Suppl):463s. abstract. [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 25. [Accessed June 4, 2010];Dacarbazine and ipilimumab vs. dacarbazine with placebo in untreated unresectable stage III or IV melanoma. ClinicalTrials.gov. at http://www.clinicaltrials.gov/ct/show/NCT00324155.
- 26.James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523–8. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 27.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 28.Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26:624–33. doi: 10.1080/07357900802027073. [DOI] [PubMed] [Google Scholar]
- 29.Barrow C, Browning J, MacGregor D, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–71. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 30.Morton DL, Mozzillo N, Thompson JF, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol. 2007;25(Suppl):474s. abstract. [Google Scholar]
- 31.Eggermont AM, Suciu S, Ruka W, et al. EORTC 18961: post-operative adjuvant ganglioside GM2-KLH21 vaccination treatment vs observation in stage II (T3-T4N0M0) melanoma: 2nd interim analysis led to an early disclosure of the results. J Clin Oncol. 2008;26(Suppl):484s. abstract. [Google Scholar]
- 32.Testori A, Richards J, Whitman E, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma. J Clin Oncol. 2008;26:955–62. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 33.Sosman JA, Unger JM, Liu PY, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: impact of HLA class I antigen expression on outcome. J Clin Oncol. 2002;20:2067–75. doi: 10.1200/JCO.2002.08.072. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 35.Eisen T, Trefzer U, Hamilton A, et al. Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer. 2010;116:146–54. doi: 10.1002/cncr.24686. [DOI] [PubMed] [Google Scholar]
- 36.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 37.Weber J. Anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–72. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 38.Lin R, Yellin MJ, Lowy I, Safferman A, Chin K, Ibrahim R. An analysis of the effectiveness of specific guidelines for the management of ipilimumab-mediated diarrhea/colitis: prevention of gastrointestinal perforation and/or colectomy. J Clin Oncol. 2008;26(Suppl):497s. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.