Abstract
Purpose
To compare the efficacy and tolerability of the mitogen-activated protein (MAP)/extracellular signal-regulated (ERK) kinase (MEK) 1/2 inhibitor selumetinib versus temozolomide in chemotherapy-naive patients with unresectable stage III/IV melanoma.
Experimental Design
This phase II, open-label, multicenter, randomized, parallel-group study examined the effect of 100 mg oral selumetinib twice daily in 28-day cycles versus oral temozolomide (200 mg/m2/d for 5 days, then 23 days off-treatment). The primary endpoint was progression-free survival.
Results
Two hundred patients were randomized. Progression-free survival did not differ significantly between selumetinib and temozolomide (median time to event 78 and 80 days, respectively; hazard ratio, 1.07; 80% confidence interval, 0.86–1.32). Objective response was observed in six (5.8%) patients receiving selumetinib and nine (9.4%) patients in the temozolomide group. Among patients with BRAF mutations, objective responses were similar between selumetinib and temozolomide groups (11.1% and 10.7%, respectively). However, five of the six selumetinib partial responders were BRAF mutated. Frequently reported adverse events with selumetinib were dermatitis acneiform (papular pustular rash; 59.6%), diarrhea (56.6%), nausea (50.5%), and peripheral edema (40.4%), whereas nausea (64.2%), constipation (47.4%), and vomiting (44.2%) were reported with temozolomide.
Conclusions
No significant difference in progression-free survival was observed between patients with unresectable stage III/IV melanoma unselected for BRAF/NRAS mutations, who received therapy with selumetinib or temozolomide. Five of six patients with partial response to selumetinib had BRAF mutant tumors.
Introduction
The Ras/Raf/MEK/ERK pathway is a key signaling cascade driving cell-cycle proliferation, differentiation, and survival (1, 2). Mutations affecting signaling molecules, including Ras and Raf, activate this pathway and contribute to malignant progression in many human cancers (3-6). BRAF and NRAS mutations are generally mutually exclusive in melanoma (7, 8). At the time of study initiation, the mutation frequencies for BRAF and NRAS were estimated as 59% (9) and 30% (10), respectively. However, recent estimates suggest that the frequency for BRAF mutations may be as low as 41% (11).
Agents targeting mutated BRAF are in development (12, 13); however, they may be associated with paradoxical activation of the mitogen-activated protein (MAP) kinase pathway in BRAF wild-type cells (14). MAP/extracellular signal-regulated (ERK) kinase (MEK) 1/2 is an attractive therapeutic target due to its key position within the Ras/Raf/MEK/ERK pathway (2, 15) and paradoxical activation effects are not expected with MEK inhibitors. Selumetinib (AZD6244/ARRY-142886) is an orally available, potent, selective, allosteric inhibitor of MEK1/2 with preclinical antitumor activity in melanoma (16), which has been shown to inhibit the growth of cell lines expressing BRAF V600E mutation (17, 18).
In a phase I trial of selumetinib including patients with melanoma (20 of 57 patients, 35%), prolonged stable disease (SD) of 5 or more months was observed in 9 patients (16%; ref. 19). The maximum tolerated dose of selumetinib was determined as 200 mg twice a day; however, the dose chosen for ongoing phase II studies was 100 mg twice a day due to the frequency of treatment-related rash with chronic administration. Consistent inhibition of ERK phosphorylation shown between pre- and posttreatment biopsies showed that this dose results in target inhibition. This study also showed that selumetinib 100 mg twice a day was considered to have a manageable toxicity profile.
The current study compared the efficacy of orally administered selumetinib and temozolomide in patients with chemotherapy-naive advanced melanoma. It is the first multicenter randomized study conducted in patients with melanoma assessed for both BRAF and NRAS mutations. At the time of initiation, there was no global standard of care for patients with chemotherapy-naive advanced melanoma; therefore, temozolomide was chosen as comparator for this study because it had been used in both clinical trials and was licensed for this indication in some countries (20). In addition, temozolomide has the same active metabolite [5-(3-methyl-1-triazeno)imidazole-4-carboxamide] as dacarbazine, an approved treatment for advanced melanoma, but temozolomide has the benefit of being administered orally. The dose of temozolomide (200 mg/m2/d for 5 days, followed by 23 days off-treatment) used in the present study is the recommended monotherapy dose (21) which was used in a large phase III study in patients with advanced melanoma (22).
Material and Methods
This phase II, open-label, multicenter, randomized, parallel-group study (clinicaltrials.gov registry number NCT00338130) enrolled patients without previous systemic chemotherapy for advanced melanoma between July 2006 and June 2007 and was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki. Thirty-four centers from 10 countries participated in this trial: Argentina (3 centers), Australia (3), Austria (2), Brazil (6), Canada (2), Denmark (1), France (3), Switzerland (1), the United Kingdom (3), and United States (10).
Patient selection
All included patients provided written informed consent and fulfilled the following criteria: age ≥18 years, histologic or cytologic confirmation of unresectable American Joint Committee on Cancer (AJCC) stage III or IV malignant melanoma, at least one measurable site of disease defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0, World Health Organization (WHO) performance status 0–2, and willingness to provide tumor biopsy (fresh or archival) for determination of BRAF and NRAS mutation status. Female patients were required to have a negative pregnancy test or be postmenopausal. To be representative of the general melanoma population, the number of patients with uveal melanoma was limited to 20 of the planned 182.
Exclusion criteria included absolute neutrophil count <1,500/mm3, platelets <100,000/mm3, hemoglobin ≤9 g/dL, serum creatinine clearance ≤30 mL/min, serum bilirubin ≥1.5 × upper limit of normal (ULN), aspartate aminotransferase ≥2.5 × ULN, alanine aminotransferase ≥2.5 × ULN, or serum creatinine ≥1.5 mg/dL, chemotherapy or radiotherapy within 5 years prior to start of study treatment (excluding palliative radiotherapy at focal sites), any systemic chemotherapy for unresectable AJCC stage III or IV melanoma, prior combination biochemotherapy for cancer, unstable brain metastasis or spinal cord compression (<3 months off steroids), and history of another primary malignancy within 5 years prior to start of study treatment (except for adequately treated basal or squamous cell skin cancer or cancer of the cervix in situ).
Study design
Patients were randomized 1:1 to selumetinib (100 mg free-base solution, twice daily in 28-day cycles) or temozolomide (200 mg/m2/d for 5 days, followed by 23 days off treatment). Assessment by RECIST criteria was conducted at weeks 6 and 12 and then every 8 weeks for progression-free survival (PFS). Local center tumor assessment was used for the primary analysis, with conclusions validated by an independent central review of scans/images. Patients could continue study treatment until objective disease progression (defined by local investigator) and were then followed for survival. Patients with progressive disease (PD), as assessed by the investigator in the temozolomide group, were permitted to crossover to selumetinib.
Data cutoff point was September 28, 2007, for PFS and objective tumor response. All other analyses, including time-to-death (TTD) used a date of June 20, 2008.
Study objectives
The primary objective was to compare the efficacy of selumetinib versus temozolomide in patients with unresectable stage III or IV malignant melanoma. The primary outcome was PFS. Secondary outcomes were TTD, objective response rate (ORR), and duration of response.
PFS, defined as the interval between the date of randomization and the first date of objective disease progression (RECIST 1.0) or death due to any cause, was to be analyzed following approximately 126 progression events. Nonprogressing patients were censored at last objective tumor assessment.
TTD was calculated from randomization until death due to any cause; surviving patients were censored at last date known to be alive, or withdrawal of consent.
Best objective response [OR; complete response (CR), partial response (PR), SD ≥ 6 weeks] or PD was calculated as the best response, using RECIST 1.0, recorded from date of randomization.
Secondary objectives included assessment of safety, tolerability, and efficacy of selumetinib versus temozolomide with respect to BRAF or NRAS mutation status.
Exploratory analyses included assessment of the treatment effect in the following subgroups; disease stage (stage III vs. stage IV), uveal melanoma versus non-uveal, mucosal melanoma versus non-mucosal, and BRAF and NRAS mutation status.
Assessment of safety
Adverse events, serious AEs (SAE), clinical laboratory evaluations, vital signs, and electrocardiograms were collected from provision of informed consent until 30 days after discontinuation of study treatment. Adverse events were collected using Common Terminology Criteria for Adverse Events (CTCAE) version 3.
Determination of BRAF and NRAS mutation status
Pathology review confirmed the presence of tumor; no enrichment by macrodissection was conducted prior to DNA extraction, given the high sensitivity of the allele-specific PCR-based method ARMS (Amplification Refractory Mutation System). The methods used for mutation detection including sequences of primers and probes for detection of BRAF and NRAS mutations have been previously described (23). The allele-specific PCR detects BRAF V600E as well as V600K and V600D (1799T>A) and the allele-specific PCR for NRAS detects NRAS Q61K mutation (C181A) and the Q61R mutation (A182G). Mutation testing was carried out centrally within the AstraZeneca Tumor Genetics Research Laboratory.
In brief, genomic DNA was extracted from thin sections totaling 40 μm by digestion in proteinase K for 48 hours, boiling in 5% chelex, phase-extracting in chloroform, ethanol-precipitating, and resuspending in 100 μL water. PCR was carried out as described previously, using cell line admixtures containing the mutation of interests to act as positive controls (23).
A mutation-positive result was only accepted if it was present in two independent PCRs generated from the same DNA sample. Normal genomic DNA was used as a negative control. Results were not designated positive unless the mutation was detected at a level above the nonspecific background noise. This was done to control for false-positive results. A “negative” result from an assay could represent no mutation present; if there was insufficient DNA extracted from the sample to identify the presence of the mutation, it was designated a fail.
Determination of GNAQ mutation status
GNAQ analysis was conducted in uveal melanoma tumors only. Codon 209 was analyzed by ARMS and direct sequencing. The same forward primer was used for both sequencing and ARMS: 5′-ACTGTAAAACGACGGCCAGTTTTTCCCTAAGTTTGTAAGTAGTGCT-3′. Reverse primers were used as follows: GNAQ sequencing reverse, 5′-ACCAGGAAACAGCTATGACCGTCTGACTCCACGAGAACTTGAT- 3′; GNAQ 209P ARMS reverse, 5′-AGTGTATCCATTZTCTTCTCTCTGACCTTP-3′; GNAQ 209L ARMS reverse, 5′-AGTGTATCCATTZTCTTCTCTCTGACCTTE-3′ [L = LNA (locked nucleic acid) modified C, P = LNA G, Z = LNA T].
Statistical and analytic methods
A sample size of 182 patients (91 per arm) was required to provide adequate power for comparison of PFS between the two treatment groups both in the overall population and in the BRAF mutant subpopulation (24). Patients with BRAF mutant tumors (assumed to be 60% of the total; ref. 9) were hypothesized to show a greater response to selumetinib than those with wild-type tumors. The target PFS HR in the BRAF mutant subgroup was 0.67 (and was to be tested as a secondary endpoint at the one-sided significance level of 20%).
To maintain statistical power for the overall population, the target HR on which the study was powered was 0.74 for PFS (i.e., a 35% delay in median time to progression assuming exponential distribution). This assumed that the BRAF wild-type subpopulation contained NRAS mutant patients (~30%), who might derive clinical benefit.
This study was designed to have 80% power to detect a true PFS HR of 0.74 in the overall population at the one-sided 20% significance level and required approximately 126 progression events. The trial was designed as a randomized screening trial to quantify the level of risk entailed for further development, and as such, the type I and type II errors were adjusted to be less constrained, so that the targeted treatment benefit may be appropriate, whereas the sample size remains reasonable (as discussed by Rubinstein and colleagues; ref. 25).
PFS and TTD were analyzed using a Cox proportional hazards model allowing for the effect of treatment and fitting for the following baseline covariates: BRAF mutation status (positive vs. negative vs. unknown), WHO performance status (0 vs. 1 or 2), tumor type [non-uveal (cutaneous, mucosal, unknown) vs. uveal], and level of lactate dehydrogenase (<2 × ULN vs. ≥2 × ULN) at baseline. These covariates were thought to be potentially prognostic, and thus the impact of these covariates is adjusted for in the statistical analyses to improve the precision of the estimated treatment effect as well as compensating for any lack of balance between groups for these baseline covariates. The model included these effects regardless of whether the inclusion of effects significantly improved the fit of the model. These analyses were prespecified in the Statistical Analysis Plan. Tumor stage (III vs. IV) was prespecified as a covariate but not included in the model as only 6 patients had an unresectable AJCC stage III tumor.
Intention-to-treat (ITT) analysis was used for all efficacy analyses. The ITT population included all randomized patients and compared the treatment groups on the basis of randomized treatment, regardless of the treatment actually received. The evaluable-for-safety population was a subset of the ITT population that included all patients who received ≥1 dose of study treatment and was used for summaries of the safety data.
Results
Demographics and other patient characteristics
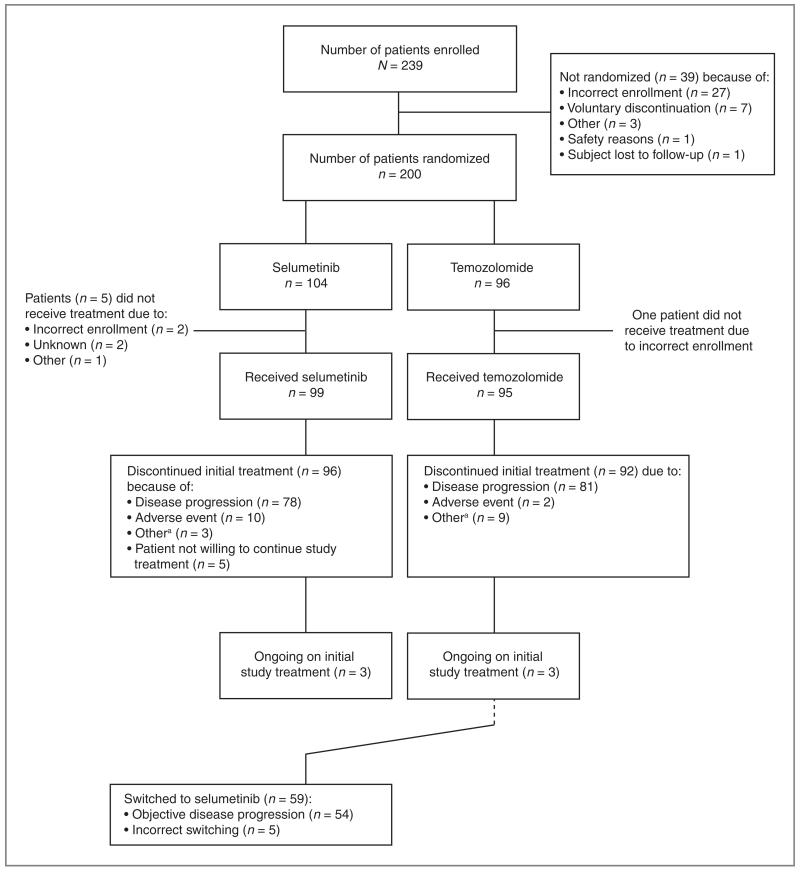
Two hundred thirty-nine patients were enrolled, of whom 200 were randomized: 104 to selumetinib and 96 to temozolomide (Fig. 1). Of these patients, 99 and 95 received selumetinib and temozolomide, respectively. Ninety-six and 92 patients, respectively, discontinued assigned treatment with selumetinib or temozolomide. Three patients in each arm were continuing assigned treatment at the time of data cutoff. Fifty-nine patients in the temozolomide arm switched to selumetinib. In total, 158 randomized patients (79.0%) had their BRAF and NRAS mutation status confirmed. BRAF mutant tumors were identified in 73 of 158 patients (46.2%; V600E, 66; V600K, 5; K601E, 1; K581S, 1); 28 of 158 patients’ tumors (17.7%) were NRAS mutated (Q61K, 15; Q61R, 12; G13R, 1). No tumors were both BRAF and NRAS mutated; therefore, 101 of 158 patients’ tumors (63.9%) were either BRAF mutant or NRAS mutant. Of the 42 patients without confirmed mutation status, 24 did not have samples to analyze and 18 had no result due to assay failure.
Figure 1.
Patient disposition.
The study population was representative of the advanced melanoma clinical population in terms of baseline and demographic characteristics; however, there were some imbalances between the treatment arms (Table 1). There was a higher percentage of women in the selumetinib group (47.1%) than in the temozolomide group (32.3%). In addition, more patients were BRAF mutant in the selumetinib group (43.3%) than in the temozolomide group (29.2%), and more patients in the selumetinib group had WHO performance status 1 or 2 than those receiving temozolomide (33.7% and 26.1%, respectively).
Table 1.
Patient demographics and baseline characteristics
| Treatment group, n (%) |
||
|---|---|---|
| Selumetinib (n = 104) |
Temozolomide (n = 96) |
|
| Sex | ||
| Male | 55 (52.9) | 65 (67.7) |
| Female | 49 (47.1) | 31 (32.3) |
| Age, y | ||
| Mean (range) | 57.1 (20–84) | 57.0 (28–84) |
| Racial origin | ||
| Caucasian | 99 (95.2) | 91 (94.8) |
| Non-Caucasiana | 3 (2.9) | 3 (3.0) |
| Unknown | 2 (1.9) | 2 (2.1) |
| WHO performance status | ||
| 0 Normal activity | 67 (64.4) | 71 (74.0) |
| 1 restricted activity | 34 (32.7) | 23 (24.0) |
| 2 in-bed ≤ 50% of the time | 1 (1.0) | 2 (2.1) |
| Unknown | 2 (1.9) | 0 (0) |
| AJCC staging | ||
| Stage III | 3 (2.9) | 3 (3.1) |
| Stage IV | 99 (95.2) | 92 (95.8) |
| M1a/b | 40 (38.5) | 36 (37.5) |
| M1c | 58 (55.8) | 54 (56.3) |
| Unknown M status | 1 (1) | 2 (2.1) |
| Unknown stage | 2 (1.9) | 1 (1.0) |
| Lactate dehydrogenase level at baseline | ||
| <2 × ULN | 79 (76.0) | 79 (82.3) |
| ≥2 × ULN | 17 (16.3) | 15 (15.6) |
| Unknown | 8 (7.7) | 2 (2.1) |
| Tumor type | ||
| Cutaneous | 75 (72.1) | 72 (75.0) |
| Uveal | 7 (6.7) | 13 (13.5) |
| Mucosal | 6 (5.8) | 0 (0.0) |
| Unknown primary tumor | 16 (15.4) | 11 (11.5) |
| Mutation status | ||
| BRAF positive | 45 (43.3) | 28 (29.2) |
| NRAS positive | 10 (9.6) | 18 (18.8) |
| Wild-type for both | 29 (27.9) | 28 (29.2) |
| Unknownb | 20 (19.2) | 22 (22.9) |
This group comprised Black, Hispanic, and Mediterranean patients.
This group comprised those patients where there was no result for both BRAF and NRAS mutation status or one of the mutation assays failed.
In the BRAF and NRAS mutant subpopulation, more patients had WHO performance status 1 or 2 in the selumetinib group (38.2%) than in the temozolomide group (23.9%). Furthermore, more BRAF or NRAS mutant patients in the selumetinib group (12.7%) had lactate dehydrogenase levels ≥2 × ULN than those in the temozolomide group (8.7%). The analyses adjusted for these factors.
Twenty (10%) patients in the study had uveal melanoma, with 15 evaluable for BRAF/NRAS mutations; no mutations in BRAF or NRAS were detected. Twelve patients with uveal melanoma had tumors with sufficient material evaluable for GNAQ mutation: 4 tumors were GNAQ mutated (3 GNAQ 209P, 1 GNAQ 209L), 8 tumors were GNAQ wild-type.
Efficacy
Progression-free survival based on investigator-assessed RECIST data
The PFS analysis was conducted after 151 progression events. No difference in PFS was observed between selumetinib and temozolomide [HR, 1.07; 80% confidence interval (CI), 0.86–1.32; 1-sided P = 0.650; 2-sided P = 0.699; Fig. 2A). In prespecified analyses, PFS was consistent across subgroups (data not shown) except for patients with uveal melanoma (HR, 0.70; 80% CI, 0.35–1.42) but no significant difference could be concluded for patients with uveal melanoma due to the small number of patients (16 events/20 patients) and wide CI. Overall, 79 (76%) patients in the selumetinib group and 72 (75%) patients randomized to temozolomide had objective disease progression or had died at the data cutoff point, with median time to event of 78 and 80 days, respectively.
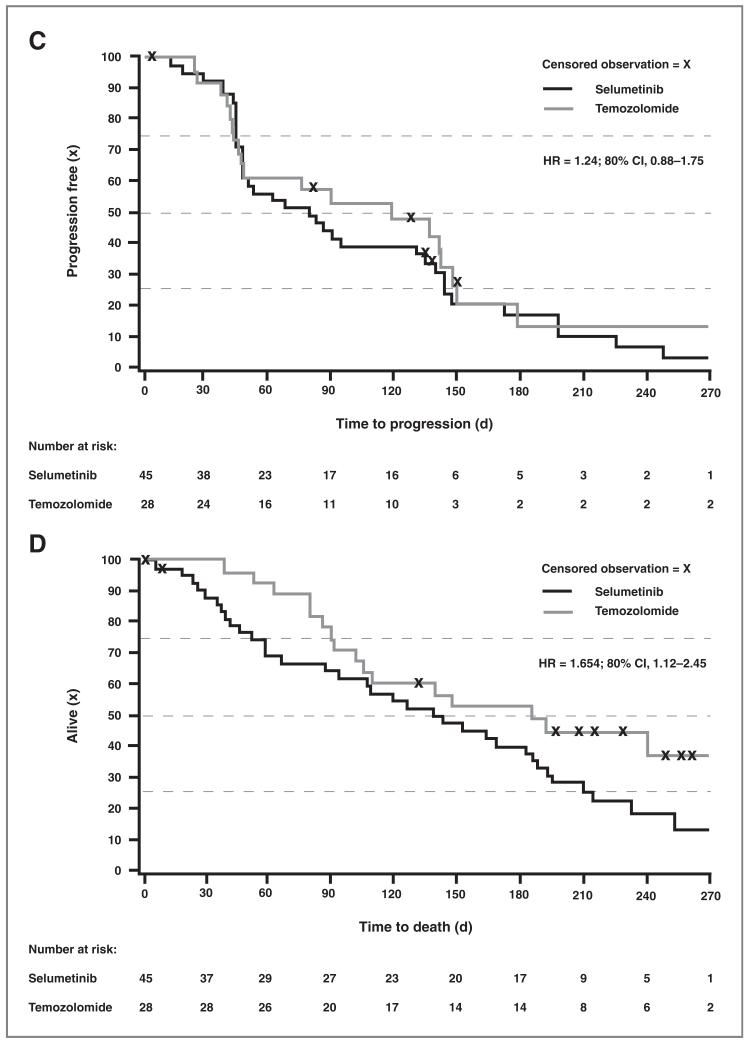
Figure 2.
Comparison of PFS (A) and (C) and of TTD (B) and (D) between selumetinib and temozolomide in the overall population (A) and (C) and BRAF mutant patients (B) and (D). A, Kaplan–Meier comparison of PFS between selumetinib and temozolomide in the overall population (ITT population). B, Kaplan–Meier comparison of TTD between selumetinib and temozolomide in the overall population (ITT population). C, Kaplan-Meier comparison of PFS between selumetinib and temozolomide in the BRAF mutant subpopulation (ITT population). D, Kaplan–Meier comparison of TTD between selumetinib and temozolomide in the BRAF mutant subpopulation (ITT population).
Because of open-label nature of the study, an independent central review was incorporated to assess consistency and ensure that conclusions were robust. Discordance between the disease status assessment in local and central review was noted, with local review more favorable for selumetinib (Supplementary Table S1; ref. 26). In 20% of cases, the discrepancy was due to different assessments of percentage change of target lesions alone and 31% were due to the identification of one or more new lesions alone (Supplementary Table S2) either by the local review or by central review. This discordance did not change the conclusions of this study.
Time to death
The final analysis of TTD was conducted after 130 deaths had occurred. The median TTD was 284 and 369 days for selumetinib and temozolomide groups, respectively (HR, 1.351; 80% CI, 1.07–1.71; 95% CI, 0.95–1.93; 1-sided P = 0.950; 2-sided P = 0.099; Table 2), suggesting improved but not statistically significant TTD for temozolomide compared with selumetinib in the overall population (Fig. 2B). A higher proportion of patients randomized to temozolomide received selumetinib following disease progression (61%) compared with those who received temozolomide or dacarbazine following progression on selumetinib (≥24%). The frequency of crossover from selumetinib to temozolomide may be an underestimation as this information was not mandatorily gathered as part of the study protocol.
Table 2.
Summary of TTD analysis for selumetinib versus temozolomide in the overall population and in BRAF and NRAS mutant patients
| Number of patients |
Number of deaths (%) |
Median time to event (d) |
HRa | CI |
P
|
|||
|---|---|---|---|---|---|---|---|---|
| Two-sided, 80% |
Two-sided, 95% |
One-sidedb | Two-sided | |||||
| Overall populationc | ||||||||
| Selumetinib | 104 | 73 (70.2) | 284 | 1.351 | 1.07, 1.71 | 0.95, 1.93 | 0.950 | 0.099 |
| Temozolomide | 96 | 57 (59.4) | 369 | |||||
| BRAF mutant subpopulationd | ||||||||
| Selumetinib | 45 | 34 (75.6) | 284 | 1.654 | 1.12, 2.45 | 0.91, 3.02 | 0.949 | 0.102 |
| Temozolomide | 28 | 16 (57.1) | 369 | |||||
| BRAF and NRAS mutant subpopulationa | ||||||||
| Selumetinib | 55 | 42 (76.4) | 275 | 1.621 | 1.18, 2.23 | 0.99, 2.65 | 0.973 | 0.053 |
| Temozolomide | 46 | 27 (58.7) | 383 | |||||
HR < 1 indicated a benefit for selumetinib.
The one-sided P indicated whether selumetinib was associated with longer TTD than temozolomide.
Analyzed using Cox proportional hazards model, adjusted for lactate dehydrogenase, BRAF mutational status, WHO performance status, and primary tumor type.
Analyzed using the Cox proportional hazards model, adjusted for lactate dehydrogenase and WHO performance status.
Objective response rate based on investigator-assessed RECIST data
Statistical comparisons of ORR and duration of response were not formally conducted because of the low number of responses. The number of patients with confirmed PR was 5.8% (6 of 104) for patients in the selumetinib group and 9.4% (9 of 96) in the temozolomide group (Table 3). No CRs were observed in either group. Forty-eight (46.2%) patients in the selumetinib group had SD of ≥6 weeks’ duration compared with 36 (37.5%) in the temozolomide group. At the time of the overall survival analysis, two new PRs were observed in patients with wild-type tumors randomized to temozolomide and one temozolomide patient with a previous PR became a CR. Of the 11 responders in the temozolomide group, the duration of response ranged from 94 to ≥420 days (3 patients were still responding at time of data cutoff). In the selumetinib group, the duration of response ranged from 130 to 358 days (all patients had progressed).
Table 3.
Objective tumor response for selumetinib and temozolomide
| Response status | Objective tumor response |
Treatment group, n (%) |
|
|---|---|---|---|
| Selumetinib | Temozolomide | ||
| Overall population | n = 104 | n = 96 | |
| Responsea | CR | 0 | 0 |
| PR | 6 (5.8) | 9 (9.4) | |
| Total | 6 (5.8) | 9 (9.4) | |
| Nonresponse | SD ≥ 6 wk | 48 (46.2) | 36 (37.5) |
| PD | 40 (38.5) | 43 (44.8) | |
| Nonevaluable | 10 (9.6) | 8 (8.3) | |
| Total | 98 (94.2) | 87 (90.6) | |
| BRAF mutant subpopulation | n = 45 | n = 28 | |
| Responsea | CR | 0 | 0 |
| PR | 5 (11.1) | 3 (10.7) | |
| Total | 5 (11.1) | 3 (10.7) | |
| Nonresponse | SD ≥ 6 wk | 18 (40.0) | 12 (42.9) |
| PD | 17 (37.8) | 11 (39.3) | |
| Nonevaluable | 5 (11.1) | 2 (7.1) | |
| Total | 40 (88.9) | 25 (89.3) | |
| BRAF and NRAS mutant subpopulation | n = 55 | n = 46 | |
| Responsea | CR | 0 | 0 |
| PR | 5 (9.1) | 4 (8.7) | |
| Total | 5 (9.1) | 4 (8.7) | |
| Nonresponse | SD ≥ 6 wk | 23 (41.8) | 21 (45.7) |
| PD | 21 (38.2) | 19 (41.3) | |
| Nonevaluable | 6 (10.9) | 2 (4.3) | |
| Total | 50 (90.9) | 42 (91.3) | |
An objective response included patients with either a confirmed CR or PR according to RECIST version 1.0.
Efficacy in patients with BRAF or NRAS mutations
There were no significant differences in PFS between the two treatment groups in the BRAF mutant (Fig. 2C) and BRAF or NRAS mutant subsets (not shown). Among patients with BRAF mutation, objective tumor response was observed in 11.1% (5 of 45) of patients receiving selumetinib and 10.7% (3 of 28) of the temozolomide group. Similarly, in the patient subpopulation with BRAF or NRAS mutations, the objective tumor responses were 9.1% (5 of 55) and 8.7% (4 of 46) in the selumetinib and temozolomide groups, respectively (Table 3). Of the 6 selumetinib responders, 5 were BRAF mutant compared with 3 of the 9 temozolomide responders. As with the overall population, BRAF mutant patients randomized to temozolomide had improved TTD compared with those randomized to selumetinib (Fig. 2D).
Efficacy in patients who switched treatment
As of September 28, 2007, 51 patients randomized initially to temozolomide had switched to selumetinib; 46 (90.2%) switched following objective disease progression and 5 patients switched incorrectly (before objective disease progression as assessed by site RECIST data). Three switches were due to an incorrect assessment of objective disease progression and the other two resulted from clinical progression alone (the latter two patients went on to progress according to RECIST 28 and 64 days after switching to AZD6244; the first patient had new lesions and the second died).
As of June 20, 2008, 59 of the 96 patients randomized to temozolomide (61%) had switched to selumetinib (54 of 59 patients after objective disease progression). No further patients had incorrectly switched prior to disease progression. One patient (2%) had PR, 25 (46%) had PD, and 8 (15%) were not evaluable. Two patients who switched exhibited PR while receiving selumetinib; however, one of these switched prior to objective disease progression on temozolomide and so is counted as a response to temozolomide. The patient with confirmed PR on selumetinib following progression to temozolomide was BRAF mutant.
Change in tumor size
Exploratory plots were produced to assess the change from baseline in tumor size by week 6 and best overall change at time of primary analysis (Supplementary Fig. S1). Overall, there was little difference between the two treatment groups for change in tumor size, either at week 6 or for best overall change.
Safety
Adverse events
Most adverse events reported in this study were CTCAE grade I or II and were manageable with drug holiday or standard supportive therapy (Table 4). Fewer patients in the temozolomide group had treatment-related adverse events, adverse events ≥ grade III, SAEs or adverse events leading to discontinuation compared with the selumetinib group. The most frequent SAEs in the selumetinib group were diarrhea (n = 3), vomiting (n = 3), and infections (n = 3). Small intestinal obstruction (n = 2) and confusional state (n = 2) were the most frequent SAEs in the temozolomide group. Three deaths were reported in patients receiving selumetinib; one death due to unknown cause occurred in the absence of tumor progression, one patient died of metastases to meninges (disease progression), and one patient experienced cardiorespiratory arrest that was attributed to selumetinib by the investigator. A further selumetinib-randomized patient died outside of the 30-day follow-up reporting period as a result of myocarditis.
Table 4.
Most frequent all-causality adverse events (occurring in at least 15% of patients in each group), SAEs, and discontinuations due to any adverse events
| Number (%) of patients |
||||
|---|---|---|---|---|
| Selumetinib (n = 99) | Temozolomide (n = 95) | |||
| Preferred term | AE | AE ≥ grade III | AE | AE ≥ grade III |
| AE | 99 (100.0) | 57 (57.6) | 92 (96.8) | 36 (37.9) |
| Dermatitis acneiform | 59 (59.6) | 12 (12.1) | 3 (3.2) | 0 (0.0) |
| Diarrhea | 56 (56.6) | 4 (4.0) | 20 (21.1) | 0 (0.0) |
| Nausea | 50 (50.5) | 3 (3.0) | 61 (64.2) | 3 (3.2) |
| Peripheral edema | 40 (40.4) | 1 (1.0) | 6 (6.3) | 3 (3.2) |
| Fatigue | 29 (29.3) | 3 (3.0) | 40 (42.1) | 4 (4.2) |
| Vomiting | 28 (28.3) | 1 (1.0) | 42 (44.2) | 6 (6.3) |
| Headache | 21 (21.2) | 3 (3.0) | 23 (24.2) | 2 (2.1) |
| Pyrexia | 16 (16.2) | 1 (1.0) | 10 (10.5) | 0 (0.0) |
| Constipation | 12 (12.1) | 0 (0.0) | 45 (47.4) | 1 (1.1) |
| SAEs | 32 (32.3) | 16 (16.8) | ||
| Discontinuations due to AEs | 10 (10.1) | 2 (2.1) | ||
NOTE: This table includes adverse events with an onset date between the date of the first dose and 30 days following the date of the last dose of study treatment (unless the patient switched to selumetinib earlier than 30 days following discontinuation of temozolomide).
Abbreviation: AE, adverse event.
The most commonly reported adverse events were dermatitis acneiform (59.6%), diarrhea (56.6%), and nausea (50.5%) in the selumetinib group, and nausea (64.2%), constipation (47.4%), and vomiting (44.2%) in the temozolomide group (Table 4).
Laboratory evaluation
Deterioration in hematology parameters of at least two grades from baseline was observed in fewer patients treated with selumetinib than with temozolomide; leukocytes (1.0% vs. 8.5%), lymphocytes (6.1% vs. 14%), neutrophils (2.0% vs. 7.5%), and platelets (1.0% vs. 11.7%).
A greater proportion of patients receiving selumetinib showed deterioration of at least two grades from baseline in clinical chemistry parameters than with temozolomide: alanine aminotransferase (13.1% vs. 3.2%), aspartate aminotransferase (11.8% vs. 2.4%), and albumin (16.3% vs. 1.1%). Bilirubin levels remained normal in both treatment groups.
A slight increase in calcium phosphate product level was observed in the selumetinib group, with 6 patients reporting levels above the predefined cutoff value (4.5 mmol/L). One of these patients had an SAE (grade III) of hyperphosphatemia.
Small increases in mean systolic (7.4 mm Hg) and diastolic (5.3 mm Hg) blood pressure, without a corresponding change in heart rate, were observed in the selumetinib group by week 8. Hypertension was reported as an adverse event by 8 (8.1%) patients in the selumetinib group and 2 (2.1%) in the temozolomide group.
Discussion
Advanced melanoma represents one of the most treatment-refractory malignancies. Despite decades of research, worldwide consensus on a standard first-line treatment has yet to be established. While dacarbazine and temozolomide are used for first-line chemotherapy of advanced melanoma, patient response to these agents is low (22, 27). The present study investigated the role of the oral MEK1/2 inhibitor selumetinib as monotherapy for patients with unresectable stage III/IV melanoma. No significant difference was seen in the primary endpoint of PFS between the 2 treatment arms for either the overall population or the subpopulations of patients with BRAF or BRAF/NRAS mutant tumors. Although some imbalances were seen between treatment groups in baseline covariates, the statistical analyses adjusted for the impact of these factors. Disease control (5.8% PR; 46.2% SD) with selumetinib monotherapy was observed. Because of the open-label nature of this trial, an independent central review of tumor assessment was incorporated to ensure consistency. However, as stated in the protocol, the primary efficacy analysis was based on the investigator-assessed RECIST data, as this was considered to be more reflective of clinical practice and the central review was not carried out in real-time. As has been reported for other studies (28), differences between local and central review were noted but this did not alter the conclusions of the primary analyses.
Tumor responses to selumetinib monotherapy have been observed in patients with melanoma and other solid tumors, suggesting antitumor activity with this MEK1/2 inhibitor. Cell lines expressing BRAF or RAS mutations (including melanoma cell lines) have increased sensitivity to selumetinib (29). This is particularly relevant to melanoma as recent estimates suggest that activating mutations of BRAF and NRAS are found in 41% and 18% of melanomas, respectively (11). Although the present study did not test for mutation status prospectively, 79% of patients had mutation status confirmed retrospectively. Retrospective mutation testing could be considered a limitation of this study, given the imbalance seen between treatment arms. However, the rationale for prespecified retrospective testing was 2-fold: first, implementing prospective testing of patients would require central testing in a time frame that might withhold treatment from patients for a prolonged period of time, and second, the primary objective was to assess efficacy in the overall population, and so making patients wait for a mutation test before starting treatment, when the result would not exclude them from entering the trial, was felt not to be in the patients’ best interests.
The observed BRAF mutation rate of 46.2% is lower than had been expected when planning this study but similar to recent reports (11, 30, 31). Of note in our study, 5 of the 6 patients showing PR with selumetinib had tumors that were BRAF mutant. This finding raises the possibility that BRAF mutation may be an important, but not exclusive, requirement for response to selumetinib. Data from other compounds in development have shown that patients with BRAF mutant tumors can show a high response rate to MEK or BRAF inhibition (12, 13, 32–34). For example, a phase I trial of the MEK inhibitor GSK112012 showed disease control in 8 of 11 patients with BRAF mutant melanoma (32). This suggests that additional genetic markers may be necessary for a cell to respond to selumetinib monotherapy. In line with this hypothesis, a transcriptional profile associated with activation of MEK and sensitivity to selumetinib preclinically has recently been identified, although this may not be predictive of clinical benefit (35). Testing conducted on samples from this study showed no correlation between this transcription profile and clinical response (AstraZeneca, data on file).
The clinical challenge is, therefore, to find ways of optimizing the efficacy of selumetinib, for example, through combination with other targeted agents or chemotherapy. In preclinical models, selumetinib in combination with docetaxel, irinotecan, gemcitabine, or temozolomide was shown to have enhanced antitumor efficacy compared with single-agent treatment (36). Preliminary clinical results from a phase I trial of selumetinib in combination with dacarbazine, docetaxel, or temsirolimus have shown objective response in 5 of 9 patients with BRAF mutation–positive tumors (37). Selumetinib is currently beinginvestigated for advanced melanoma in combination with dacarbazine for patients with prospectively determined BRAF mutant tumors (NCT00936221).
Possible theories for the nonsignificant improved survival of patients initially assigned to temozolomide versus selumetinib (other than a chance finding) were examined but no clear explanation was found. First, selumetinib might have had a detrimental effect in relation to survival, but no differences in other efficacy endpoints (PFS, ORR, change in tumor size) and safety data did not suggest this, either in this trial or a separate comparative phase II trial measuring TTD (38). Second, an imbalance in prognostic factors might have contributed to this outcome, but this is unlikely as imbalances in a range of prognostic factors (lactate dehydrogenase, WHO performance status, BRAF mutation status, and tumor type) had already been accounted for in the TTD analysis. Third, an imbalance in the number of patients that crossed over from temozolomide to selumetinib, or vice versa (61% temozolomide arm vs. ~25% selumetinib arm), could have affected the outcome; for example, the possibility that the sequential administration of two equally active agents prolonged survival (selumetinib has activity that is preliminarily in the range of temozolomide and PFS curves are not dissimilar). However, the relative activity of these agents in the first- and second-line setting is unknown. In addition, the relative activity of non-study treatments that non-crossover patients went on to receive after temozolomide and selumetinib is unknown.
To be representative of the general melanoma population, patients with uveal melanoma were included in this study. It was felt that these patients have the potential to benefit from MEK inhibition because they may carry somatic mutations such as GNAQ and GNA11 (39). Analysis of efficacy in these patients was an exploratory endpoint that did not translate into a significant clinical benefit in this trial. However, on the basis of anecdotal evidence from this and a phase I study (19), a phase II study of selumetinib in patients with uveal melanoma has been initiated, NCT01143402.
Selumetinib was generally well tolerated; the reported adverse events were consistent with prior reports (19, 40), and no new clinically significant safety issues were identified in the present study. There was a higher reported incidence of dermatitis acneiform, diarrhea, and peripheral and periorbital edema with selumetinib than with temozolomide. Nausea, vomiting, constipation, dyspnea, and fatigue were more commonly reported in the temozolomide group than in the selumetinib group, which is consistent with the prescribing information for temozolomide. Hematologic toxicities were not an issue with selumetinib.
Dermatologic toxicities with selumetinib resemble those observed with epidermal growth factor receptor inhibitors (41) in their clinical presentation (dermatitis acneiform, xerosis cutis, paronychia; ref. 40). These skin toxicities can be ameliorated by topical corticosteroid and/or antibacterial therapy (40, 42) and responded to dose interruptions or discontinuation of therapy. In the present study, selumetinib-associated dermatologic conditions were manageable; only one patient discontinued study treatment due to dermatitis acneiform. It has been suggested that there may be a link between rash and the signal transduction pathway. An exploratory (unplanned) analysis of rash (maximum grade on treatment) and efficacy (maximum change in tumor size) found no relationship (AstraZeneca, data on file).
The toxicity profile of selumetinib in this trial therefore appears to be manageable. However, it is possible that the acceptable tolerability of selumetinib may be a consequence of underdosing in this study and could, therefore, explain the low number of responses observed. During development of selumetinib, the dose-limiting toxicities and maximum tolerated dose were based on the frequency of rash. It is possible that in the subsequent development of newer MEK inhibitors, lessons were learnt from these early trials of selumetinib and other MEK inhibitors, and the management of rash that results from administration of this type of drug is now more effective. For example, in a phase I trial of the MEK inhibitor GSK1120212 which had disease control rate of 73%, the frequency of rash was 77% (43). However, the frequency of grade III rash was lower than that seen in our study, suggesting that although the incidence of rash may be higher overall, it could be better controlled with optimal supportive care. It is therefore possible that the dose of selumetinib used in this phase II study was overcautious with regard to toxicity and that the maximal dosage range was not explored in full. It should therefore be noted that ongoing and future trials of selumetinib will use a 75-mg hydrazine sulfate tablet formulation which shows statistically significantly higher plasma exposure as well as oral bioavailability 197% that of the 100-mg free-base suspension used in this study (44).
In conclusion, the oral MEK1/2 inhibitor selumetinib showed modest activity with no significant difference in PFS compared with temozolomide in patients with chemotherapy-naive advanced melanoma unselected for BRAF mutations. The objective tumor responses observed were comparable in both the overall and BRAF and NRAS mutant populations; however, 5 of 6 selumetinib responders had BRAF mutant tumors. Further development of selumetinib in this disease will therefore focus on combination with other agents and upon the selection of patients for therapy, using BRAF mutation status.
Supplementary Material
Translational Relevance.
The Ras/Raf/MEK/ERK pathway is a key signaling cascade driving cell-cycle proliferation, differentiation, and survival. Activations in this pathway contribute to malignant progression in many human cancers. Selumetinib (AZD6244/ARRY-142886) is an oral inhibitor of mitogen-activated protein (MAP)/extracellular signal-regulated (ERK) kinase (MEK1/2) currently in clinical development for a number of tumor types. Preclinical studies have shown antitumor activity of selumetinib in melanoma xenograft models particularly those harboring BRAF mutations. In this study, there was no significant difference in efficacy between selumetinib and temozolomide as first-line therapy for patients with advanced melanoma not selected for activating BRAF mutations. Of note, 5 of the 6 patients showing partial response with selumetinib had tumors that were BRAF mutant. On the basis of these results, and preclinical data, a phase II study of selumetinib in combination with chemotherapy for patients with BRAF-mutated melanoma has been initiated.
Acknowledgments
The authors thank the investigators from all 34 sites that took part in this study, in particular, Dr. Paul Chapman for his editorial input. Moh Tadayyon (MediTech Media) and Ewen Buckling (iMed Comms) provided medical writing support funded by AstraZeneca.
Grant Support
This study was sponsored by AstraZeneca. The clinical research of R. Dummer was supported by the Gottfried and Julia Bangerter Rhyner Stiftung and the research of John M. Kirkwood was supported by the NCI SPORE in Skin Cancer (P50 CA121973).
Footnotes
Disclosure of Potential Conflicts of Interest
L. Bastholt has honoraria from Speakers’ Board and is a consultant/advisory board member of Merck. C. Robert is a consultant/advisory board member of Roche. M. Middleton has commercial research support and is a consultant/advisory board member of AstraZeneca. M. Cantarini, V. Zazulina, and K. Kemsley are employed by AstraZeneca. R. Dummer is a consultant/advisory board member of AstraZeneca.
No potential conflicts of interest were disclosed by the other authors.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Boerner SA, Winkler JD, LoRusso PM. Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta. 2007;1773:1248–55. doi: 10.1016/j.bbamcr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Jilaveanu LB, Zito CR, Aziz SA, Conrad PJ, Schmitz JC, Sznol M, et al. C-Raf is associated with disease progression and cell proliferation in a subset of melanomas. Clin Cancer Res. 2009;15:5704–13. doi: 10.1158/1078-0432.CCR-09-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 6.Zebisch A, Czernilofsky AP, Keri G, Smigelskaite J, Sill H, Troppmair J. Signaling through RAS-RAF-MEK-ERK: from basics to bedside. Curr Med Chem. 2007;14:601–23. doi: 10.2174/092986707780059670. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Angelini S, Hemminki K. Activating BRAF and N-Ras mutations in sporadic primary melanomas: an inverse association with allelic loss on chromosome 9. Oncogene. 2003;22:9217–24. doi: 10.1038/sj.onc.1206909. [DOI] [PubMed] [Google Scholar]
- 8.Platz A, Egyhazi S, Ringborg U, Hansson J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1:395–405. doi: 10.1016/j.molonc.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–8. [PubMed] [Google Scholar]
- 10.Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res. 2002;8:3468–74. [PubMed] [Google Scholar]
- 11.Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011;164:776–84. doi: 10.1111/j.1365-2133.2010.10185.x. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kefford R, Arkenau H, Brown MP, Millward M, Infante JR, Long GV, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28(suppl):15s. abstr 8503. [Google Scholar]
- 14.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang JY, Wilcoxen KM, Nomoto K, Wu S. Recent advances of MEK inhibitors and their clinical progress. Curr Top Med Chem. 2007;7:1364–78. doi: 10.2174/156802607781696837. [DOI] [PubMed] [Google Scholar]
- 16.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–83. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 17.Friday BB, Yu C, Dy GK, Smith PD, Wang L, Thibodeau SN, et al. BRAF V600E disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and Raf proteins. Cancer Res. 2008;68:6145–53. doi: 10.1158/0008-5472.CAN-08-1430. [DOI] [PubMed] [Google Scholar]
- 18.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 19.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann R, Spieth K, Leiter U, Mauch C, von den Driesch P, Vogt T, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. J Clin Oncol. 2005;23:9001–7. doi: 10.1200/JCO.2005.01.1551. [DOI] [PubMed] [Google Scholar]
- 21.SP Europe UK summary of product characteristics, temodal capsules. 2011 Jun 14; updated. cited 2011 Jul 19. Available from: http://www.medicines.org.uk/EMC/medicine/7027/SPC/Temodal+Capsules/
- 22.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 23.Ellison G, Donald E, McWalter G, Knight L, Fletcher L, Sherwood J, et al. A comparison of ARMS and DNA sequencing for mutation analysis in clinical biopsy samples. J Exp Clin Cancer Res. 2010;29:132. doi: 10.1186/1756-9966-29-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004;10:6759–63. doi: 10.1158/1078-0432.CCR-04-0496. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 26.Kemsley K, Ghiorghiu D, Schmitt N, Wilson D, Young H, Bohnsack O, et al. Use of progression-free survival in advanced melanoma: comparison of central and site review of RECIST data in a randomised phase II trial. Perspectives in Melanoma XII; 2–4 Oct 2008. The Hague; The Netherlands: [Google Scholar]
- 27.Patel PM, Suciu S, Mortier L, Kruit WH, Robert C, Schadendorf D, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) Eur J Cancer. 2011;47:1476–83. doi: 10.1016/j.ejca.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Tang PA, Pond GR, Chen EX. Influence of an independent review committee on assessment of response rate and progression-free survival in phase III clinical trials. Ann Oncol. 2010;21:19–26. doi: 10.1093/annonc/mdp478. [DOI] [PubMed] [Google Scholar]
- 29.Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 30.Jakob JA, Bassett RL, Ng CS, Lazar AJF, Alvarado GC, Rohlfs ML, et al. Clinical characteristics and outcomes associated with BRAF and NRAS mutations in metastatic melanoma. J Clin Oncol. 2011;29(suppl):15s. abstr 8500. [Google Scholar]
- 31.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 32.Infante JR, Fecher LA, Nallapareddy S, Gordon MS, Flaherty KT, Cox DS, et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. J Clin Oncol. 2010;28(suppl):15s. abstr 2503. [Google Scholar]
- 33.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribas A, Kim KB, Schuchter LM, Gonzalez R, Pavlick AC, Weber JS, et al. BRIM-2: an open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. J Clin Oncol. 2011;29(suppl):15s. abstr 8509. [Google Scholar]
- 35.Dry JR, Pavey S, Pratilas CA, Harbron C, Runswick S, Hodgson D, et al. Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244) Cancer Res. 2010;70:2264–73. doi: 10.1158/0008-5472.CAN-09-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt SV, Logie A, Davies BR, Cockerhill M, Haupt N, Curtis NJ, et al. 265 POSTER A MEK1/2 inhibitor, AZD6244 (ARRY-142886), shows beneficial effects when combined with standards of care or novel therapies - mechanistic characterisation suggests a role for apoptosis. Eur J Cancer Suppl. 2008;6:86. abstract. [Google Scholar]
- 37.Patel SP, Lazar AJ, Mahoney S, Vaughn C, Gonzalez N, Papadopoulos NE, et al. Clinical responses to AZD6244 (ARRY-142886)-based combination therapy stratified by gene mutations in patients with metastatic melanoma. J Clin Oncol. 2010;28(suppl):15s. doi: 10.1002/cncr.27790. abstr 8501. [DOI] [PubMed] [Google Scholar]
- 38.Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2011 May 19; doi: 10.1007/s10637-011-9687-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Zuidervaart W, van NF, Stark M, Dijkman R, Packer L, Borgstein AM, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer. 2005;92:2032–8. doi: 10.1038/sj.bjc.6602598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desar IM, Bovenschen HJ, Timmer-Bonte AJ, Cantarini MV, Van Der Graaf WT, Van Rossum MM, et al. Case studies showing clinical signs and management of cutaneous toxicity of the MEK1/2 inhibitor AZD6244 (ARRY-142886) in patients with solid tumours. Acta Oncol. 2009;49:110–3. doi: 10.3109/02841860903104152. [DOI] [PubMed] [Google Scholar]
- 41.Segaert S, Van CE. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425–33. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 42.Schad K, Baumann CK, Zipser MC, Enderlin V, Kamarashev J, French LE, et al. Mitogen-activated protein/extracellular signal-regulated kinase kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clin Cancer Res. 2010;16:1058–64. doi: 10.1158/1078-0432.CCR-09-1766. [DOI] [PubMed] [Google Scholar]
- 43.Infante JR, Falchook GS, Lawrence DP, Weber JS, Kefford RF, Bendell JC, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436) J Clin Oncol. 2011;29(suppl) abstr CRA8503. [Google Scholar]
- 44.Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–23. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.