Abstract
Objective
To test the hypotheses that: 1) the transient receptor potential vanilloid 4 (TRPV4) ion channel is protective in the obesity-model of osteoarthritis (OA), resulting in more severe obesity-induced OA in Trpv4 knockout (Trpv4−/−) mice; and 2) loss of TRPV4 alters mesodermal stem cell differentiation.
Methods
Male Trpv4−/− and wild-type (Trpv4+/+)mice were fed a control or high-fat diet (10% kcal and 60% kcal from fat, respectively) for 22 weeks, at which time spontaneous cage activity and severity of knee OA were evaluated. In addition, the adipogenic, osteogenic, and chondrogenic potential of bone marrow-derived (MSC) and adipose-derived (ASC) stem cells from Trpv4−/− and Trpv4+/+ mice were compared.
Results
A high-fat diet significantly increased knee OA scores and reduced spontaneous cage activity in Trpv4−/− mice, while also increasing weight gain and adiposity. MSCs from Trpv4−/− mice had decreased adipogenic and osteogenic differentiation potential versus Trpv4+/+ MSCs. ASCs from Trpv4−/− mice had increased adipogenic and osteogenic and reduced chondrogenic differentiation potential versus Trpv4+/+ ASCs.
Conclusion
Pan-Trpv4−/− mice develop more severe OA with high-fat feeding, potentially due to more severe diet-induced obesity. The altered differentiation potential of Trpv4−/− progenitor cells may reflect the importance of this ion channel in the maintenance and turnover of mesodermally-derived tissues.
Keywords: Articular cartilage, cellular mechanotransduction, arthritis, inflammation, mesenchymal stem cell
INTRODUCTION
Obesity is one of the most significant and modifiable risk factors for osteoarthritis (OA).[1] However, local biomechanical factors associated with changes in the onset and progression of knee OA in the obese population[2,3] cannot explain the relationship between obesity and OA in non-load bearing joints.[4] Obesity and related metabolic syndromes are associated with chronic low-grade inflammation and systemic tissue damage.[5] Recent studies suggest that these systemic metabolic factors participate in the development of OA in both weight bearing and non-weight bearing joints;[6] however, the mechanisms by which these systemic factors alter the course of OA remain unclear.[7]
The transient receptor potential vanilloid 4 (TRPV4) ion channel is a Ca2+-preferred cation channel, originally characterized as a transducer of osmotic stress.[8,9] TRPV4-mediated Ca2+ signaling in response to osmotic fluctuations in the cartilage is one potential mechanism by which chondrocytes sense and respond to joint loading.[10] Recent findings indicate that TRPV4 signaling plays a crucial role in skeletal development[11,12], while genetically-encoded deletion of TRPV4 in mice leads to accelerated joint degeneration with aging.[13] More recent findings also suggest that chondrocyte TRPV4 could be a multi-modally modulated channel, interacting with pro-inflammatory mediators and cytokines to mediate catabolic signaling and nociception.[14,15]
We hypothesized that the absence of TRPV4-mediated signaling in the presence of the catabolic, biomechanical and inflammatory factors of obesity would accelerate OA progression in the high-fat diet model of OA. To examine the link between the observed phenotype of Trpv4−/− mice and function of TRPV4 at the cellular level, we measured the effects of TRPV4 deficiency on the intrinsic capabilities of bone marrow-derived (MSCs) and adipose-derived (ASCs) stem cells, isolated from Trpv4−/− and Trpv4+/+ mice, to differentiate towards the adipogenic, osteogenic, and chondrogenic lineages.
MATERIALS AND METHODS
Detailed methods are available as supplementary material (available online only).
Animal Handling
At 10 weeks of age, male pan-Trpv4 knockout (Trpv4−/−) and wild-type (Trpv4+/+) mice were placed on either a high-fat (60% kcal) or control diet (10% kcal) for 22 weeks, at which time, spontaneous locomotor activity was measured.
Body Composition
Immediately following sacrifice, total body fat of each mouse was measured using Dual Energy X-ray absorptiometry.[6]
Histologic Evaluation of OA
Sections of hind limb joints were stained with Hematoxylin, Safranin-O and Fast Green and scored for degenerative changes using a modified Mankin system by three blinded graders.[16] Sections of subcutaneous fat tissue from 10-week old Trpv4−/− and Trpv4+/+ mice were also taken and stained with Hematoxylin and Eosin.
Stem Cell Isolation, Purification, and Expansion
Bone marrow derived stem cells (MSC) and subcutaneous adipose-derived stem cells (ASC) were isolated from the femurs and tibias (MSC), and the inguinal fat pad (ASC) of Trpv4−/− and Trpv4+/+ mice (8–10 weeks old), using a recently described method involving FACS to obtain cells with specific cell markers, [17,18] and expanded to P3.
Tridifferentiation
Passage 3 MSCs and ASCs were induced towards the adipogenic, osteogenic, or chondrogenic lineages and assayed for differentiation capacity. Adipogenesis was quantified by Oil-Red-O release, while osteogenesis was quantified by Alizarin stain release. Chondrogenic differentiation was assessed by Alcian blue staining and glycosaminoglycan content.
Statistical Analysis
Normality was tested, and data were log-transformed before analysis if necessary. Statistical analysis was performed using multiple-factor analysis of variance (ANOVA) for comparison of multiple groups, with Fisher LSD post-hoc analysis using α=0.05. Significant differences were reported at the 95% confidence interval unless otherwise noted. Data are presented as mean±standard error of the mean.
RESULTS
Trpv4−/− mice are more susceptible to diet-induced obesity than Trpv4+/+ mice
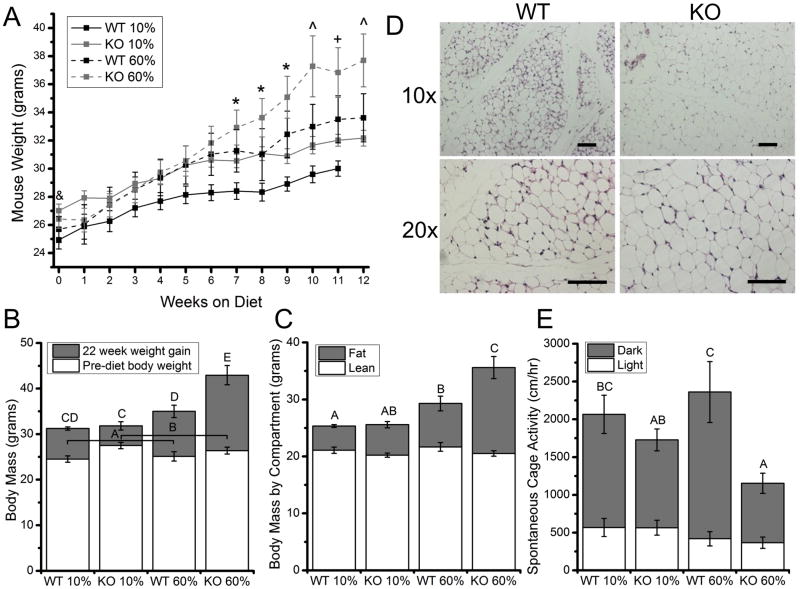
Trpv4−/− mice weighed significantly more than Trpv4+/+ mice at 10 weeks of age (Fig. 1A, B). After being fed a high-fat diet, Trpv4−/− mice gained significantly more weight than Trpv4+/+ mice (Fig. 1A, B). DXA measurements after high-fat feeding revealed that the differences in body mass with genotype and diet were due to body fat, with Trpv4−/− mice gaining significantly more body fat than Trpv4+/+ mice following high-fat feeding (Fig. 1C). To further examine the obese phenotype of Trpv4−/− mice, histological sections were taken of the inguinal fat pad of 10-week old normally fed mice and showed that even prior to high-fat feeding, Trpv4−/− mice may possess larger adipocytes than Trpv4+/+ controls (Fig. 1D).
Figure 1. Trpv4−/− mice show increased adiposity in response to high-fat feeding.
A. High-fat fed mice weighed significantly more than control diet mice by week 7. By week 10, knockout (KO) 60% mice weigh significantly more than all other groups (p=0.022) & Trpv4 KO >WT (p<0.05), *60% diet >10% diet (p<0.05), ^60% KO >all other groups (p<0.05), +60% KO >10% WT (p<0.05). B, Wild-type (WT) 60% mice gained (insignificantly) more weight after 22 weeks of high-fat feeding compared to WT 10% mice (p=0.0837), whereas KO 60% mice gained more weight than all other groups (p=0.003). C, Post-diet, mice did not differ in the amount of lean body mass. WT 60% mice had significantly more body fat than WT 10% mice, but KO 60% had more body fat than all other groups (p<0.001). D, Histological sections of subcutaneous adipose tissue from 10-week-old mice, with KO adipocytes appearing larger than WT adipocytes, Scale bar=100μm. E, When fed a high-fat diet, Trpv4−/−mice were 40% as active during the dark cycle as Trpv4+/+ mice (diet: p=0.833, genotype: p=0.003, genotype*diet: p=0.091). Data are shown as mean ±SEM. Data not sharing a common superscript letter indicate a significant difference (p<0.05).
High-fat fed Trpv4−/− mice have lower cage activity than high-fat fed Trpv4+/+ mice
To further investigate the relationship between Trpv4 deficiency and increased weight gain, spontaneous cage activity was measured after three days of habituation. For mice fed a control fat diet, genotype had no effect on dark cycle locomotor activity (p=0.332); yet, when fed a high-fat diet, Trpv4−/− mice were 40% as active as Trpv4+/+ mice (Fig. 1E).
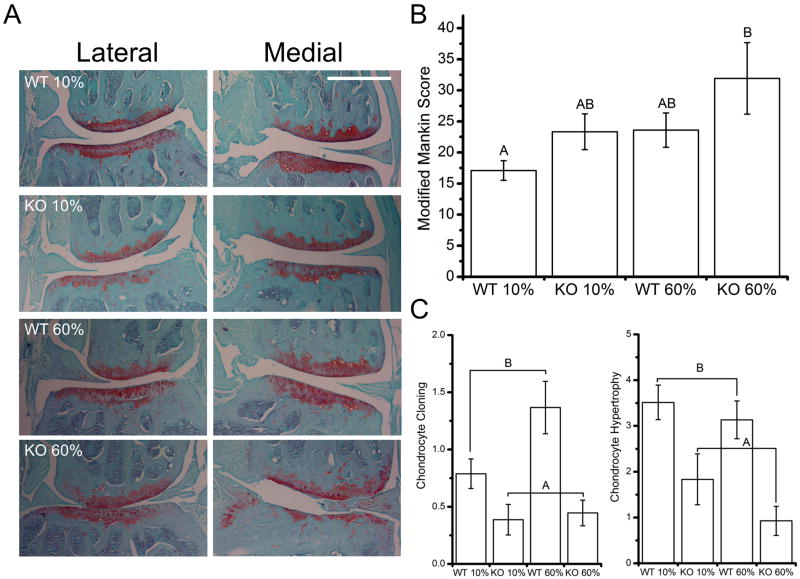
Trpv4 deficiency increases knee osteoarthritis following high-fat feeding
A modified Mankin score was tabulated and analyzed that combined the score for cartilage structural degeneration and proteoglycan loss as recommended in reference[19]. Neither Trpv4−/− nor high-fat feeding alone increased joint degeneration; however, the combination of the two factors increased OA severity (Fig. 2A, B). Trpv4−/− mice also demonstrated altered chondrocyte histomorphology, with reduced chondrocyte cloning and chondrocyte hypertrophy (Fig. 2C, Fig. S1 available online).
Figure 2. Trpv4−/− mice have more severe diet-induced osteoarthritis and altered chondrocyte histomorphology.
A. Representative histological images, scale bar = 500 μm. B. KO 60% mice have more severe joint degeneration than WT 10% mice (genotype: p=0.057, diet: p=0.049, genotype*diet: p=0.779). C. Trpv4−/− joints have less chondrocyte cloning and chondrocyte hypertrophy (p<0.001). Data are shown as mean±SEM. Data not sharing a common superscript letter indicate a significant difference (p<0.05).
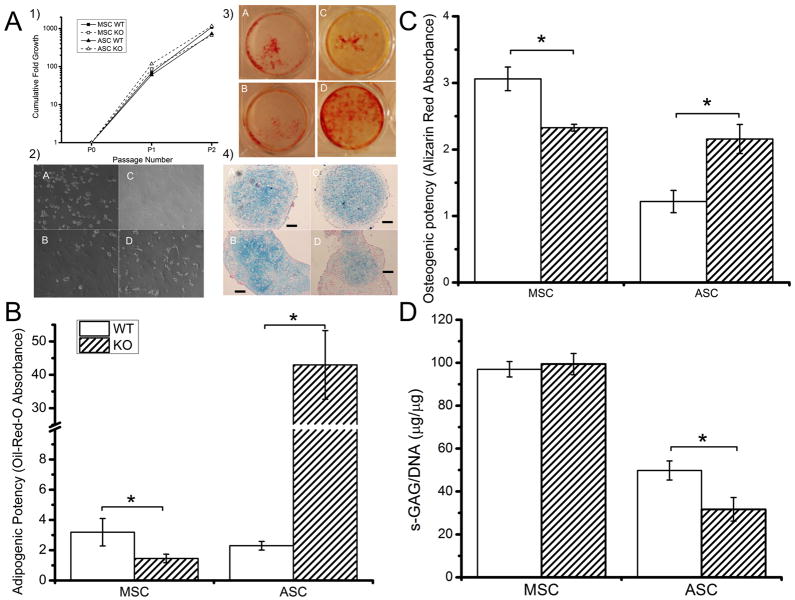
Altered in vitro differentiation of Trpv4−/− progenitor cells
MSCs and ASCs were isolated from 10-week old Trpv4−/− and Trpv4+/+ mice and expanded to P3. No effect of genotype on expansion rate was observed (Fig. 3A1). Trpv4−/− MSCs exhibited a reduced adipogenic differentiation potential compared to MSCs isolated from Trpv4+/+ mice, whereas Trpv4−/− ASCs demonstrated a largely increased adipogenic differentiation potential compared to Trpv4+/+ ASCs (Fig. 3A2, 3B). Similarly, Trpv4−/− MSCs demonstrated a reduced osteogenic differentiation potential while Trpv4−/− ASCs had an increased osteogenic differentiation potential (Fig. 3A3, 3C). No effect of Trpv4 deficiency was observed with MSC chondrogenic differentiation. However, Trpv4−/−ASCs demonstrated a reduced chondrogenic differentiation potential compared to Trpv4+/+ ASCs, as indicated by less GAG/DNA (Fig. 3D) and Alcian Blue staining (Fig 3A4).
Figure 3. Adult stem cells from Trpv4−/− mice exhibit altered differentiation potential.
MSCs and ASCs were purified and expanded as described previously (17,18). Data for adipogensis and osteogenesis were normalized to DNA content and to the staining of cells cultured in control media. A. 1. MSCs and ASC from Trpv4−/− mice expanded equally rapidly in hypoxic culture. A2–4. A: WT MSC B: WT ASC C: KO MSC D: KO ASC. A2. Cell morphology at day 7 of adipogenic differentiation. A3. Alizarin Red staining at day 14 osteogenesis. A4. Chondrogenically induced cell pellets (Alcian Blue/Nuclear Fast Red). B. Bone marrow derived MSCs have a reduced ability to differentiate when cultured in adipogenic media (p=0.008), while ASCs show a large increase (p<0.001). C. Bone marrow derived MSCs have a reduced ability to differentiate when cultured in osteogenic media (p<0.001), while ASCs show an increased ability (p<0.001). D. Trpv4 deficiency does not affect in vitro MSC chondrogenesis. However, Trpv4−/− ASCs have decreased GAG accumulation (p=0.036) and Alcian Blue staining. D. Data are shown as mean±SEM. * indicates significant difference (p<0.05).
DISCUSSION
Our findings indicate that TRPV4 exhibits a chondroprotective role in diet-induced OA. Trpv4−/− mice exhibited an increased susceptibility to high fat diet-induced OA, potentially due in part to an increased susceptibility to diet-induced obesity. MSCs and ASCs from these mice also demonstrated altered differentiation potential, with ASCs from Trpv4−/− mice exhibiting significantly higher adipogenic potential and decreased chondrogenic potential.
Our observations that very high-fat fed Trpv4−/− mice are particularly prone to obesity stands in contrast with the findings by Kusodu et al. that pan-Trpv4−/− mice are protected from diet-induced obesity.[20] However, substantial differences exist between these two studies regarding, including age at diet initiation, composition, and duration of high-fat feeding. In this study, mice were fed a 60% high-fat diet for 22 weeks beginning at 10 weeks, compared to a 42% kcal diet beginning at 16 weeks and lasting 12 weeks in [20]. In addition, although we did not measure food consumption or energy expenditure in our study (no effect of Trpv4 knockout was found in [20]), we observed reduced cage activity of high-fat fed Trpv4−/− mice. It is unclear, however, whether this represent a cause and/or a consequence of the additional weight gain of Trpv4−/− mice. Additionally, given the chondroprotective effect of activity in the setting of diet-induced obesity, [21] it is even possible that this decrease in activity increased the severity of joint degeneration in the Trpv4−/− mice directly.
While neither high-fat feeding nor Trpv4 deficiency alone increased OA severity at 8 months of age, a combination of these two factors did. This finding is generally consistent with previous work showing that Trpv4−/− mice exhibit significant spontaneous OA changes at 9 months.[13] Similarly, high-fat diet feeding alone did not produce a significant increase in OA, but is consistent with previous similarly designed studies. Though this study supports the hypothesis that TRPV4 plays a role in the pathogenesis of obesity-associated OA, further investigation is needed to fully describe the cartilage-specific role of TRPV4 in obesity and OA.
Trpv4−/− mice at 32 weeks of age exhibited a chondrocyte morphology that is distinct from that of Trpv4+/+ mice, with less chondrocyte cloning and hypertrophy observed in the articular cartilage of Trpv4−/− mice. The cause of chondrocyte cloning (or chondrocyte cluster formation) in osteoarthritic cartilage and its influence on cartilage disease progression is unknown, but may signify a proliferative repair response.[22] Chondrocyte hypertrophy in articular cartilage also signifies altered metabolic activity by chondrocytes following tissue damage and inflammation.[23,24] Further studies will be needed to determine if TRPV4 mediates these or other chondrocyte responses to joint insult.
We observed an altered metabolic and osteoarthritic response of Trpv4−/− mice to high-fat feeding. In an attempt to better understand the role of this channel in these mesodermally-derived tissues, we examined whether adult stem cells would also exhibit altered growth or differentiation characteristics in vitro that reflect the tissue characteristics observed in vivo. Isolated MSCs from Trpv4−/− mice exhibited reduced adipogenesis, while ASCs revealed significantly increased adipogenesis. Consistent with these in vitro findings, adipocytes in Trpv4−/− mice appeared larger than those of Trpv4+/+ mice at 10 weeks of age. Determining the role of TRPV4 in adipose tissue function, as is being actively pursued with other TRP channels, [25] could yield important insight into both metabolic and inflammatory-associated diseases.
The role of TRPV4 in bone metabolism is also evident in Trpv4−/− mice, which display increased bone volume that may in fact contribute to increased cartilage degeneration directly.[13] While the skeletal phenotype of Trpv4−/− mice has been largely attributed to impaired osteoclast function, [26] we found that Trpv4−/− MSCs exhibited reduced osteogenesis, while Trpv4−/− ASCs exhibited increased osteogenesis. Future investigations are needed to fully deconstruct the role of TRPV4 in bone development, remodeling, and repair.
Though we found no effect of TRPV4 in MSC chondrogenesis, it is plausible that the potent application of growth factors required to induce in vitro chondrogenesis (TGF-β3, BMP-6) may have overpowered the effect of basal TRPV4 signaling in these cells. TRPV4 activation in chondroprogenitors has been shown to enhance Sox9 expression in a Ca2+-dependent manner, one of the main regulators of cartilage-specific expression of matrix molecules such as collagen type II and aggrecan.[27] Interestingly, we observed that chondrogenesis was diminished in Trpv4−/− ASC, possibly due to the altered phenotype and metabolism of the pan-Trpv4−/− adipose tissue, rather than indicating a direct effect of loss of TRPV4 signaling with chondrogenic differentiation. Further studies are necessary to establish the role of TRPV4 in adipose tissue that led to the pro-obesity phenotype in our Trpv4−/− mice.
In conclusion, global loss of Trpv4 increases knee OA severity in response to high-fat feeding in a manner that is associated with increased weight gain. However, the effects of pan-Trpv4 deletion, such alterations in bone remodeling, [13,26] and energy metabolism, complicates conclusions regarding the in vivo role of various progenitor cells in this model system. There may be other systemic effects of pan-Trpv4 knockout as well, included altered nociception, given the involvement of TRPV4 in joint inflammation and pain.[15,28] Use of tissue-targeted Cre-lox systems[29–31] may help to define the tissue-specific effects of TRPV4 signaling with respect to obesity, joint inflammation, pain, and OA. Determining the cartilage-specific role of TRPV4 in the many etiologies and models of OA, including obesity-induced OA, should provide new insight into molecular mechanisms that link biomechanical and inflammatory factors of OA, hopefully leading to new preventions and treatments for this prevalent disease.
Supplementary Material
White arrows indicate chondrocyte hypertrophy, black arrows indicate chondrocyte cloning (cluster formation).
Acknowledgments
We thank Bridgette Furman and Holly Leddy for help with histological grading and statistical analysis, Brian Diekman and Chia-Lung Wu for assistance with stem cell isolations, and Steven Johnson and Francisco Cordero for their technical support. We would like to thank Drs. William Westel and Ramona Rodriguiz for assistance and advice on the study.
FUNDING: This work was supported by grants from the NIH (AR48182, AR50245, AG15768, AR48852, AG40868, DE018549 and GM08719) and the Arthritis Foundation.
Footnotes
LISCENCE FOR PUBLICATION: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/producs/journals/instructions-for-authors/licence-forms).
COMPETING INTERESTS: None declared.
REFERENCE LIST
- 1.Teichtahl AJ, Wang Y, Wluka AE, et al. Obesity and knee osteoarthritis: new insights provided by body composition studies. Obesity (Silver Spring) 2008;16:232–40. doi: 10.1038/oby.2007.30. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann of Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 3.Runhaar J, Koes BW, Clockaerts S, et al. A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis. Obes Rev. 2011;12:1071–82. doi: 10.1111/j.1467-789X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 4.Oliveria SA, Felson DT, Cirillo PA, et al. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10:161–6. [PubMed] [Google Scholar]
- 5.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 6.Griffin TM, Fermor B, Huebner JL, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12:130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45:387–98. [PMC free article] [PubMed] [Google Scholar]
- 8.Liedtke W, Choe Y, Marti-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strotmann R, Harteneck C, Nunnenmacher K, et al. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Bio. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 10.Phan MN, Leddy HA, Votta BJ, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–37. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock MJ, Prenen J, Funari VA, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nature Genet. 2008;40:999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilius B, Owsianik G. Channelopathies converge on TRPV4. Nature Genet. 2010;42:98–100. doi: 10.1038/ng0210-98. [DOI] [PubMed] [Google Scholar]
- 13.Clark AL, Votta BJ, Kumar S, et al. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62:2973–83. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochukov MY, McNearney TA, Yin H, et al. Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes. Mol Pain. 2009;5:49. doi: 10.1186/1744-8069-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denadai-Souza A, Martin L, Vieira de Paula MA, et al. Role of transient receptor potential vanilloid 4 in joint inflammation. Arthritis Rheum. 2011;64:1848–58. doi: 10.1002/art.34345. [DOI] [PubMed] [Google Scholar]
- 16.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20:719–25. doi: 10.1097/01.bot.0000211160.05864.14. [DOI] [PubMed] [Google Scholar]
- 17.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–96. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diekman BO, Wu CL, Furman BD, Huebner JL, Kraus VB, Olson SA, et al. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents post-traumatic arthritis. Cell Transplant. 2012 Aug 10; doi: 10.3727/096368912X653264. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusudo T, Wang Z, Mizuno A, et al. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J Appl Physiol. 2012;112:1223–32. doi: 10.1152/japplphysiol.01070.2011. [DOI] [PubMed] [Google Scholar]
- 19.Glasson S, Chambers M, Van Den Berg W, et al. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Griffin TM, Huebner JL, Kraus VB, et al. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64:443–53. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura T, Okada A, Yatabe T, et al. RECK is up-regulated and involved in chondrocyte cloning in human osteoarthritic cartilage. Am J Pathol. 2010;176:2858–67. doi: 10.2353/ajpath.2010.091003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406–15. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Luo Z, Ma S, et al. TRP channels and their implications in metabolic diseases. Pflugers Arch. 2011;461:211–23. doi: 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
- 26.Masuyama R, Vriens J, Voets T, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8:257–65. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu S, Wakabayashi M, Ohno T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282:32158–67. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- 28.Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Barlow C, Schroeder M, Lekstrom-Himes J, et al. Targeted expression of Cre recombinase to adipose tissue of transgenic mice directs adipose-specific excision of loxP-flanked gene segments. Nucleic Acids Res. 1997;25:2543–5. doi: 10.1093/nar/25.12.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Lichtler AC, Sheu TJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau J, Minett MS, Zhao J, et al. Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse. Mol Pain. 2011;7:100. doi: 10.1186/1744-8069-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
White arrows indicate chondrocyte hypertrophy, black arrows indicate chondrocyte cloning (cluster formation).