Abstract
Background
Recent studies have identified an acute traumatic coagulopathy that is present on admission to the hospital and is independent of iatrogenic causes. We have previously reported that this coagulopathy is due to the association of severe injury and shock and is characterized by a decrease in plasma protein C levels. Whether this early coagulopathy and later propensity to infection, MOF and mortality are associated with the activation of protein C pathway has not been demonstrated and constitutes the aim of this study.
Methods and Findings
This was a prospective cohort study of 203 major trauma patients. Serial blood samples were drawn on arrival in the ED, and at 6, 12, and 24 hours after admission to the hospital. PT, PTT, Va, VIIIa, PC aPC t-PA and D-Dimer levels were assayed. Comprehensive injury, resuscitation and outcome data were prospectively collected.
A total of 203 patients were enrolled. Patients with tissue hypoperfusion and severe traumatic injury showed a strong activation of the protein C which was associated with a coagulopathy characterized by inactivation of the coagulation factors V and VIII and a derepression of the fibrinolysis with high plasma levels of plasminogen activator and high D-dimers. Elevated plasma levels of activated protein C were significantly associated with increased mortality, organ injury, increased blood transfusion requirements, and reduced ICU ventilator-free days. Finally early depletion of protein C after trauma is associated with a propensity to post-traumatic ventilator-associated pneumonia.
Conclusions
Acute traumatic coagulopathy occurs in the presence of tissue hypoperfusion and severe traumatic injury and is mediated by activation of the protein C pathway. Higher plasma levels of aPC upon admission are predictive of poor clinical outcomes following major trauma. After activation, patients who fail to recover physiologic plasma values of protein C have an increased propensity to later nosocomial lung infection.
Introduction
Trauma remains the leading cause of death and disability between the ages of 1 and 44, eclipsing ischemic heart disease, cerebrovascular disease and HIV/AIDS.[1] Worldwide, one in seven deaths is due to injury, and this is expected to rise to 1 in 5 in the next 15 years, despite continuing advances in resuscitation, trauma surgery, and critical care. Hemorrhage is the major mechanism responsible for death during the first 24 hours after trauma, and efforts to control hemorrhage and restore circulatory homeostasis form the core of the early therapeutic approach to traumatic injuries[2-5].
Perturbations in blood coagulation are common following major trauma and are associated with poor outcomes [6, 7]. Classically, coagulopathy associated with trauma is thought to be due to the consumption of coagulation factors, dilution from intravenous blood and fluid therapy, or hypothermia [8].The traditional post-injury resuscitative protocol involves large volumes of relatively “cold” fluid (dilution), exposure of the patient (hypothermia), and prolonged surgery (more exposure, hypothermia and continued bleeding), all of which precipitate metabolic failure (acidosis) [2, 5]. These abnormalities have been characterized in animal models and clinical human research. There is also an extensive literature exploring the ideal resuscitative protocol and treatment of this iatrogenic coagulopathy [2, 8]. Consistent with this concept, recent retrospective studies from the military and from civilian trauma centers have shown that an increased plasma: red cell ratio (> 1:2) is associated with decreased mortality and improved long term outcomes in massively transfused trauma patients [9].
It has recently been recognized that approximately one quarter of the severely traumatized patients present with an acute traumatic coagulopathy on arrival in the emergency department that is physiologically and mechanistically distinct from the classical iatrogenic posttraumatic coagulopathy. Two studies have described that this acute traumatic coagulopathy is associated with higher transfusion requirements, a greater incidence of Multiple Organ Dysfunction Syndrome (MODS), longer ICU and hospital stays, and a four-fold increase in mortality in coagulopathic patients compared to those with normal coagulation[10]. In two recent clinical studies, we have further characterized this early traumatic coagulopathy and have reported that when traumatic injury is combined with tissue hypoperfusion (shock), the resultant coagulopathy is characterized by a significant decrease in plasma protein C (PC) levels and an derepression of fibrinolysis [11-13]. Whether this early coagulopathy and protein C decrease is associated with the activation of protein C pathway has not been demonstrated and constitutes the first aim of this study.
Along with its anticoagulant effect, activated protein C has newly understood cytoprotective functions. Several studies have shown a beneficial effect of aPC on organ injury and mortality after onset of sepsis in both experimental and clinical investigations[14-18]. The reduction in plasma levels of activated protein C observed in severe sepsis may contribute to endothelial and epithelial cell dysfunction and a pro-coagulant milieu. Whether similar changes in the protein C pathway follow severe trauma is still unknown. We therefore hypothesized that the early activation of the protein C pathway in patients with severe trauma might be followed by a depletion of plasma levels of protein C much earlier than currently understood and that this early depletion would be associated with loss of cytoprotectivity. Hence, the second aim of the study is to determine whether there is an association between activated protein C (aPC)-mediated acute traumatic coagulopathy and later propensity to infection, organ failure and mortality after severe trauma in humans.
Methods
The Institutional Review Board of the University of California at San Francisco approved the research protocol for this prospective cohort study and granted a waiver of consent for the blood sampling as a minimal risk intervention.
Patients
Consecutive major trauma patients admitted to the San Francisco General Hospital (level 1 trauma center) were studied. All adult patients who met criteria for highest-level trauma team activation by physiologic and anatomic triage criteria were eligible for enrollment. To qualify for highest-level activation patients must (and are therefore enrolled in the study) meet either physiologic criteria including at least one pre hospital or hospital SBP<90 or HR >110, reduced GCS, or anatomic criteria including penetrating torso trauma and evidence high-energy blunt trauma. Patients can also be activated at the clinical discretion of pre hospital medics or hospital emergency department senior triage nursing or attending ED and Trauma physicians. Patients were excluded if they were less than 18 years old, were prisoners, were pregnant or were transferred from other hospitals or had significant resuscitation prior to trauma activation. Patients were retrospectively excluded if they were later found to be on anticoagulant medications or possessing a preexisting bleeding diathesis.
Sample collection and measurements
Our sampling protocol and methodology has been described previously in detail.[12] Briefly, serial 10 ml samples of blood were drawn in citrated tubes upon arrival in the emergency department, 6, 12 and 24 hours after admission to the hospital. The samples were immediately transferred to the central laboratory, centrifuged and the plasma extracted and stored at −80°C. Samples were analyzed at the conclusion of the study by researchers who were blinded to all patients’ data. Factors Va, VIIIa (activity), Protein C activity (PC), tissue plasminogen activator (t-PA), and D-Dimers were measured with a Stago Compact (Diagnostica Stago Inc., Parsippany, NJ). All tests were performed in accordance with the manufacturer’s instructions. Activated protein C measurements were performed from blood collected in tubes containing benzamidine, using an enzyme capture assay that we have successfully established in our laboratory.
Data collection, outcome measures
Data were collected prospectively on patient demographics, the injury time, mechanism (blunt or penetrating) and severity, pre-hospital fluid administration, time of arrival in the trauma room and admission vital signs. The Injury Severity Score (ISS) was used as a measure of the degree of tissue injury[19]. An arterial blood gas was drawn at the same time as the research sample as part of the standard management of major trauma patients. The base deficit was used as a measure of the degree of tissue hypoperfusion. Admission base deficit is a clinically useful early marker of tissue hypoperfusion in trauma patients, and an admission base deficit greater than 6 mmol/l has previously been identified as predictive of worse outcome in trauma patients[20, 21]. Ventilator-associated pneumonia (VAP) was diagnosed by blinded investigators from daily laboratory measurements, bacteriological culture results, and daily chest x-rays which were obtained as standard of care at SFGH. Diagnosis was made by Centers for Disease Control and Prevention criteria[22-24].
Outcome measures
Patients were followed until hospital discharge or death. For mortality analysis, patients surviving to hospital discharge were assumed to still be alive. Secondary outcome measures were also recorded for 28-day ventilator-free days, acute lung injury (American-European consensus conference definition)[23], VAP and acute renal injury (Acute Dialysis Quality Initiative consensus conference definition)[25] and blood transfusions required in the first 24 hours.
Statistical analysis
Data analysis was performed by the investigators and the Department of Biostatistics at UCSF. Normal-quantile plots were used to test for normal distribution. Relationships between injury quartiles and quartiles of activated protein C and continuous variables were tested with the Kruskall-Wallis test followed by a non-parametric test for trend. Correlation was assessed by Spearman correlation coefficients. Linear regression was used to test the relationship between injury and protein C and aPC and then between protein C and coagulopathy and continuous outcomes. Logistic regression was used to examine the relationship between the protein C system and dichotomous outcomes. A p-value of ≤ 0.05 was chosen to represent statistical significance. To define depletion of protein C after injury a slope of change was calculated between 0 and 12 hours and again between 12 and 24 hours. Depleters were defined as having a slope more negative than the median during both time periods. Balanced depleters had a positive slope in both time periods and depletion with recovery was defined as a negative slope between 0-12 hours and a positive slope between 12 and 24 hours.
Results
The trauma patients included in the study were severely injured with an average Injury Severity Score of 25±13 and average Base Deficit of −7± 1. Table 1 summarizes the type of injury and the demographics of the patients. As defined by an INR>1.3, 19.3% of patients were coagulopathic after injury. Because of the short transport times in San Francisco and predisposition toward minimal pre hospital fluid resuscitation, there was minimal pre hospital (and therefore pre blood sampling) fluid given. There was no difference in pre hospital fluid between the non coagulopathic patients and coagulopathic patients (233±312 cc vs 112± 207 cc p=NS).
Table 1.
Injury and Demographics
| VARIABLE | Mean | SD |
|---|---|---|
| AGE | 41.0 | 18.8 |
| Gender Male | 79% | |
| Mechanism Blunt | 62% | |
| Mechanism Penetrating | 38% | |
| ISS | 25.2 | 13.8 |
| Base Deficit | 7.0 | 5.6 |
| Pre sampling Resuscitation | 217cc | 319cc |
ISS: Injury severity score
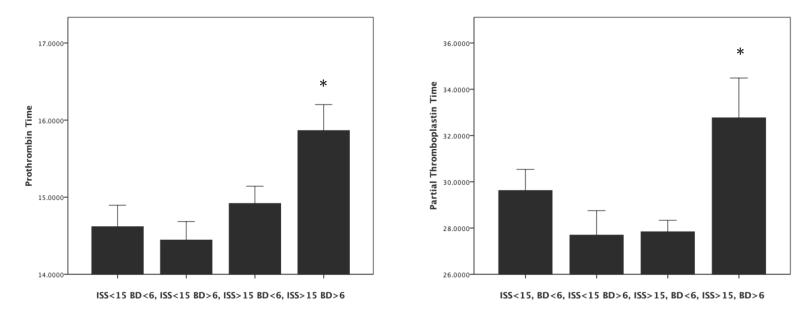
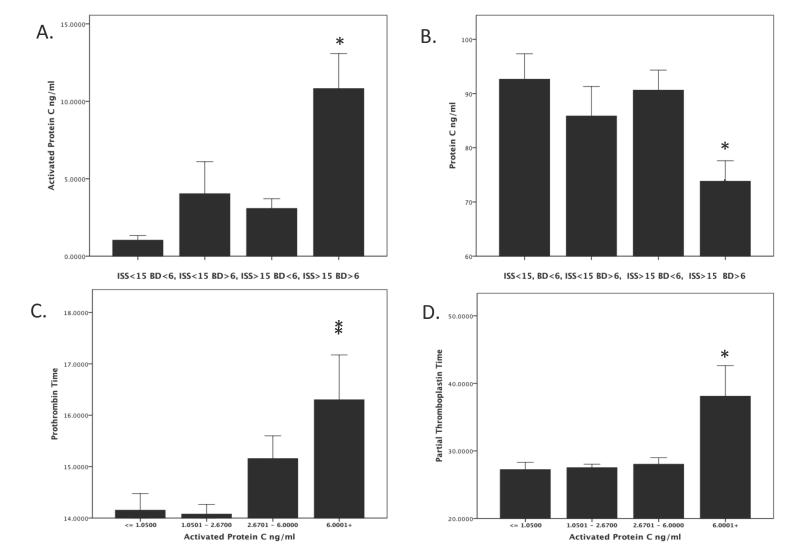
Based on previous work and accepted definitions of tissue injury and shock (BD) (ref), we divided our patients into four equal groups based on ISS and base deficit. Figure 1 shows that the combination of severe injury severity (ISS>15) and shock (BD>6) resulted in an increased PT (p=.01) and PTT (p=.03) upon arrival to the emergency department. Figure 2 indicates that there was a strong activation of the protein C pathway only in patients who presented with the combination of severe tissue injury and shock. Plasma levels of aPC were elevated while plasma levels of PC were low in the trauma patients with shock and elevated ISS compared with the other groups of patients (Figure 2A&B p<.0001). There was a significant inverse correlation between initial plasma levels of PC and aPC (r = .313 p <.0001). Figure 2C&D show a significant relationship between increasing aPC and PT (p=.007) and aPC and PTT (p = .002). As expected, there was also a significant correlation between aPC and PT (r = .31 p =.003) and PTT (r =.287 p=.006) and an inverse correlation between PC and PT (r = −.39 p<.0001) and PTT (r =−.16 p =.05).
Figure 1. A combination of tissue injury and shock result in coagulopathy in trauma patients.
Patients were divided into groups using previously described definitions of injury severity based on Injury Severity Score and Shock based on Base Deficit. This resulted in 4 groups; minimal injury, no shock (ISS<15 BD<6); minimal injury, shock (ISS>15 BD >6); severe injury, no shock (ISS>15 BD<6); and severe injury and shock (ISS >15, BD >6). Prothrombin time (PT) and partial thromboplastin time (PTT) were assayed. Patients with severe injury and shock had elevated PT (Panel A) and PTT (Panel B). All p<.05 by Kruskall Wallis rank test.
Figure 2. Tissue injury and shock result in a systemic activation of protein C pathway associated with coagulopathy in trauma patients.
Patients were divided into groups using previously described definitions of injury severity based on Injury Severity Score and Shock based on Base Deficit. This resulted in 4 groups; minimal injury, no shock (ISS<15 BD<6); minimal injury, shock (ISS>15 BD >6); severe injury, no shock (ISS>15 BD<6); and severe injury and shock (ISS >15, BD >6). Plasma levels of activated protein C (aPC) and protein C (PC) were assayed, as described in the Methods. Patients with severe injury and shock had elevation of plasma levels of aPC (Panel A) and a concomitant decrease in levels of PC (Panel B). aPC levels were then divided into quartiles. Patients with the highest quartile of plasma levels of aPC had elevated PT (Panel C) and PTT (Panel D). All p<.05 by Kruskall Wallis rank test.
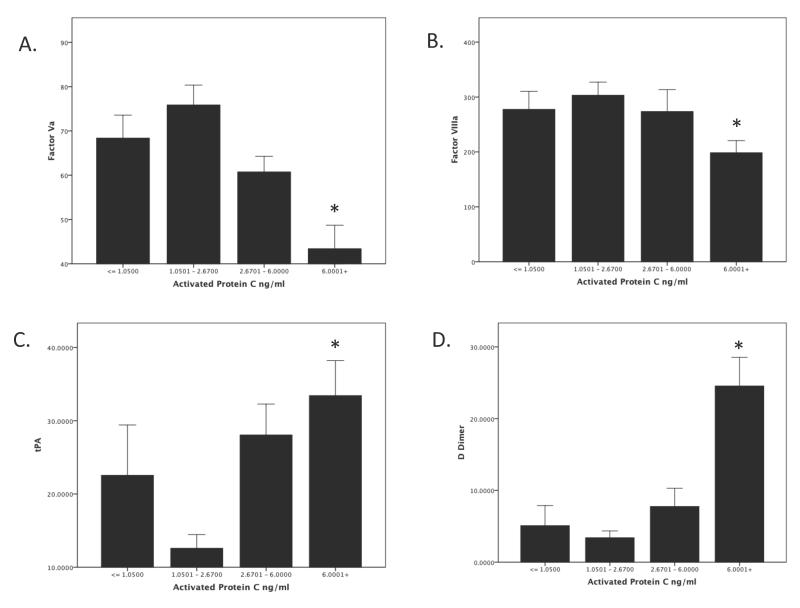
Exploring further the correlative mechanisms for this coagulopathy, Figure 3 shows the relationship between activated protein C and coagulation factors and fibrinolysis. Figure 3 A&B show an inverse relationship between protein C activation and deactivation of factors Va (3A p = <.0001) and VIIIa (3B p = <.04). There is also a relationship between aPC and fibrinolysis. Figure 3C&D shows that increasing levels of aPC are associated with increased tPA (p = .005) and D-Dimers (p =<.0001). Spearman correlation showed a significant inverse relationship between aPC and Factor Va (r=−.34 p = .001) aPC and factor VIIIa (r=−.21 p= .05) and a positive relationship between aPC and tPA (r=.41 p=.008) and aPC and d-Dimers (r= .58 p = <.0001).
Figure 3. Systemic Activation of the protein C pathway is associated with the inactivation of factors Va and VIIIa and derepression of fibrinolysis in trauma patients.
Patients were divided into quartiles based on plasma levels of activated protein C. Patients with the highest activation of protein C in the plasma (highest quartile) had a coagulopathy caused by deactivation of factor Va (Panel A) and factor VIIIa (Panel B). In addition, patients with the highest plasma level of aPC had a derepression of the fibrinolysis, as evidenced by elevated tPA and d-Dimers (Panel C&D).
Linear regression analysis confirmed a significant relationship between aPC and coagulopathy. Specifically, for every log increase in aPC there is a .57 increase in INR (95% CI 0.18-0.97; p= .005) and a 2.4 second increase in PT (95% CI 0.87-3.9; p =.005). This same relationship exists in reverse for non-activated protein C, where for each log decrease in PC there is a 1.2 increase in INR (95% CI 0.3 – 2.1; p =.01) and a 3.3 second increase in PTT (95% CI 0.04-6.7; p =.04). Taken together, these results demonstrate that severe trauma and hemorrhagic shock are associated with an activation of the protein C pathway that correlates with a coagulopathy characterized by a deactivation of the coagulation factors Va and VIIIa and a derepression of the fibrinolysis. Because we have previously reported that blocking the anticoagulant domain of aPC corrects the coagulopathy in a mouse model of trauma-hemorrhage with elevated plasma levels of aPC [26], it is likely that the activation of the protein C pathway mediates the early coagulopathy observed in trauma patients.
The second aim of the study was to determine whether there was a relationship between early activation of the protein C system and outcome from severe trauma. Because there is variability in baseline non-activated protein C levels, we used aPC to PC ratio to reflect the degree of activation of the protein C pathway at the time of admission to the hospital. Table 2 shows a strong association between activation of protein C and transfusion requirements. Protein C activation was further associated with an increased odds of ventilator associated pneumonia (OR 2.4, 95% CI 1.06-2.4; P=.02), an 1.6 fold increased odds of multiple organ failure (95% CI 1.0-2.5; p= .05), a 1.9 fold increased odds of acute lung injury (95% CI 1.2-3.1; p=.01) and an 2.1 fold increase in the odds of mortality (95% CI 1.3-2.3; p=.0007) (Table 3). In addition, patients with activation of protein C also spent significantly longer on the ventilator and had longer ICU and hospital stays (Table 4). Lastly logistic regression showed that there was no statistically significant effect of blood product transfusion (PRBC, FFP or platelets on outcomes (VAP, MOF, Mortality) (all p = NS)
Table 2.
Linear regression testing the effect of activation of protein C on transfusion and resuscitation requirements.
| Predictor | Dependent | B | OR | p value |
|---|---|---|---|---|
| aPC/PC ratio | 6h PRBC (Units) |
6.7 | 2.5 - 20.9 | .003 |
| aPC/PC ratio | 6h FFP (Units) | 5.3 | 2.4 - 8.1 | <.0001 |
| aPC/PC ratio | 6h Platelets (Units) |
.57 | .15 - .99 | .009 |
| aPC/PC ratio | 6h Crystalloid (Liters) |
2.2 | .155 - 4.2 | .035 |
aPC/PC ratio: Ratio between plasma levels of activated protein C and protein C zymogen at the admission to the hospital.
Table 3.
Logistic regression of protein C system effects on outcome.
| Predictor | Dependent | OR | CI | P |
|---|---|---|---|---|
| aPC/PC ratio | VAP | 2.4 | 1.6 - 2.62 | .024 |
| aPC/PC ratio | MOF | 1.585 | 1.0 - 2.5 | .050 |
| aPC/PC ratio | ALI | 1.894 | 1.1 - 3.1 | .01 |
| aPC/PC ratio | Mortality | 2.1 | 1.4 -3.3 | .0007 |
aPC/PC ratio: Ratio between plasma levels of activated protein C and protein C zymogen at the admission to the hospital. VAP: Ventilator-associated pneumonia. MOF: Multiple organ failure. ALI: Acute lung injury.
Table 4.
Activation of protein C and hospital outcome
| Predictor | Dependent | B | CI | P |
|---|---|---|---|---|
| aPC/PC ratio | Ventilator days | 5.63 | 3.49-7.77 | <.0001 |
| aPC/PC ratio | ICU days | 5.26 | 3.23-7.30 | <.0001 |
| aPC/PC ratio | Hospital days | 4.17 | 2.40-5.94 | <.0001 |
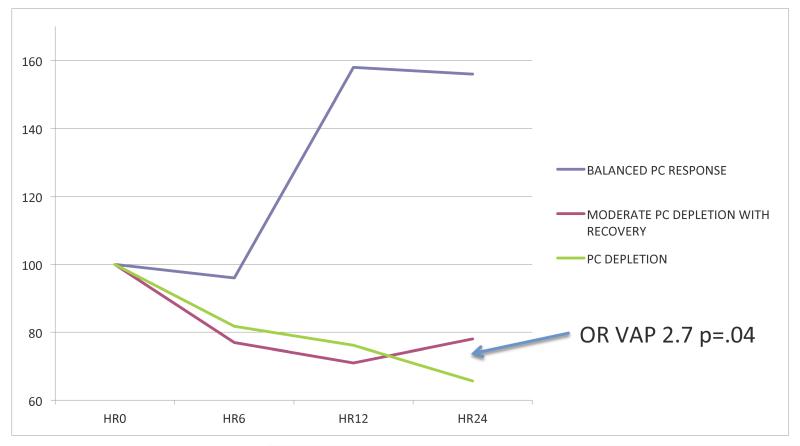
In the last series of analyses, we sought to determine whether the trauma-related activation of the protein C pathway was followed by the recovery of physiological plasma levels of protein C. We thus measured plasma levels of protein C at 0, 6, 12 and 24 hours after admission to the hospital. We then calculated the individual and median slopes between plasma protein C values measured at these different time points. We finally divided our cohort of patients into 3 groups based on whether changes over time in their plasma levels of protein C were above or below the median values of these slopes. We found that the patient group with the greatest depletion in the first 12 hours followed by no recovery or further depletion from 12-24 (most negative slope) had an 2.7 times higher risk of VAP than the patients who did not change their plasma levels of protein C during the first 24 hours after admission to the hospital (95% CI 1.05-6.8; P= .03). (Table/Figure 4) Taken together, these results indicate that the activation of the protein C pathway early after severe injury and hemorrhagic shock is associated with worse outcome in trauma patients. Furthermore, the inability to recover physiologic plasma levels of protein C within 24 hours after trauma is associated with an increased risk to develop VAP.
Figure 4. Protein C depletion is associated with an increased incidence of nosocomial lung infection in trauma patients.
Patients were divided in three groups based on changes in plasma levels of protein C during the first 24h after trauma. In one group of patients there was a significant increase in the plasma levels of protein C zymogen within 12 hours after trauma. The rest of the patients who had a decrease in the plasma levels of proten C zymogen were divided into two groups characterized either by a moderate decrease of plasma levels of PC with recovery or by a significant decrease in plasma levels of PC without recovery during the first 24 hours after trauma. Patients who decreased their plasma levels of PC without any recovery had a 2.7 times increase in nosocomial lung infection than those we show some recovery of the plasma levels of PC between 12 and 24 hours after trauma (OR 2.7 CI 1.05 −6.8 p = .04).
Discussion
We present the first evidence that the development of acute traumatic coagulopathy correlates with the activation of protein C pathway. We further report that after this initial activation of protein C, there is in some patients a rapid depletion of the plasma levels of the protein C zymogen that is associated with a significantly increased risk for developing nosocomial lung infection. These results suggest that the maintenance of adequate plasma levels of the protein C after severe trauma may protect against the development of later lung infection and represent a putative biological link between coagulopathic bleeding after injury and later infectious complications in the same trauma patients.
Coagulopathy after trauma is a common, and some degree of coagulopathic perturbation affects most severely injured patients at some point during their surgical and resuscitative course. For decades, it was commonly believed that any hypocoagulable state after trauma was due to iatrogenic mechanisms. Indeed, the accepted concept within the trauma community had long been that any coagulopathy after injury was secondary to hypothermia, dilution and metabolic acidosis that resulted from resuscitation and surgical care. Anecdotally however, several investigators had observed coagulopathy that took place before significant resuscitation, dilution and hypothermia. These observations were finally systematically studied in 2003 by Brohi and Macleod in two separate reports who documented acute traumatic coagulopathy 1 hour after injury in approximately 30% of patients[10, 11]. To test potential mechanisms to explain this coagulopathy, we performed a prospective study of 209 severely injured patients admitted to San Francisco General Hospital and reported that this acute traumatic coagulopathy was associated with a depletion of plasma levels of protein C zymogen at the admission to the hospital[12]. Subsequent work showed that this effect was also present in patients with isolated traumatic brain injury[13]. However, we did not measure in that study plasma levels of activated protein C. Furthermore, we did not collect longitudinal sampling and we therefore were unable to examine the relationship between early activation, later depletion of the protein C pathway and subsequent propensity to organ failure or infection. Our new data presented here shows that patients with a combination of severe tissue injury (elevated ISS) and shock (elevated BD) are coagulopathic nearly immediately after their injury. This coagulopathy is strongly associated with the activation of protein C pathway. Further supporting protein C activation are the strong inverse correlation between plasma levels of aPC and factor Va and VIIIa inactivation and the derepression of fibrinolysis. As expected, this activation of protein C and resultant coagulopathy is associated with increased fluid and blood product resuscitation and poorer outcome (MOF, VAP and Death).
The second significant finding our study is the relationship between early coagulopathy, subsequent depletion of the protein C system and propensity toward infection. Indeed, our new data indicates that, as early as 6 hours after trauma (and initial coagulopathy), patients begin to segregate into those who are ‘depleters’ defined by depletion of protein C stores and those who maintain physiologic plasma levels of protein C zymogen. Those who deplete and do not recover their protein C plasma levels have a significant propensity to later infectious complications.
Along with its well-described anticoagulant functions, activated protein C also has newly described cytoprotective effects[27, 28]. Recombinant aPC has been shown to protect baboons and mice from sepsis and to attenuate LPS-mediated inflammatory signaling in monocytes [14, 15]. This non-anticoagulant effect of protein C is mediated through PAR-1 and EPCR, and multiple downstream signaling pathways including Rac-1 and NF-kappaB. Evidence for a link between protein C depletion and sepsis in humans has been described. Indeed, a well-established correlation between diminished plasma levels of protein C and worsened outcome exists in patients with septic shock.[29] The understanding that a dysfunction of the protein C pathway is a likely mechanism for the end-organ damage observed after sepsis led to several groups testing the efficacy protein C, sTM, EPCR and activated protein C as therapy in septic animals and humans.[30, 31] [16]. Finally, recent work from our laboratory has shown that the administration of murine activated protein C attenuated lung injury induced by P. aeruginosa in a mouse model of pneumonia. [18]
Along with abundant data that septic shock is associated with protein C depletion, there is also evidence linking other shock states with protein C activation and depletion. Adrie et al studied survivors of cardiac arrest and showed that patients in shock from cardiac arrest had increased plasma levels of aPC followed by later depletion to undetectable levels. [32] Along with increased plasma levels of aPC, these patients also were hypocoagulable early after cardiac arrest. Because perturbations in the activity of the protein C pathway are present in both septic and cardiogenic shock, it is not surprising that trauma and hemorrhagic shock would be associated with similar abnormalities in the protein C pathway.
There are several limitations of and questions raised by this study. First, because we cannot obtain a baseline (pre injury) sample, it is impossible to know the true level of protein C activation for an individual patient. We attempted to control for this by using the aPC /PC ratio; however, we are aware that this is a best case approximation of true activation of the protein C pathway for each patient. Secondly, it is difficult to know how severe the depletion of activated protein C has to be to affect its ability to protect the vascular endothelium after severe trauma. It is possible that the propensity towards higher rate of lung infection in the protein C depletion group results from the inability to activate protein C zymogen in response to colonization of the distal airways by nosocomial bacteria. Indeed, a recently published work from our laboratory has demonstrated that the administration of activated protein C significantly attenuates P. aeruginosa-mediated damage to the alveolar-capillary barrier via its non-anticoagulant cytoprotective domain.[18] Of additional interest is the relative contribution of non-iatrogenic acute biological mechanisms (aPC) and iatrogenic (dilution, hypothermia) coagulopathy to the overall coagulopathy of trauma. Our data here support a larger role for non-iatrogenic coagulopathy early after major trauma. Indeed, our relative short pre hospital transport times and predisposition toward minimal pre hospital resuscitation make our first ED sample not confounded by excessive dilution or by the well-described effects of chloride containing resuscitation fluids. Interestingly our non-coagulopathic group received nearly a double prehospital fluid volume compared to the coagulopathic group potentially strengthening the argument for an acute biological mechanism, although the fluid resuscitation volumes in both groups were minimal and not statistically distinct. Also understood is that fact that many of the correlations between coagulation mediators while statistically significant are not statistically strong. While we strive for and achieve the highest level of standardization in our sampling and measurement, these patients are extremely heterogenous in their demographics, injuries, and physiology. This is layered on sampling in a very chaotic environment. Despite this difficulty, we have shown that we can achieve reliable sampling to characterize acute traumatic coagulopathy. To truly mechanistically test these correlations require laboratory models in which the plasma levels of activated protein C can be manipulated. Indeed, we have previously reported that activated protein C drives acute traumatic coagulopathy a mouse model of injury and hemorrhagic shock [26]. Lastly, The effect of blood transfusion on the outcomes studied also deserves discussion. While others have published direct effects of blood transfusion on outcome after severe trauma[33-35], our data did not demonstrate a direct association between blood transfusion and outcome in the present study. There are several putative reasons for this negative finding. First, our study was not designed to study the effect of blood transfusion on outcome parameters after trauma and may thus have been underpowered to detect such an association. Second, over the past several years several investigators have called into question the deleterious effects of blood transfusion and have proposed that earlier blood product transfusion in ratios closer to whole blood are in fact beneficial to the survival of trauma patients[36, 37]. During the completion of our study, blood transfusion was not standardized at our institution, although high ratio of FFP to packed red cells are usually administered to our trauma patients. In addition we do not have the data necessary to determine the reasons or granular timing for transfusion of blood products. Therefore, we were unable to determine the transfusion ratios and unable to discern whether the lack of association between the amount of blood products and outcome was in fact due to the lack of biological effect or rather due to the fact that our study was not designed to test this association. A multicenter study is currently underway to test the relationship between acute traumatic coagulopathy, blood product transfusion and outcome.
In conclusion, we present here the first evidence for an early activation of the protein C pathway that is associated with the acute coagulopathy observed in severely traumatized patients. In addition, we found an association between this early coagulopathy, later depletion of protein C stores and propensity to develop nosocomial lung infection, a common complication in severely injured patients who survive their initial injury. The, combination of the present clinical data with our previously published data on the mechanistic role of the protein C pathway in the development of coagulopathy associated with trauma-hemorrhage in mice [26] indicate that the anticoagulant function of activated protein C represents an unfortunate side-effect of a profound anti-inflammatory response being released by the body in response to severe trauma and shock. In our previous mouse work, the cytoprotective effect of aPC was necessary for survival through the acute phase while the anticoagulant function when blocked had no effect on survival. Taken together with these data it seems that acute traumatic coagulopathy represents a maladaptive response to severe injury. Indeed, after severe trauma and tissue hypoperfusion, the body is likely releasing a large amount of an antiinflammaroy and cytoprotective molecule (aPC) in an attempt to prevent the development of a lethal microvascular thrombosis and endothelial and epithelial destruction. In a perfect example of maladaptive response, activation of the protein C pathway after severe injury has a profound anticoagulant effect which results in the development of a clinically significant coagulopathy. Shortly after this ‘to much of a good thing’ response which is complicated by a bleeding diathesis, there is n certain trauma patients, a depletion of the response (‘to little of a good thing later’) and a propensity to orgain injury and infection. The implications of this connection between early acute traumatic coagulopathy and later organ dysfunction and propensity to infection are significant. Indeed, future studies are warranted to identify drivers of both the early coagulopathy and later depletion of the protein C system. From this knowledge, putative future clinical intervention could involve blocking of the anticoagulant domain of aPC early after trauma, which would correct the early posttraumatic coagulopathy, while maintaining the cytoprotective effect of that protein that is critical for the homeostasis of the vascular endothelium. Later, augmentation of the depleted protein C response by the administration of a protein C mutant that does not have the anticoagulant effect of the wild-type protein could be considered. There is thus a need for additional experimental and clinical studies to fully understand the role the protein C pathway after severe trauma.
Table 5.
Relationship between depletion of protein C over time and outcome
| Predictor | Dependent | OR | CI | P |
|---|---|---|---|---|
| 12 hour PC depletion |
VAP | .985 | .97 - .99 | .04 |
| Balanced PC response |
VAP | Referent | ||
| Moderate Depleters | VAP | 1.6 | .7 - 4.1 | .2 |
| PC Depleters | VAP | 2.7 | 1.05 – 6.8 | .04 |
VAP: Ventilator-associated pneumonia.
Acknowledgments
This work was supported by NIH GM085689 (Cohen) the AAST Hemostasis and Resuscitation Scholarship (Cohen), NIH HL 090833 (Calfee)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Injury Chart Book. World Health Orginization; Geneva: [Google Scholar]
- 2.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. The Journal of trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, et al. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001-2004. Annals of surgery. 2007;245(6):986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96(2):111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46(5):685–686. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gando S, Nanzaki S, Kemmotsu O. Disseminated intravascular coagulation and sustained systemic inflammatory response syndrome predict organ dysfunctions after trauma: application of clinical decision analysis. Annals of surgery. 1999;229(1):121–127. doi: 10.1097/00000658-199901000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfusion medicine reviews. 2003;17(3):223–231. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 9.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. The Journal of trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 10.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 11.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Current opinion in critical care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 12.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of surgery. 2007;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. The Journal of trauma. 2007;63(6):1254–1261. doi: 10.1097/TA.0b013e318156ee4c. discussion 1261-1252. [DOI] [PubMed] [Google Scholar]
- 14.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Seminars in thrombosis and hemostasis. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 15.Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E, Griffin JH, Riewald M. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. The Journal of biological chemistry. 2006;281(29):20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. The New England journal of medicine. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 17.Choi G, Schultz MJ, Levi M, van der Poll T, Millo JL, Garrard CS. Protein C in pneumonia. Thorax. 2005;60(8):705–706. doi: 10.1136/thx.2004.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bir NLM, Howard M, Goolaerts A, Roux J, Carles M, Cohen MJ, Iles KE, Fernández JA, Griffin JH, Pittet JF. Cytoprotective-selective Activated Protein C Attenuates P. aeruginosa-induced Lung Injury in Mice. American journal of respiratory cell and molecular biology. 2011 doi: 10.1165/rcmb.2010-0397OC. (Jan 21 E Pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker SP, O’Neill B, Haddon W, Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. The Journal of trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 20.Davis JW, Parks SN, Kaups KL, Gladen HE, O’Donnell-Nicol S. Admission base deficit predicts transfusion requirements and risk of complications. The Journal of trauma. 1996;41(5):769–774. doi: 10.1097/00005373-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford EJ, Morris JA, Jr., Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. The Journal of trauma. 1992;33(3):417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 22. [ http://www.cdc.gov/nhsn/psc_da.html ]
- 23.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 24.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 25.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care (London, England) 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet JF, Cohen MJ. Shock. 6. Vol. 32. Augusta, Ga: 2009. Increase in activated protein C mediates acute traumatic coagulopathy in mice; pp. 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 28.Esmon CT. Is APC activation of endothelial cell PAR1 important in severe sepsis?: No. J Thromb Haemost. 2005;3(9):1910–1911. doi: 10.1111/j.1538-7836.2005.01573.x. [DOI] [PubMed] [Google Scholar]
- 29.Liaw PC, Esmon CT, Kahnamoui K, Schmidt S, Kahnamoui S, Ferrell G, Beaudin S, Julian JA, Weitz JI, Crowther M, et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood. 2004;104(13):3958–3964. doi: 10.1182/blood-2004-03-1203. [DOI] [PubMed] [Google Scholar]
- 30.Dhainaut JF. Introduction: rationale for using drotrecogin alfa (activated) in patients with severe sepsis. American journal of surgery. 2002;184(6A Suppl):S5–10. doi: 10.1016/s0002-9610(02)01137-6. [DOI] [PubMed] [Google Scholar]
- 31.Dhainaut JF, Yan SB, Cariou A, Mira JP. Soluble thrombomodulin, plasma-derived unactivated protein C, and recombinant human activated protein C in sepsis. Critical care medicine. 2002;30(5 Suppl):S318–324. doi: 10.1097/00003246-200205001-00023. [DOI] [PubMed] [Google Scholar]
- 32.Adrie C, Monchi M, Laurent I, Um S, Yan SB, Thuong M, Cariou A, Charpentier J, Dhainaut JF. Coagulopathy after successful cardiopulmonary resuscitation following cardiac arrest: implication of the protein C anticoagulant pathway. Journal of the American College of Cardiology. 2005;46(1):21–28. doi: 10.1016/j.jacc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Benson AB, Moss M. Trauma and acute respiratory distress syndrome: weighing the risks and benefits of blood transfusions. Anesthesiology. 2009;110(2):216–217. doi: 10.1097/ALN.0b013e3181948ac0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarrete-Navarro P, Rivera-Fernandez R, Rincon-Ferrari MD, Garcia-Delgado M, Munoz A, Jimenez JM, Ortega FJ, Garcia DM. Early markers of acute respiratory distress syndrome development in severe trauma patients. Journal of critical care. 2006;21(3):253–258. doi: 10.1016/j.jcrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. The Journal of trauma. 2005;59(3):717–723. [PubMed] [Google Scholar]
- 36.Holcomb JB. Traditional transfusion practices are changing. Critical care (London, England) 14(3):162. doi: 10.1186/cc9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Annals of surgery. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]