Abstract
Chemically self-assembled antibody nanorings (CSANs) displaying multiple copies of single chain variable fragments (scFv) can be prepared from dihydrofolate reductase (DHFR) fusion proteins and bismethotrexate (BisMTX). We have designed and synthesized a BisMTX chemical dimerizer (bisMTX-NH2) that contains a third linker arm that can be conjugated to fluorophores, radiolabels and drugs. Mono-, divalent and higher order AntiCD3 CSANs were assembled with a FITC labeled bis-methotrexate ligand (bisMTX-FITC) and found to undergo rapid internalization and trafficking by the HPB-MLT, a CD3+ T-leukemia cell line, to the early and late endosome and lysosome. Since the fluorescence of bisMTX-FITC when incorporated into CSANs was found to be significantly greater than the free ligand, the stability of the endocytosed AntiCD3 CSANs could be monitored. The internalized CSANs were found to be stable for several hours, while treatment with the non-toxic DHFR inhibitor trimethoprim (TMP) resulted in a rapid loss (>80%) of cellular fluorescence within minutes, consistent with efficient intracellular disassembly of the nanorings. Over longer time periods (24 h) cellular fluorescence decreased by 75%–90%, whether or not cells had been treated with DMSO or trimethoprim. Although BisMTX is a potent inhibitor of DHFR, it was found to be non-toxic (GI50 >20uM) to HPB-MLT cells. In contrast, AntiCD3 CSANs prepared with BisMTX were found to be at least 13- fold more cytotoxic (GI50=0.5–1.5 uM) than BisMTX at 72 hours. Consistent with our findings from CSANs stability studies, no increase in cytotoxicity was observed upon treatment with trimethoprim. Taken together, our results suggest that cell receptor targeting CSANs prepared with trifunctional BisMTX could be used as potential tissue selective drug carriers
Keywords: Antibody, Drug Delivery, Nanotechnology, Cancer
Introduction
Antibody drug conjugates (ADCs) have received considerable attention due to promising clinical results, with a therapeutic recently receiving FDA approval.1,2 The specific cell targeting, as directed by the antibody, allows for the use of extremely toxic small molecules, with the targeting resulting in reduced off target side effects. These properties have led to the possibility of resurrecting small molecule therapeutics, which have been discarded from the development pipeline due to unfavorable toxicities or pharmacokinetics. To date the predominant method of attaching drugs to antibodies has been via a covalent linkage; some of which can be cleaved intracellularly through chemical or enzymatic reactions.1 The specificity afforded by antibodies (and antibody fragments) have also resulted in their use for the targeted delivery of liposomes,3 dendrimers,4 metallic nanoparticles5 and nanoparticles of non-metallic composition.6 These species have been exploited for the delivery of therapeutic and imaging agents either through non-covalent encapsulation7 or covalent attachment.4 In some cases e.g. gold nanoparticles and quantum dots the nanoparticle may also be the therapeutic or imaging agent.8,9
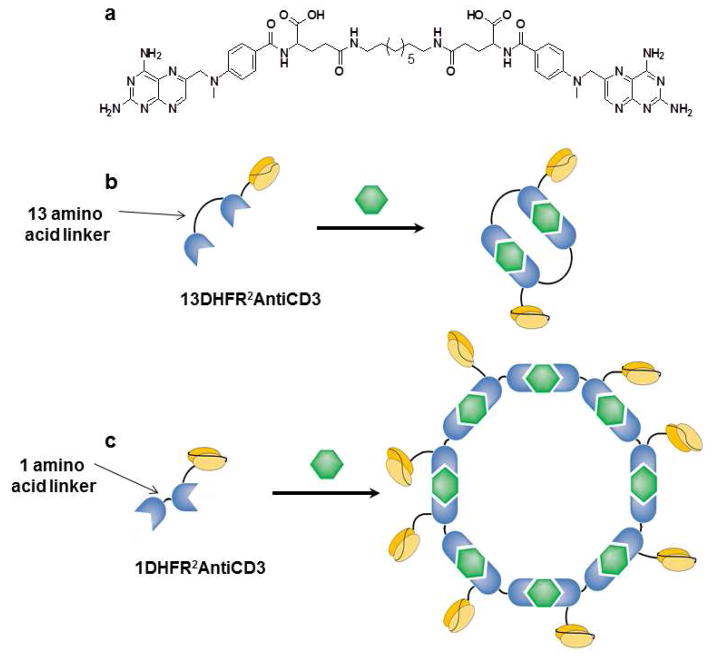
Chemically self-assembled antibody nanorings (CSANs) can be formed through the oligomerization of dimeric dihydrofolate reductase antiCD3 single chain variable fragment (scFv) fusion proteins (DHFR2antiCD3) with a bis-methotrexate (bisMTX) ligand (Figure 1).10,11 We have previously shown that antiCD3 CSANs are endocytosed upon binding to CD3+ T cells via a clathrin dependent mechanism, in a manner similar to that of the parental antiCD3 monoclonal antibody (mAb), UCHT-1.11 We hypothesized that antiCD3 CSANs could be used for the delivery of dyes and drugs to T-leukemia cells for imaging and therapeutic purposes (Figure 2). Control over ring size, and hence the valency of the displayed scFv, is possible through modification of the length and composition of the amino acid linker between the DHFR proteins: a 13 amino acid linker (13DHFR2antiCD3) results in a mixture of monomeric and dimeric species; a shorter single glycine linker (1DHFR2antiCD3) leads to predominantly octameric rings.10–12 This offers the possibility of controlling the number of therapeutic molecules delivered by the CSANs, as well as tuning the affinity for the targeted cells. Further the modular nature of the CSAN construct allows changes in size, valency, and loading of cargo. As these properties can modulate cellular endocytosis.13,14 CSANs may prove an interesting construct for which to study these effects.
Figure 1.
a) Structure of bisMTX b) Formation of dimeric CSANs by incubation of 13DHFR2antiCD3, with DHFR in blue and antiCD3 in yellow, with bisMTX, in green c) Formation of octameric CSANs by incubation of 1DHFR2antiCD3 with bisMTX..
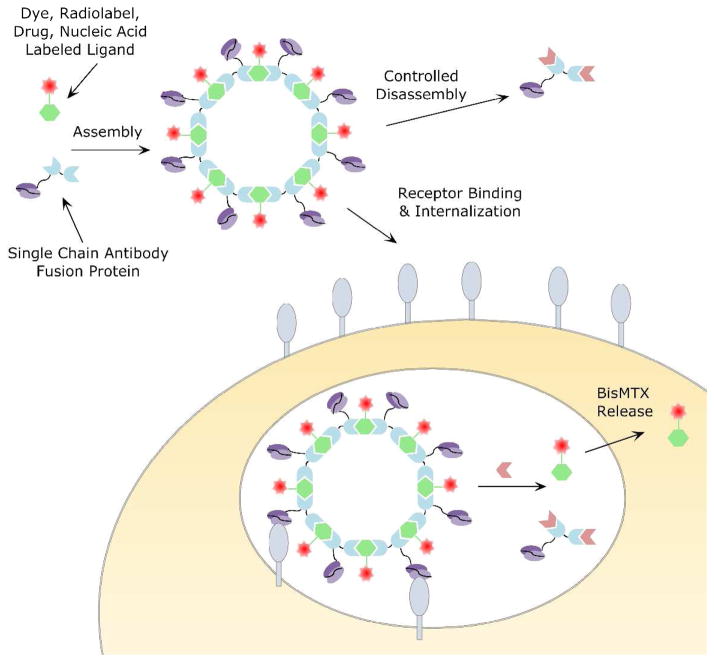
Figure 2.
Schematic showing the formation of CSAN with a branched bisMTX ligand, functionalized with a therapeutic or imaging agent. CSAN binding and endocytosis by cell surface receptors carries the cargo inside the cells where it can be released. CSANs can be disassembled by small molecule DHFR inhibitors.
Nevertheless, despite the ability of the antiCD3 CSANs to selectively bind and undergo endocytosis, little is known about the intracellular behavior of these unique nanoparticles, or their ability to carry cargoes into cells. To address this issue, we have designed and synthesized a unique trivalent bisMTX ligand to which we can attach dyes and potentially radiolabels or drugs. The attachment of a fluorophore has allowed us to verify the ability of CSANs to act as a carrier. Also because the fluorescence of the dyes is quenched upon release from the CSANs, we are able to monitor the intracellular stability of the CSANs under a variety of conditions. Further, we have shown that CSANs can be disassembled intracellularly by DHFR inhibitors. To probe the potential for endosomal escape, we have determined that while bisMTX is non-toxic to HPB-MLT (a T leukemia cancer cell line) antiCD3 CSAN mediated delivery resulted in an increase in cytotoxicity of at least 13-fold. Taken together, these results demonstrate that T-leukemia cells endocytose intact antiCD3 CSANs, which have a significant life-time in the cell, and that upon disassembly bisMTX is released into the cytoplasm.
Experimental Section
General Methods
13DHFR2antiCD3 and 1DHFR2antiCD3 were prepared as described previously.10,11 13DHFR2antiCD22 was prepared in a similar manner. Experimental details on plasmid construction and protein expression will be reported elsewhere. BisMTX was synthesized as described previously.15 HPB-MLT (T leukemia) and Raji (B lymphoma) cells were cultured in RPMI-1640 media (Lonza) supplemented with 10 % (v/v) fetal bovine serum, L-Glutamine (2 mM final concentration), penicillin (100 units/mL), and streptomycin (100 μg/mL) in a humidified incubator with 5 % CO2 at 37°C.
Size exclusion chromatography
BisMTX-FITC (3 eq) was mixed with either 13DHFR2antiCD3 or 1DHFR2antiCD3 in P500 buffer (0.5 M NaCl, 50 mM KH2PO4, 1 mM EDTA, pH 7) and incubated at room temperature for approximately 10 minutes. The CSAN solution was injected onto a Superdex G200 size exclusion column (Amersham Biosciences, USA) and eluted with P500 buffer at 0.5 mL/min.
Fluorescence Confocal Microscopy
BisMTX-FITC (or BisMTX-PG) and the appropriate DHFR fusion protein were mixed (250 nM) and added to 0.5 × 106 HPB-MLT cells at either 4 or 37 °C for 1 hr in RPMI media. Cells were then pelleted by centrifugation (400 × g, 5 min). After being washed twice with PBS (phosphate buffered saline) cells were incubated on Poly-Prep slides coated with poly-L-Lysine (Sigma) at 4 or 37 °C for 30 mins. Cells were then fixed with 4 % paraformaldehyde solution for 10 mins and washed thrice with PBS. Finally, cells were treated with ProLong Gold Antifade reagent with DAPI (Invitrogen), a cover slip was applied.
After overnight incubation images were taken within inner sections of the cells by sequential scanning using a fluorescence confocal microscopy using an Olympus FluoView 1000 BX2 Upright Confocal microscope. Resulting images are the compression of 3–5 z-axis slices. For disassembly experiments after the initial one hour incubation cells were washed twice with PBS before being resuspended in media containing 2 mM trimethoprim, 0.5 mM pyrimethamine or DMSO for three hours at 37 °C after which time they where processed as above. Early endosome co-localization experiments were performed by incubating cells with antiCD3 CSANs or FITC labeled UCHT-1 with Alexa Fluor 594 labeled transferrin (Life Technologies) for 30 minutes at 37 °C before washing and continuing slide preparation as above. Lysosomes were labeled by incubating cells with media containing LysoTracker red DND-99 (Life Technologies) for 1 hour at 37 °C, in the presence of CSANs or FITC labeled UCHT-1, before washing and slide preparation as above.
Flow Cytometry
1 × 106 HPB-MLT (or Raji) cells were treated with BisMTX-FITC containing CSAN constructs at the required concentrations at 4 °C for 1 hr in PBS buffer (containing 0.05 % BSA and 0.1 % sodium azide). Cells were centrifuged (400 × g, 10 min), washed twice with PBS/BSA/sodium azide buffer before being resuspended in the same buffer. Their fluorescence was analyzed with a FACSCalibur flow cytometer (BD Biosciences). For competition experiments, HPB-MLT cells were incubated with 40 nM UCHT-1 on ice for 10 mins prior to addition BisMTX-FITC CSAN constructs anti-CD3 constructs (100 nM) followed by washing as above. For disassembly experiments HPB-MLT cells were treated with 150 nM CSANs or 3.95 nM UCHT-1 FITC for 1 hour at 37 °C before being washed twice with RPMI media and finally resuspended in media. The cells (0.5 × 106) were aliquoted into 1 mL of media containing either 2 mM trimethoprim or DMSO and incubated at 37 °C for the required period of time. Cells were then pelleted, washed once with PBS buffer and fixed with 0.5 % paraformaldehyde in PBS buffer. Samples were stored at 4 °C in the dark until analyzed.
Cytotoxicity Analysis
2.5 × 104 HPB-MLT cells were mixed with the appropriate concentration of antiCD3 CSANs in a total volume of 90 uL and incubated at 37 °C for one hour. Then 10 uL media containing trimethoprim (or an equivalent volume of DMSO) was added to give a final concentration of 250 μM before the cells were incubated at 37 °C for 72 hours. Control cells were incubated with either 250 μM trimethoprim or DMSO. Cell viability was determined using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega).
Results and Discussion
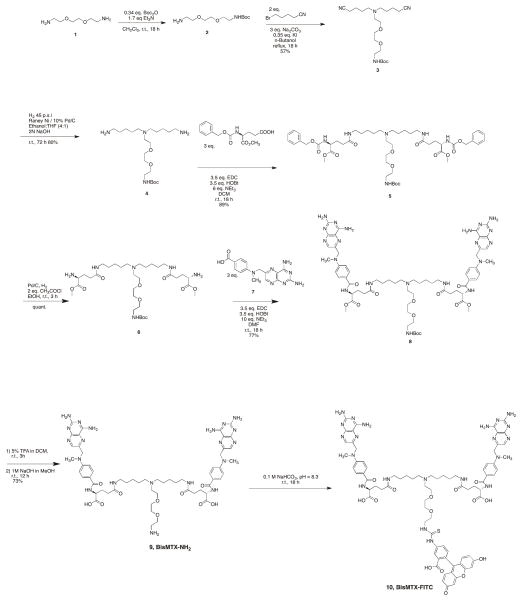
We designed and synthesized a branched bisMTX ligand with the third arm containing a primary amine 9, for the attachment of dyes (Scheme 1). The synthesis of the bis-MTX amine trilinker was achieved within seven convenient steps (Scheme 1). Initially the compound 2,2- (ethylenedioxy)bis(ethylamine) (1) was mono amine protected with di-tert-butyl dicarbonate (0.34 eq) and triethyl amine (1.7 eq) in dichloromethane. The resulting product (2) was refluxed with 5- bromovaleronitrile (2 eq) in the presence of potassium iodide (0.35 eq) and sodium carbonate (3 eq) in n-butanol to yield the tri-linker core assembly (3). Reductive hydrogenation of compound 3 with Raney-Ni did not go to completion despite several attempts with increasing amounts of the catalyst. However, the combination of Raney Ni and 10% Pd on C with 2 N NaOH in ethanol:tetrahydrofuran (4:1) completely reduced the nitrile to afford the diamine (4). The coupling of the diamine with N-carbobenzyloxy- L-glutamic acid 1-methyl ester (3 eq) in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, 3.5 eq), 1-hydroxybenzotriazole (HOBt, 3.5 eq) and triethyl amine (10 eq) in dichloromethane provided the compound 5. Carboxybenzyl amine protections were then removed by Pd catalyzed hydrogenation to yield the compound 6. Subsequent EDC mediated coupling of compound 6 with pteroic acid derivative15 (7) provided the fully protected trilinker (8). Deprotection of the tert-butyl carbonate groups with 5% trifluoroacetic acid in dichloromethane followed by the removal of methyl ester protections with 1M NaOH in methanol afforded the fully deprotected trilinker (9) in a total yield of 17%. The terminal primary amino acid in 9 can be used for attachment of a variety of moieties, via a stable amide bond. Initially, 9 was derivatized with fluorescein, through reaction with FITC to yield 10 (bisMTX-FITC).
Scheme 1.
Synthetic scheme for the synthesis of bisMTX-NH2, 9, and bisMTX-FITC, 10.
After incubation of 13DHFR2antiCD3 (the protein with the 13 amino acid linker between the DHFR proteins) with bisMTX-FITC size exclusion chromatography (SEC) analysis shows a mixture of dimeric and monomeric species similar to that observed upon incubation of the same protein with bisMTX (Figure 3).11 Analysis of the SEC trace at 494 nm, the absorbance maximum for bisMTX-FITC, revealed that the monomer peak contained this absorbance, suggesting formation of an intramolecular macrocycle, comprising one 13DHFR2antiCD3 protein and one bisMTX-FITC ligand (Figure 3). Predominantly octameric CSANs are formed when bisMTX is mixed with 1DHFR2antiCD3 however, when bisMTX-FITC is used as the assembly agent, a distribution of smaller rings, ranging in size from dimer to hexamers were observable (Supporting Information Figure S1). When compared to both CSANS and antiCD3-CSANS, the antiCD3 CSANS prepared from FITC-bisMTX preferentially form smaller rings. This behavior is likely due to the effect of increased effective molarity on the binding of FITC to a low affinity hydrophobic site near the bisMTX binding site of DHFR2. The theoretical analysis by Ercolani and co-workers of the critical importance of effective molarity on macromolecular non-covalent macrocyclization lends support to this possibility.13 In addition, the discrete ring sizes observed for antiCD3-CSANS prepared from bisMTX linked to hydrophilic molecules, such as diethylene triamine pentaacetic acid (DTPA), 1,4,7,10- tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and adenine arabinoside monophosphate (araAMP) have been found to be identical to those for antiCD3-CSANS prepared from bisMTX alone. (S. C. Kumarapperuma and C. R. Wagner, unpublished result) The molecular basis for the observed affect of FITC and other hydrophobic molecules tethered to bisMTX is under investigation and will be reported in due course.
Figure 3.
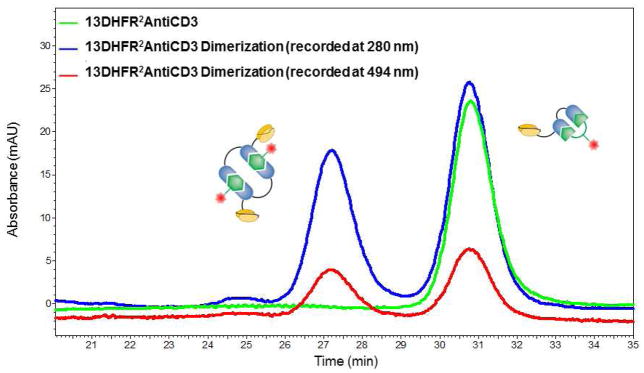
Size exclusion chromatography trace showing the formation of dimeric and monomeric species upon incubation of 13DHFR2antiCD3 with bisMTX-FITC.
In order to evaluate the interactions of bisMTX-FITC containing antiCD3 CSANs with CD3 positive cells, mixtures of bisMTX-FITC with either 13DHFR2antiCD3 or 1DHFR2antiCD3 were incubated with HPB-MLT cells. After washing, the cells were analyzed by flow cytometry (Figure 4). Increased fluorescence was observed for HPB-MLT cells that had been incubated with CSANs composed of bisMTX-FITC with either 13DHFR2antiCD3 or 1DHFR2antiCD3, relative to unstained HPB-MLT cells. The change in fluorescence was shown to be dependent on the concentration of the bisMTX-FITC containing CSANs, which were incubated with the HPB-MLT cells (Supporting Information Figure S2). Incubation of CSANs lacking antiCD3 scFvs (i.e. 13DHFR2) with HPB-MLT cells does not result in increased fluorescence, showing the necessity, as previously demonstrated, of antiCD3 scFvs for binding to the CD3+ cells (Figure 4).10,11 When antiCD3 CSANs were incubated with CD3 negative Raji (B) cells no increased fluorescence was observed (Supporting Information Figure S3). Further evidence of the specific nature of the antiCD3 CSANs interactions with cell surface CD3 receptor was confirmed by the greatly reduced fluorescence observed when HPB-MLT cells were incubated with unlabeled monoclonal antiCD3, prior to addition of bisMTX-FITC containing CSANs (Supporting Information Figure S4). Together these experiments demonstrate that antiCD3 CSANs formed with bisMTX-FITC specifically bind to HPB-MLT cells via scFv targeted interactions with cell surface CD3.
Figure 4.
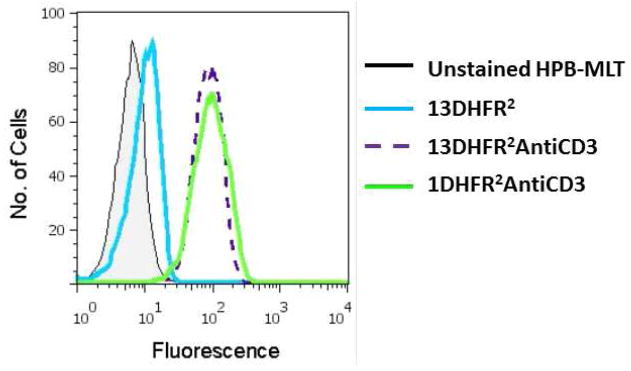
Flow cytometry traces showing the interaction of CSANs assembled with bisMTX-FITC interacting with HPB-MLT cells, recorded at either 280 or 494 nm (as described in the legend).
To explore whether CSANs could deliver bisMTX-FITC intracellularly, HPB-MLT cells were incubated with a mixture of 13DHFR2antiCD3 and bisMTX-FITC at 37 or 4 °C and then visualized by fluorescence confocal microscopy. Cells incubated with the CSANs at 37 °C show internalized green punctates due to bisMTX-FITC fluorescence (Figure 5). The presence of intracellular green punctates is consistent with antiCD3 mediated endocytosis of the FITC containing CSANs. This is similar to what is observed when FITC labeled UCHT-1 mAb is incubated with HPB-MLT cells.10,11 When CSANs are incubated with HPB-MLT cells at 4 °C the green fluorescence is observed on the cell membrane and no endocytosis is observed. This is consistent with an energy dependent endocytosis mechanism, which occurs upon scFv (or mAb) binding to CD3 cell surface receptors.10,11 Monomeric and dimeric antiCD3 CSAN species as observed by SEC (Figure 2) were purified and separately incubated with HPB-MLT cells. Similar patterns of internalization were observed for both monomeric and dimeric antiCD3 species (Supporting Information Figure S5). Incubation of bisMTX-FITC alone or CSANs formed by 13DHFR2antiCD22 (a B-cell specific scFv) and bisMTX-FITC with HPB-MLT cells resulted in no observable internalized fluorescence (Figure 5). CSANs formed by mixing 1DHFR2antiCD3 and bisMTX-FITC were also endocytosed by HPB-MLT cells, as visualized by the presence of internal green punctates (Supporting Information Figure S6). When bisMTX-FITC containing antiCD3 CSANs were incubated with HPB-MLT cells at 37 °C in the presence of Alexa Fluor 594 labeled transferrin (a marker of early endosomes17) co-localization of red and green fluorescent punctuates was observed, indicating that antiCD3 CSANs carry the bisMTX-FITC into early endosomes (Supporting Information Figure S7). Further confocal experiments using a red dye to label lysosomes (LysoTracker red DND-99) revealed that the green punctates and lysosomes co-localize in a similar manner to FITC labeled UCHT-111 (Supporting Information Figure S8) Thus, bisMTX-FITC was found to be internalized by the antiCD3 CSANs.
Figure 5.
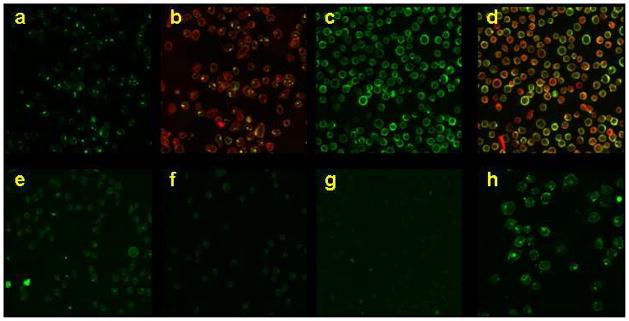
Fluorescence confocal microscopy images showing a) 13DHFR2antiCD3 and bisMTX-FITC with HPB-MLT cells incubated at 37 °C b) overlayed with Concanavalin A-Alexa Fluor 594 labeling the cell membranes c) 13DHFR2antiCD3 and bisMTX-FITC with HPB-MLT cells incubated at 4 °C d) overlayed with Concanavalin A-Alexa Fluor 594 labeling the cell membranes e) 13DHFR2antiCD22 (a B-cell specific scFv) and bisMTX-FITC with HPB-MLT cells incubated at 37 °C f) 13DHFR2antiCD22 and bisMTX-FITC with HPB-MLT cells incubated at 4 °C g) bisMTX-FITC with HPB-MLT cells incubated at 37 °C h) 13DHFR2antiCD22 and bisMTX-FITC with CD22+ Raji cells incubated at 37 °C.
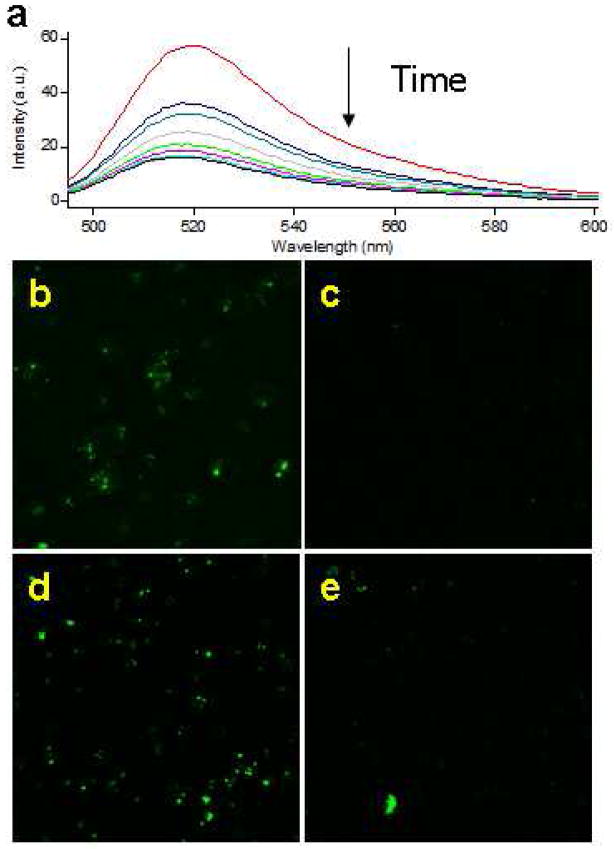
Previously, we had demonstrated that dimeric antiCD3 CSANs prepared with bisMTX could undergo rapid disassembly in the presence of an excess of the DHFR inhibitor, trimethoprim (TMP) in PBS, pH 7.0.11 Similarly, treatment of CSANs prepared from bisMTX-FITC and 13DHFR2antiCD3 with 2 mM trimethoprim resulted in conversion to the monomeric species as determined by SEC (Data not shown). During the course of our investigations, we discovered that that in solution the fluorescence of bisMTX-FITC relative to fluorescein, was greatly quenched possibly due to hydrophobic stacking interactions between FITC and the two MTX moieties15 (Data not shown). However, after mixing 1DHFR2antiCD3 with bisMTX-FITC, a 2-fold increase in FITC fluorescence was observed, which could be eliminated in a time dependent manner by the subsequent addition of excess trimethoprim (Figure 6a). In the absence of trimethoprim, no decrease in fluorescence was observed over the same time period. Consequently, the assembly and disassembly of CSANs prepared from bisMTX-FITC can be monitored by observing the modulation of the incorporated fluorescein fluorescence.
Figure 6.
(a) Addition of 2 mM trimethoprim to FITC containing CSANs results in a time dependent loss of fluorescence, due to CSAN disassembly. Conversely, addition of DHFR to bisMTX-FITC results in a concentration dependent increase in FITC fluorescence (data not shown) (b) Treatment of HPB-MLT cells with 2 mM trimethoprim results in loss of green punctates showing intracellular disassembly of CSANs for either 13DHFR2antiCD3 and bisMTX-FITC (c) or 1DHFR2antiCD3 and bisMTX-FITC (e) while DMSO treated cells show no loss of fluorescence (b and d, respectively).
Because trimethoprim is cell permeable, we hypothesized that treatment of endocytosed CSANs prepared with bisMTX-FITC with trimethoprim would allow us to observe their intracellular disassembly and release of bisMTX-FITC. After treating HPB-MLT cells with bisMTX-FITC containing CSANs, cells were washed and further incubated with non-toxic concentrations of trimethoprim (2 mM) for 3 hours at 37 °C, before being visualized by fluorescence confocal microscopy. Cells treated with trimethoprim showed loss of green punctates while intracellular green fluorescent punctates were still observable in untreated cells (Figure 6b–e). A similar loss of green punctates was observed when cells were treated with 0.5 mM pyrimethamine, a potent non-toxic inhibitor of bacterial and parasitic DHFR (data not shown).
Due to the pH dependent nature of FITC fluorescence, it is possible that acidification of the endosomes during the incubation results in the decreased fluorescence. Extracellular experiments have shown that the fluorescence of bisMTX-FITC containing CSANs is reduced at pH 5.5, as compared to pH 7.0 (Supporting Information Figure S9). However, this is unlikely the major cause of the observed decrease fluorescence, as the green fluorescence of the punctates remained stable for control cells that were not treated with trimethoprim. To further probe the possibility of pH dependence on the internalized CSANs, we labeled 9 with Pennsylvania Green (bisMTX-PG), a fluorescein analogue with pH independent fluorescent properties.18 A similar loss of fluorescence was observed with CSANs carrying bisMTX-PG when treated with trimethoprim or pyrimethamine, compared to untreated control cells (Supporting Information, Figure S10).
To further examine the disassembly kinetics of bisMTX-FITC containing CSANs we performed a flow cytometry experiment analyzing the time dependent decrease in fluorescence in the presence and absence of trimethoprim. HPB-MLT cells were treated with 150 nM CSANs composed of either 1DHFR2antiCD3 or 13DHFR2antiCD3 and bisMTX-FITC for one hour before being washed and incubated with either 2 mM trimethoprim or an equivalent volume of DMSO (as a control) at 37 °C for various time periods after which they were washed and fixed. Cells that were not treated with trimethoprim or DMSO, but were fixed after washing, were taken as time 0 and assumed to have 100% fluorescence. A rapid loss of fluorescence was observed for cells treated with bisMTX-FITC containing CSANs followed by trimethoprim (Figure 7). Within 15 minutes, the fluorescence for cells treated with 13DHFR2antiCD3 and bisMTX-FITC CSANs followed by trimethoprim was reduced by 85% while a 60% loss of fluorescence was observed for cells that had been treated with 1DHFR2antiCD3 and bisMTX-FITC CSANs followed by trimethoprim. By comparison, cells that were treated with DMSO, instead of trimethoprim, showed a significantly slower rate of decrease in fluorescence, with a loss of fluorescence of only 45% after 4 hours, whether previously treated with 1DHFR2antiCD3 or 13DHFR2antiCD3 bisMTX-FITC CSANs. In comparison, only 12% of the cellular fluorescence was lost over the same time period for HPB-MLT cells treated with fluorescein labeled parental monoclonal antibody, UCHT-1 (as a separate control), whether they were treated with DMSO or trimethoprim. In contrast, when cells that had internalized 1DHFR2antiCD3 or 13DHFR2antiCD3 bisMTX-FITC CSANs were treated with DMSO or trimethoprim for 24 hr, a similar decrease in fluorescence of 75% to 90%, respectively, was observed. These results imply that over time, disassembly of the endocytosed CSANs and release of bisMTX-FITC can occur in the absence of trimethoprim (Supporting Information Figure S11). In contrast, only a 50% loss of fluorescence was observed for HPB-MLT cells treated with FITC labeled UCHT-1 for 24 hours.
Figure 7.
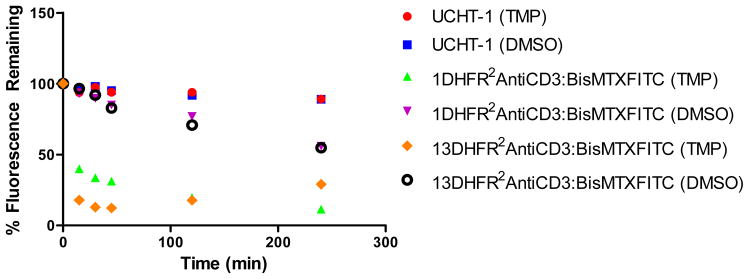
After treatment of HPB-MLT cells with bisMTX-FITC containing CSANs, incubation with trimethoprim causes a rapid decrease in fluorescence, as compared to a slow decrease for untreated cells or cells treated with FITC labeled UCHT-1 as observed by flow cytometry.
Taken together these experiments provide evidence that antiCD3 CSANs containing bisMTX-FITC are endocytosed by HPB-MLT cells and are partially stable intracellularly over 3–4 hours. In addition, treatment with small molecule inhibitors of DHFR results in the rapid intracellular disassembly of CSANs, while significant intracellular trimethoprim independent disassembly and release of bisMTX-FITC is observed over 24 hours. Nevertheless, the fluorescence quenching observed for bisMTX-FITC upon CSANs disassembly prevents us from monitoring bisMTX-FITC endosomal escape.
Previously, we had demonstrated that bisMTX (Ki = 48 pM) is nearly as potent an inhibitor of murine DHFR as MTX (Ki = 33 pM).19
Since murine DHFR is 95% sequence identical to human DHFR, we evaluated, in comparison to MTX, the cytotoxicity of both 13DHFR2antiCD3 and 1DHFR2antiCD3-bisMTX CSANs to HPB-MLT cells as an indicator of bisMTX endosomal escape upon CSAN disassembly (Figure 1A). Treatment of HPB-MLT with bisMTX alone resulted in no observed toxicity after 72 h (GI50 > 20 uM), while MTX inhibited cellular proliferation with an IC50 = 0.7 uM and IC80 > 50 uM (Figure 8, and Supporting Information). The inability of bisMTX to undergo folate receptor mediated internalization is likely responsible for its lack of activity relative to MTX, which may result from the strong preference of bisMTX for a folded conformation in aqueous solution.15 By comparison treatment for 72 h with 13DHFR2antiCD3 or 1DHFR2antiCD3 CSANs inhibited cellular proliferation with an IC50 = 0.5–1.5 uM and an IC80 ~ 2.5 uM. Consistent with the flow cytometry experiment showing that after 24 h the disassembly of CSANs is not significantly different for cells incubated in the presence or absence of trimethoprim (vide supra), treatment of the cells with trimethoprim following incubation of the cells with the CSANs for one hour had no affect on the observed cytotoxicity (Data not shown). These results are consistent with the release of bisMTX from the endosomal compartment, upon CSANs disassembly, thus resulting in likely DHFR inhibition and the observed cytotoxicity. The similar GI50 values determined for both the dimeric and octameric CSANs may be due to differences in the levels of internalization, which may be affected by the size of the constructs.14
Figure 8.
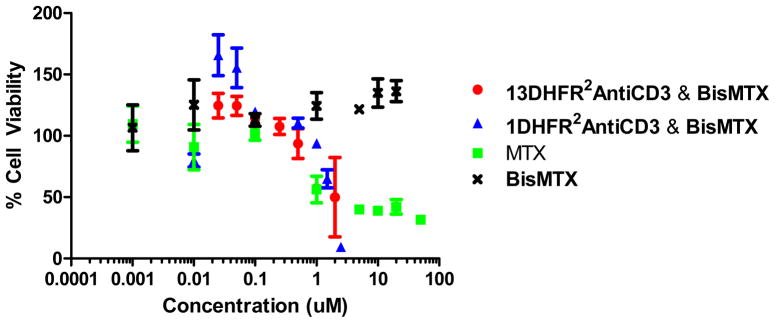
Cytotoxicity of MTX (green square), bisMTX (black cross) and 13DHFR2AntiCD3 (red circle) or 1DHFR2AntiCD3 (blue triangle) delivered bisMTX to HPB-MLT cells at 72 hrs, as determined by MTS assay.
In summary, we have prepared a new water soluble bisMTX dimerizer, bis-MTX-NH2 (9) and demonstrated that it can be FITC labeled yielding bisMTX-FITC. Similar to bisMTX, mono- di- and multivalent CSANs fused to an anti-CD3 scFv were prepared with bisMTX-FITC.11 Once internalized, the bisMTX-FITC CSANs were shown to traffic to the early and late endosome and lysosome. Because bisMTX-FITC has a much higher fluorescence when incorporated into CSANs, the intracellular life time of the nanorings could be monitored. Disassembly of the CSANs and release of bisMTX-FITC could be achieved in minutes when the cells were treated with the non-toxic DHFR inhibitors, trimethoprim or pyrimethamine. If not treated with a DHFR antagonist, the CSANs were shown to persist in the endosomal compartments for several hours. While bisMTX, a potent DHFR inhibitor, was found to be non-toxic to T-leukemia cells expressing CD3, when incorporated into a mono- di- and multivalent antiCD3 CSANs, potent cytotoxicity was observable and thus consistent with endosomal release of bisMTX. Given the lack of bisMTX toxicity, we are tempted to hypothesize that bisMTX may have a decreased ability to cause MTX associated toxicities if inadvertently released from the CSANs during systemic circulation. Ongoing in vivo studies will clarify the validity of this hypothesis. We will also be studying the in vivo stability of the CSANs, as well as their biodistribution. In addition, results from studies of the design of CSANs prepared from bisMTX-NH2 conjugated to an additional and releasable drug are in progress and will be reported in due course.
Supplementary Material
Acknowledgments
We would like to thank Dr. Blake Peterson for the gift of the Pennsylvania Green N-hydroxysuccinimide. Financial support for these studies through NIH Grants CA120116 (C.R.W.) and CA125360 (C.R.W.) is gratefully acknowledged.
Abbreviations
- CSAN
chemically self-assembled antibody nanorings
- Cbz
carboxybenzyl
- DMSO
dimethyl sulfoxide
- FITC
fluorescein isothiocyanate
- mAb
monoclonal antibody
- MTX
methotrexate
- Pyr
pyrimethamine
- SEC
size exclusion chromatography
- TMP
trimethoprim
Footnotes
Supporting Information Available. Full synthetic procedures for the preparation of 9 and 10, formation and characterization of CSANs and additional figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Alley SC, Okeley NM, Senter PD. Antibody–drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Bio. 2010;14:529–37. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 2.Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35) Clin Cancer Res. 2011;17:6428–36. doi: 10.1158/1078-0432.CCR-11-0488. [DOI] [PubMed] [Google Scholar]
- 3.Cheng WW, Allen TM. The use of single chain Fv as targeting agents for immunoliposomes: an update on immunoliposomal drugs for cancer treatment. Expert Opin Drug Deliv. 2010;7:461–78. doi: 10.1517/17425240903579963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.New K, Milenic DE, Ray GL, Kim YS, Brechbiel MW. Preparation of cystamine core dendrimer and antibody-dendrimer conjugates for MRI angiography. Mol Pharm. 2012;9:374–81. doi: 10.1021/mp2003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chattopadhyay N, Fonge H, Cai Z, Scollard D, Lechtman E, Done SJ, Pignol JP, Reilly RM. Role of Antibody-Mediated Tumor Targeting and Route of Administration in Nanoparticle Tumor Accumulation in Vivo. Mol Pharm. 2012;9:2168–79. doi: 10.1021/mp300016p. [DOI] [PubMed] [Google Scholar]
- 6.Tivnan A, Orr WS, Gubala V, Nooney R, Williams DE, McDonagh C, Prenter S, Harvey H, Domingo-Fernández R, Bray IM, Piskareva O, Ng CY, Lode HN, Davidoff AM, Stallings RL. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS One. 2012;7:e38129. doi: 10.1371/journal.pone.0038129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehtinen J, Raki M, Bergström KA, Uutela P, Lehtinen K, Hiltunen A, Pikkarainen J, Liang H, Pitkänen S, Määttä AM, Ketola RA, Yliperttula M, Wirth T, Urtti A. Pre-targeting and direct immunotargeting of liposomal drug carriers to ovarian carcinoma. PLoS One. 2012;7:e41410. doi: 10.1371/journal.pone.0041410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melancon MP, Lu W, Zhong M, Zhou M, Liang G, Elliott AM, Hazle JD, Myers JN, Li C, Stafford RJ. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials. 2011;32:7600–8. doi: 10.1016/j.biomaterials.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan J, Song H, Qian Q, Li C, Wang K, Bao C, Cui D. HER2 monoclonal antibody conjugated RNase-A-associated CdTe quantum dots for targeted imaging and therapy of gastric cancer. Biomaterials. 2012;33:7093–102. doi: 10.1016/j.biomaterials.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Hapka D, Chen H, Vallera DA, Wagner CR. Self-assembly of antibodies by chemical induction. Angew Chem Int Ed. 2008;47:10179–82. doi: 10.1002/anie.200803507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, So CR, Fegan A, Cody V, Sarikaya M, Vallera DA, Wagner CR. Chemically Self-Assembled Antibody Nanorings (CSANs): Design and Characterization of an Anti-CD3 IgM Biomimetic. J Am Chem Soc. 2010;132:17247–57. doi: 10.1021/ja107153a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson JCT, Jena SS, Flenniken M, Chou TF, Siegel RA, Wagner CR. Chemically Controlled Self-Assembly of Protein Nanorings. J Am Chem Soc. 2006;128:7630–8. doi: 10.1021/ja060631e. [DOI] [PubMed] [Google Scholar]
- 13.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A. 1999;96:2379–84. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99:912–22. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 15.Carlson JCT, Kanter A, Thuduppathy GR, Cody V, Pineda PE, McIvor RS, Wagner CR. Designing Protein Dimerizers: The Importance of Ligand Conformational Equilibria. J Am Chem Soc. 2003;125:1501–7. doi: 10.1021/ja026264y. [DOI] [PubMed] [Google Scholar]
- 16.Ercolani G. J Phys Chem B. 2003;107:5052–5057. [Google Scholar]
- 17.Dautry-Varsat A, Ciechanover A, Lodish HF. pH therecycling of transferrin during receptor-mediated endocytosis. Proc Nat Acad Sci USA. 1983;80:2258–62. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mottram LF, Boonyarattanakalin S, Kovel RE, Peterson BR. The Pennsylvania Green Fluorophore: a hybrid of Oregon Green and Tokyo Green for the construction of hydrophobic and pH-insensitive molecular probes. Org Lett. 2006;8:581–4. doi: 10.1021/ol052655g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineda P, Kanter A, McIvor RS, Benkovic SJ, Rosowsky A, Wagner CR. Dihydrofolate reductase mutant with exceptional resistance to methotrexate but not to trimetrexate. J Med Chem. 2003;46:2816–8. doi: 10.1021/jm034057i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.