Abstract
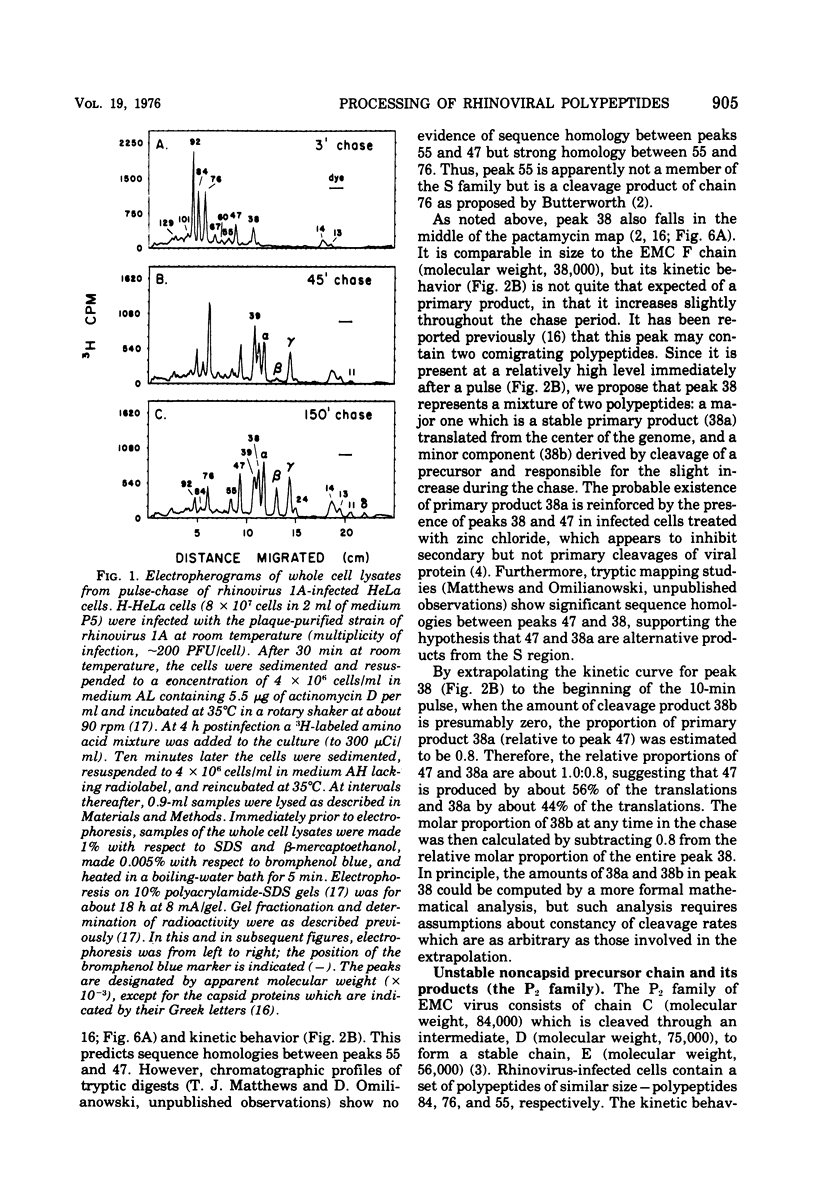
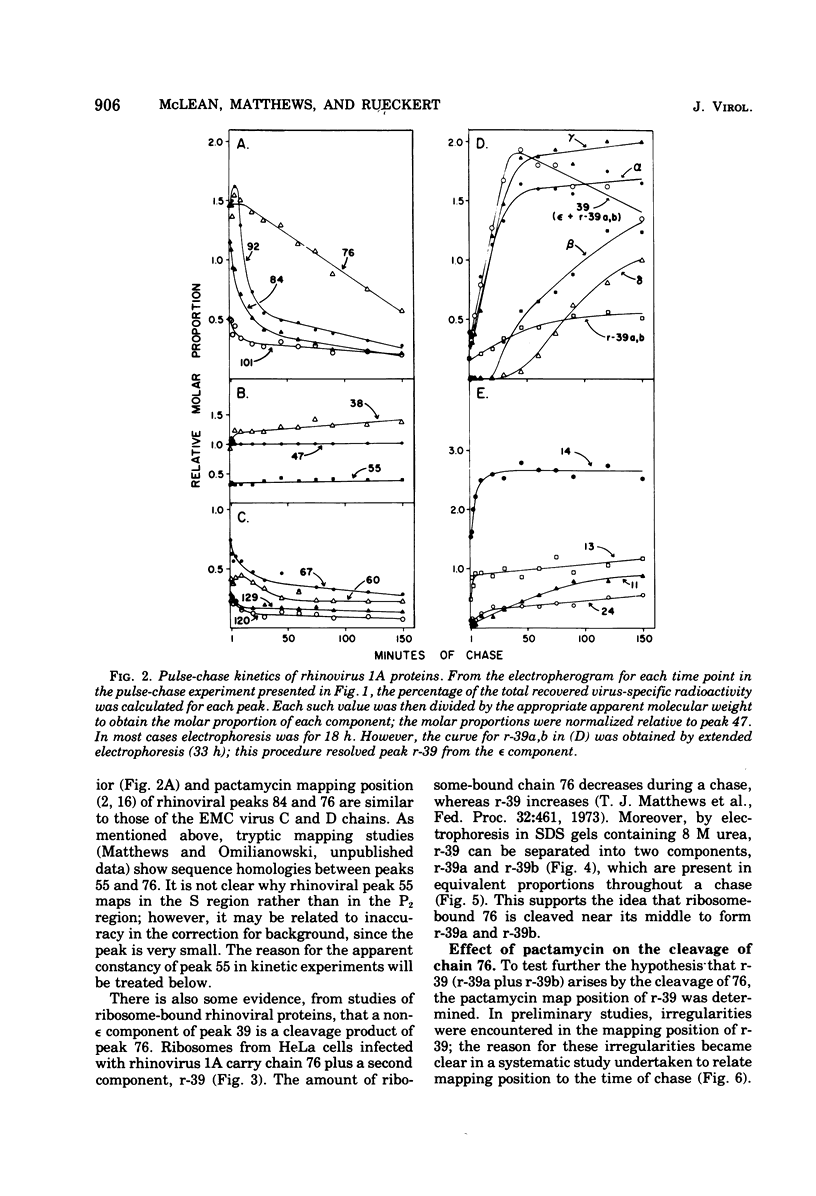
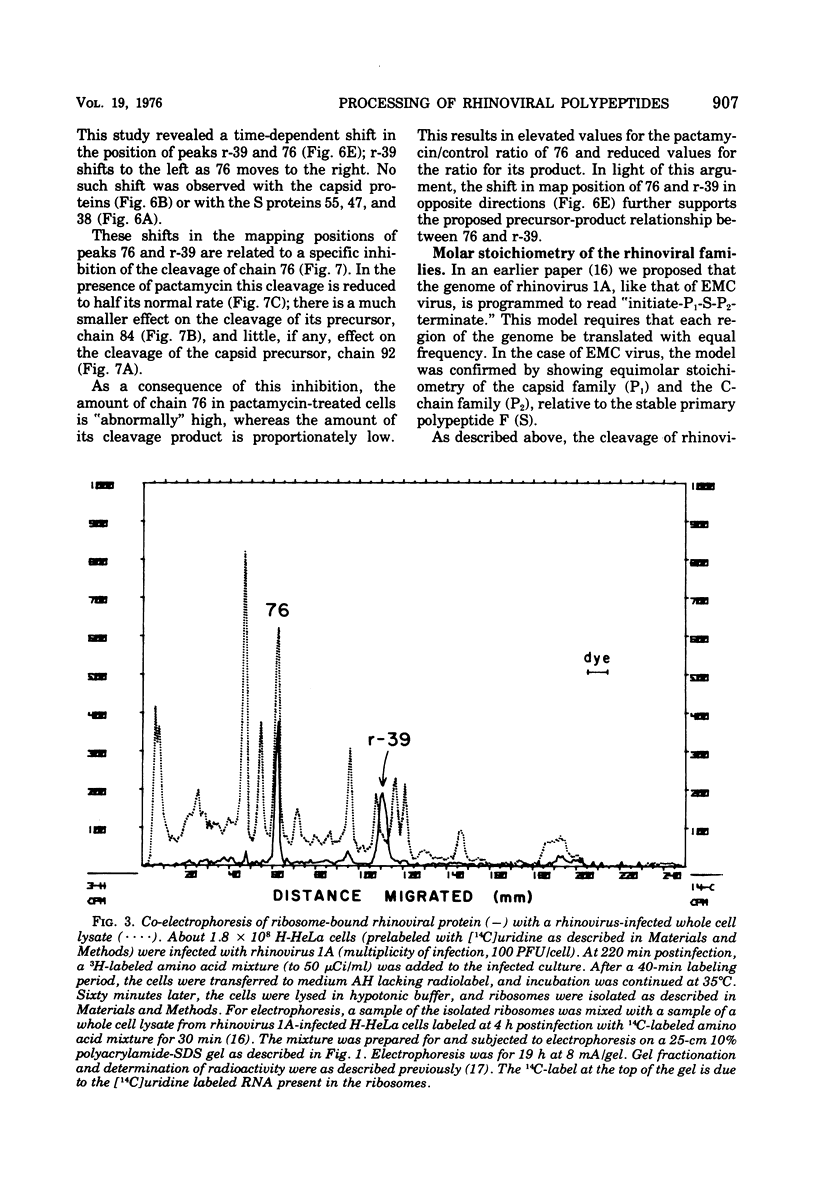
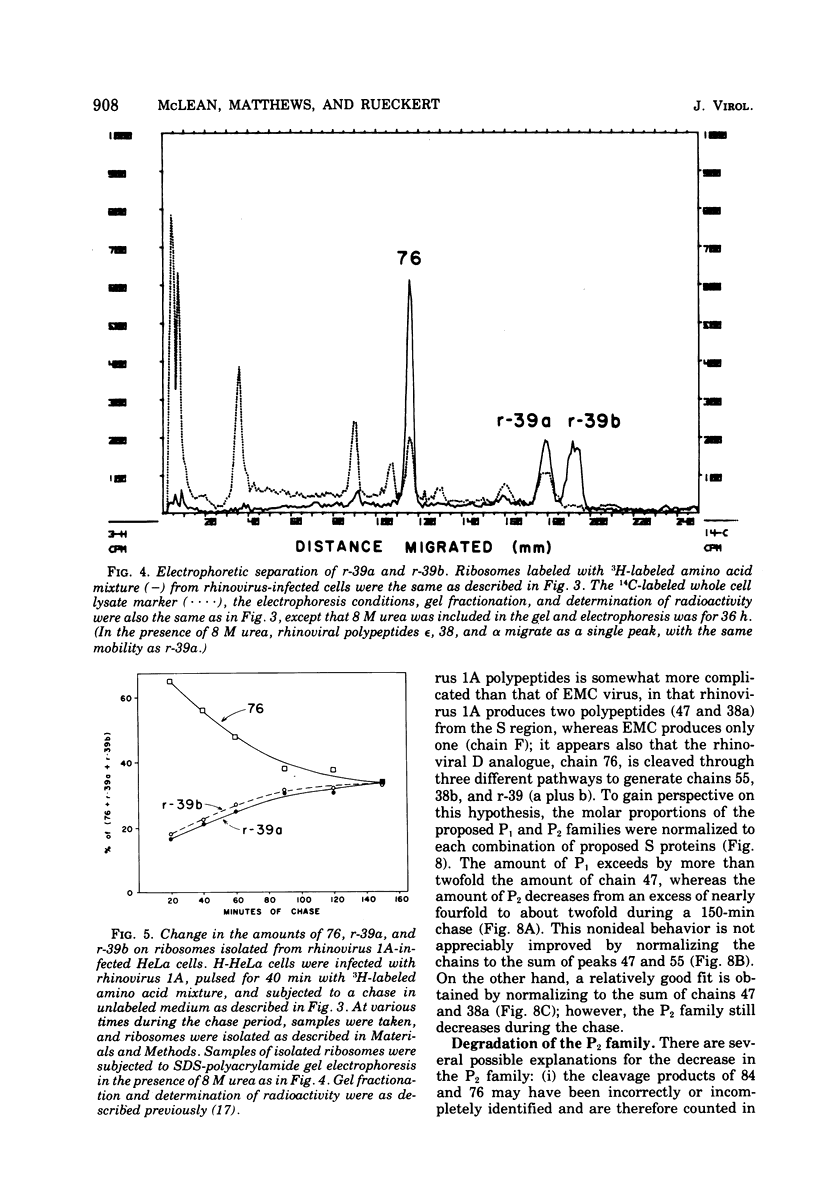
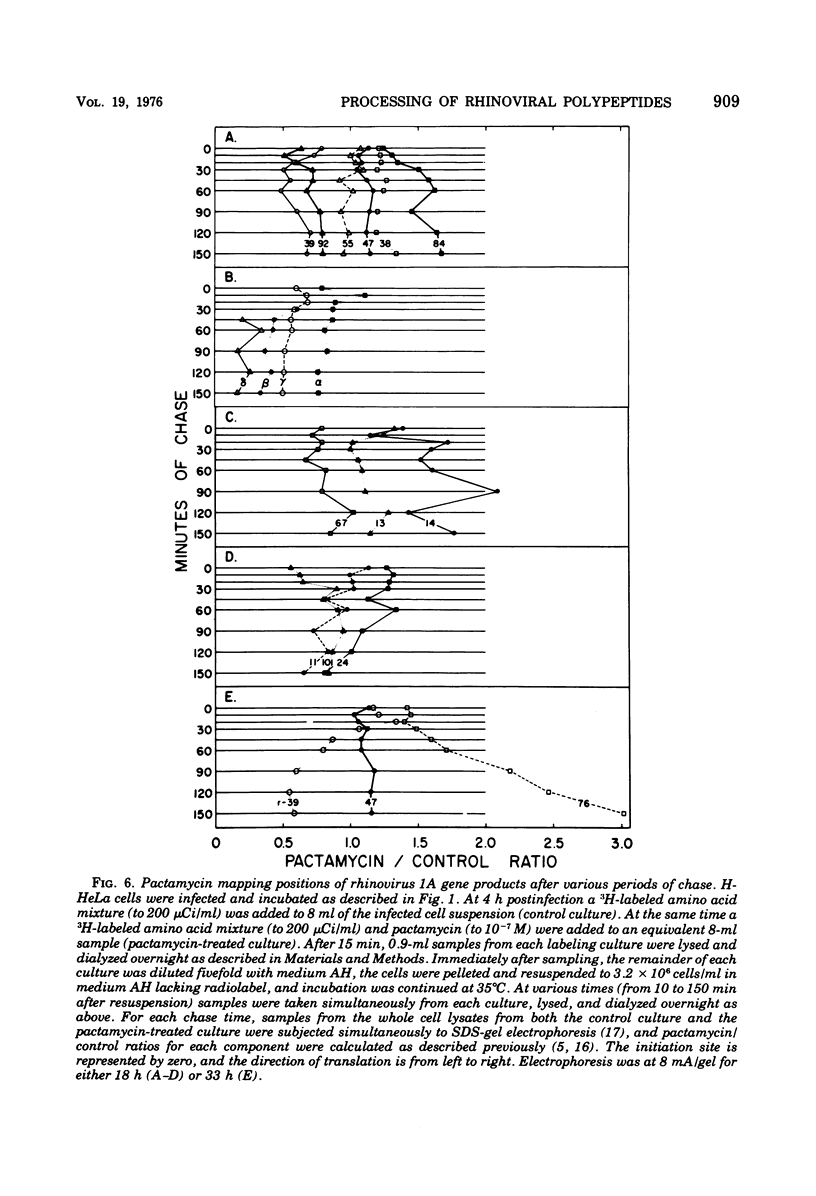
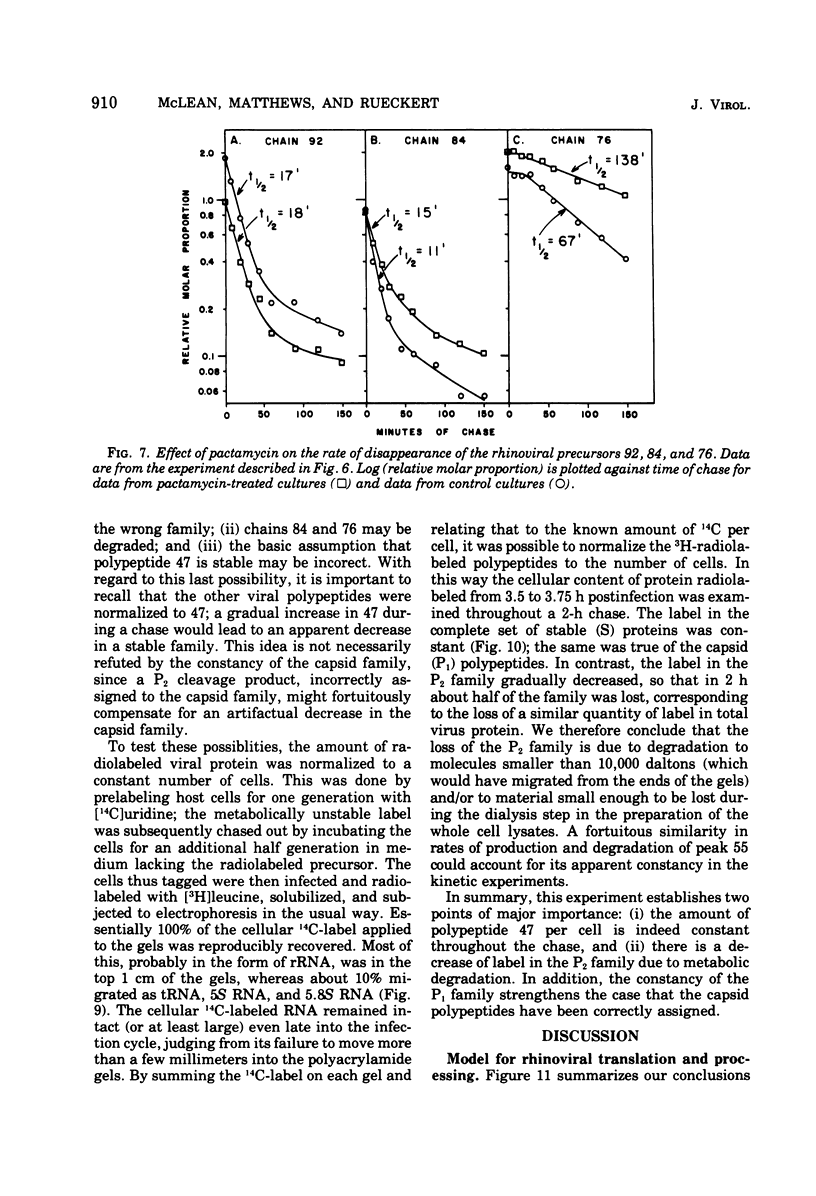
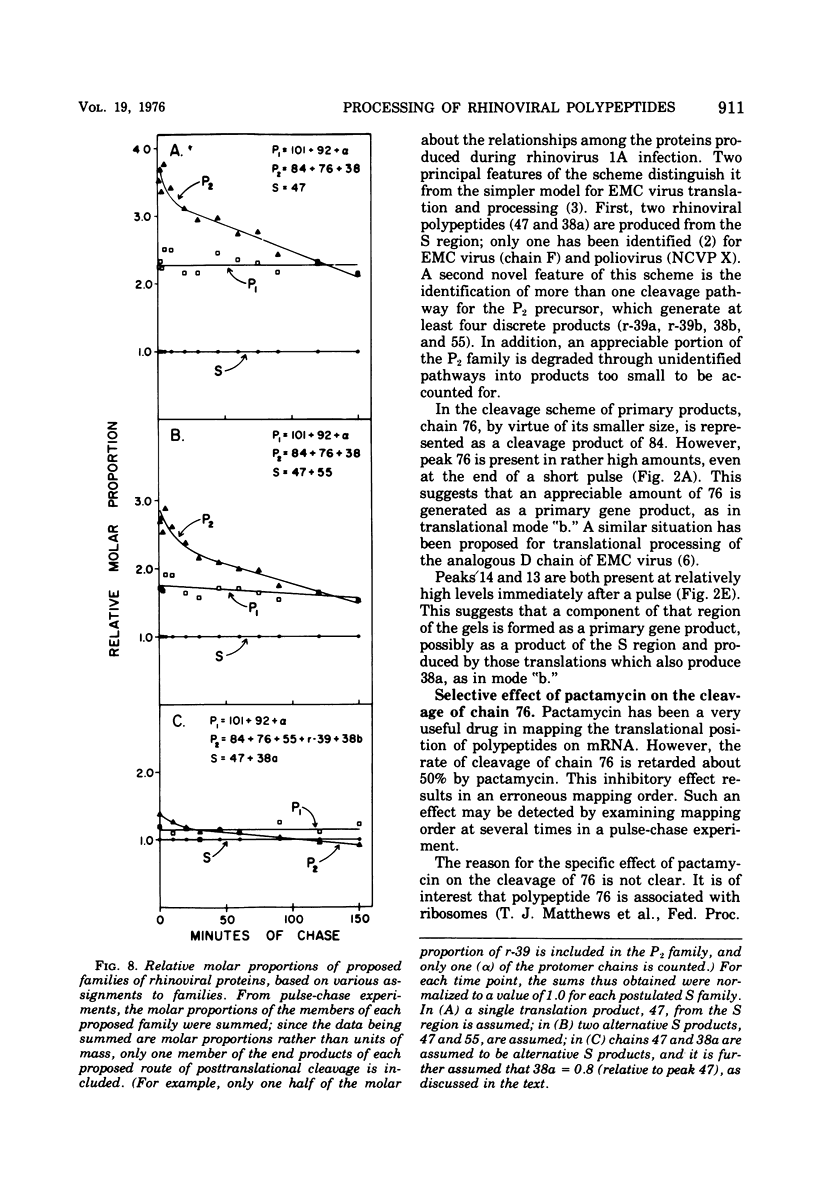
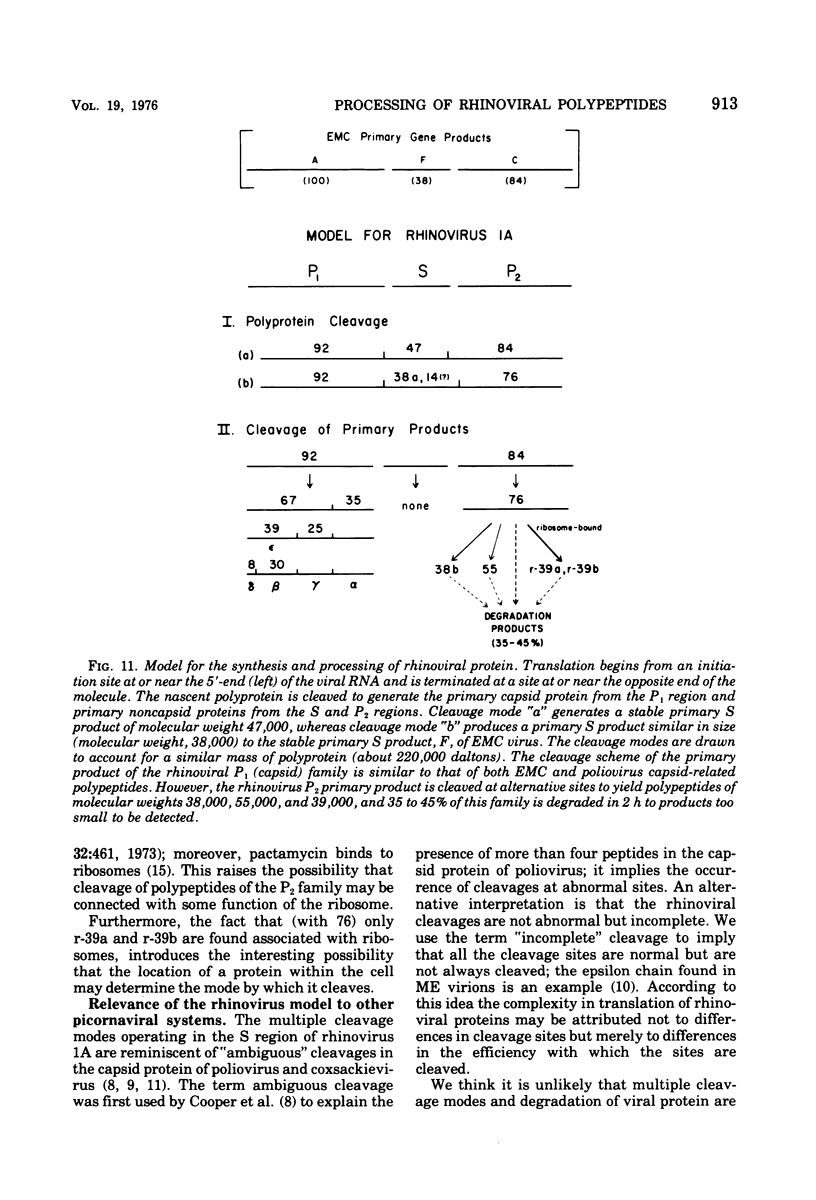
Pulse-chase kinetics and extensive pactamycin mapping studies show that the translation of rhinovirus 1A proceeds in the order: initiate-P1-S-P2-terminate, where P1 is the precursor to the capsid proteins, S is a stable primary gene product, and P2 is the precursor to a family of noncapsid products. Initial examination of the molar stoichiometry of the families of rhinoviral proteins in infected cells suggested that both the P1 and P2 regions were translated more frequently than the S region. However, we show that this apparent asymmetry in translation is an artifact arising from two phenomena: (i) ambiguous cleavage sites which result in two alternative products from the S region, having apparent molecular weights of 47,000 and 38,000, and (ii) several fates for the P2 precursors, including degradation of 35 to 45% of the P2 family to small unidentifiable products. Another artifact, a time-dependent shift in the pactamycin mapping position of polypeptide r-39, was traced to a selective inhibition of the rate of cleavage of its precursor (peak 76). The processing rate of the capsid precursor (peak 92) was not retarded by pactamycin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Cooper P. D. Relations between poliovirus polypeptides as shown by tryptic peptide analysis. J Gen Virol. 1975 Nov;29(2):215–221. doi: 10.1099/0022-1317-29-2-215. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Summers D. F., Maizel J. V. Evidence for ambiguity in the posttranslational cleavage of poliovirus proteins. Virology. 1970 Jul;41(3):408–418. doi: 10.1016/0042-6822(70)90161-3. [DOI] [PubMed] [Google Scholar]
- Cords C. E., James C. G., McLaren L. C. Alteration of capsid proteins of coxsackievirus A13 by low ionic concentrations. J Virol. 1975 Feb;15(2):244–252. doi: 10.1128/jvi.15.2.244-252.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Fragments generated by pH dissociation of ME-virus and their relation to the structure of the virion. J Mol Biol. 1971 May 28;58(1):217–235. doi: 10.1016/0022-2836(71)90242-7. [DOI] [PubMed] [Google Scholar]
- Fennell R., Phillips B. A. Polypeptide composition of urea- and heat-resistant mutants of poliovirus types 1 and 2. J Virol. 1974 Oct;14(4):821–833. doi: 10.1128/jvi.14.4.821-833.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkle D. B., Tershak D. R. Degradation of poliovirus polypeptides in vivo. Nat New Biol. 1972 Aug 16;238(85):206–208. doi: 10.1038/newbio238206a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Cleavage of mengovirus polyproteins in vivo. J Virol. 1974 Aug;14(2):261–269. doi: 10.1128/jvi.14.2.261-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J. S., Goldberg I. H. An effect of pactamycin on the initiation of protein synthesis in reticulocytes. Biochem Biophys Res Commun. 1970 Oct 9;41(1):1–8. doi: 10.1016/0006-291x(70)90460-2. [DOI] [PubMed] [Google Scholar]
- McLean C., Rueckert R. R. Picornaviral gene order: comparison of a rhinovirus with a cardiovirus. J Virol. 1973 Feb;11(2):341–344. doi: 10.1128/jvi.11.2.341-344.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Oberg B. F., Shatkin A. J. Initiation of picornavirus protein synthesis in ascites cell extracts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3589–3593. doi: 10.1073/pnas.69.12.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Colter J. S. Evidence for control of translation of the viral genome during replication of Mengo virus and poliovirus. Virology. 1975 Sep;67(1):300–305. doi: 10.1016/0042-6822(75)90430-4. [DOI] [PubMed] [Google Scholar]
- Paucha E., Seehafer J., Colter J. S. Synthesis of viral-specific polypeptides in Mengo virus-infected L cells: evidence for asymmetric translation of the viral genome. Virology. 1974 Oct;61(2):315–326. doi: 10.1016/0042-6822(74)90269-4. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



