Abstract
Myeloablative hematopoietic cell transplantation (HCT) is a common treatment for hematological malignancy. Delayed immune reconstitution following HCT is a major impediment to recovery with patients being most vulnerable during the first month after transplant. HCT is a highly stressful process. Because psychological distress has been associated with down regulation of immune function we examined the effect of pre-transplant distress on white blood cell (WBC) count among 70 adult autologous HCT patients during the first 3 weeks after transplant. The participants were on average 38 years old; 93% Caucasian, non-Hispanic and 55% male. Pre-transplant distress was measured 2–14 days before admission using the Cancer and Treatment Distress (CTXD) scale, and the Symptom Checklist-90-R (SCL-90-R) anxiety and depression subscales. WBC count was measured during initial immune recovery on days 5 through 22 post-transplant. Linear mixed model regression analyses controlling for gender and treatment-related variables revealed a significant effect of the mean pre-transplant SCL Depression-Anxiety score on WBC recovery. We found no significant effect of pre-transplant CTXD on WBC recovery. In general, higher levels of pre-treatment depression and anxiety were associated with slower WBC recovery. Psychological modulation of WBC recovery during HCT suggests a unique mechanism by which psychological distress can exert influence over the immune system. Given that WBC recovery is essential to survival for HCT patients, these data provide a rationale for treating anxiety and depression in HCT patients.
Keywords: hematopoietic cell transplantation, immune reconstitution, cancer treatment-related distress, depression, anxiety, hematologic malignancy, cancer
Introduction
Myeloablative hematopoietic cell transplantation (HCT) is a common treatment for leukemia, lymphoma and other hematological malignancies, and is also used to treat non-malignant disease such as aplastic anemia. Over 15,000 allogeneic and 30,000 autologous HCT occur each year worldwide (Copelan, 2006). The process, which was first introduced in the late 1960’s, has improved to the point that 5-year survival rates can range from 80% to below 20% depending on the type of transplant and diagnosis (Copelan, 2006). But the treatment is demanding both physically (Copelan, 2006 ) and emotionally (Andrykowski et al., 2005; Fann et al., 2007; Hoodin et al., 2006; Syrjala et al., 2004).
During the autologous HCT process, the patient first has their own hematopoietic stem cells harvested and stored. Patients then receive supra-lethal doses of chemotherapy, sometimes combined with total body irradiation, which results in eradication of the immune system. The patient is then “rescued” from certain death with infusion of their own stored stem cells (autologous). After stem cell infusion, 10–30 days are required for the patient’s circulating white blood cell (WBC) counts to rise from close to zero at the time of transplant to levels adequate to begin to protect them from acute symptoms and infection vulnerability. Immune reconstitution continues during a 3-month period with return of innate immunity followed later by the return of specific immunity. Complete immune recovery can take many months (Peggs and Mackinnon, 2004).
Transplant-related immune deficiency results in significant morbidity and mortality among HCT patients (Peggs and Mackinnon, 2004), and patients are particularly vulnerable to infection during the first weeks after transplant when WBC counts are low (Storek et al., 2003). More rapid return of immune function has been associated with fewer post-transplant side effects and better overall and disease-free (Porrata and Markovic, 2004); (Auletta and Lazarus, 2005; Porrata et al., 2008; Porrata and Markovic, 2004)
The HCT process also takes an emotional toll on patients. Patients arrive at transplant with differing diseases, histories of treatment, levels of physical function, and psychological, social, informational, and financial resources. All of these factors can contribute to the distress an individual experiences before, during, and after transplant. Psychosocial predictors of outcome have been studied extensively, with clear, replicated findings that pre-transplant physical and psychological function predict post-transplant physical and psychological outcomes (Andorsky et al., 2006; Lee et al., 2001; Syrjala et al., 2004). Pre-transplant treatment-related distress, in particular, predicts acute post-transplant symptom course, especially for pain and general distress (Schulz-Kindermann et al., 2002; Syrjala and Chapko, 1995). Psychosocial needs are greatest just prior to transplant if judged by the percent of patients with clinically significant anxiety or depression at different phases of treatment and recovery (Broers et al., 2000; Fife et al., 2000; Syrjala et al., 2004). Studies also document that pre-transplant psychological status predicts survival (Andrykowski et al., 1994; Loberiza et al., 2002; Prieto et al., 2005), although the mechanisms through which increased mortality occurs remain largely unexamined.
There is a large body of literature documenting that psychological distress can impair immune function, including innate and specific immunity (e.g., Burns et al., 2003; McEwen, 1998; Yin et al., 2000). Biological mediators of the effect of psychological distress on immune function include cortisol, via activation of the hypothalamic pituitary adrenal (HPA) axis, and catecholamines and neuropeptides via activation of the sympathetic nervous system(SNS) (McEwen, 1998). There is also a literature to support the hypothesis that psychological distress could influence immune recovery post-HCT.
Hematopoietic stem cells (HSCs), which ultimately mature to form all the different cells found in peripheral blood, reside in specific niches in the bone marrow. In this specialized environment, tight control of proliferation and differentiation is accomplished through a variety of physical (cell-cell) and molecular pathways (Adams and Scadden, 2006). It has long been known that bone marrow and other immune system organs are highly innervated by the SNS (Afan et al., 1997; Bellinger et al., 1992; Ehninger and Trumpp, 2011; Felten et al., 1985; Felten and Felten, 1994; Yamazaki et al., 2011), and that this innervation has functional significance (Broome and Miyan, 2000; Kohm et al., 2000; Maestroni, 2000; Mendez-Ferrer et al., 2008; Steidl et al., 2004). For example, SNS innervation of bone marrow happens immediately before the onset of hematopoiesis in utero (Afan et al., 1997), and growth of early hematopoietic progenitor cells is impaired in patients with spinal cord injury who thus have interrupted transmission of SNS activity below the site of the injury (Iversen et al., 2000). There are now animal model data that show HSCs respond to stressors occurring at the organismal level via adrenergic signals originating from the SNS. Katayama et al. showed that signals from the SNS control the attraction of stem cells to their niche in the bone marrow (Katayama et al., 2006). Taken together, these data provide strong evidence that alterations in SNS tone (e.g., in response to a stressor) may influence proliferation and mobilization of hematopoietic stem cells.
Given the importance of immune reconstitution for HCT patients and the stressful nature of the HCT process, we wondered if psychological distress, with its well-known influences on SNS tone, might be associated with changes in immune reconstitution after HCT. If psychological distress is associated with slower WBC recovery, it would strengthen the rationale for using psychological intervention prior to HCT to reduce distress and improve return of immune competence among survivors.
The purpose of the present study was to examine the relationship between psychological distress and immune reconstitution in the first weeks after autologous HCT. We hypothesized that elevated psychological distress prior to the transplant would be associated with slower rates of WBC reconstitution.
Method
Participants
The present study is a secondary analysis of data from a cohort of 70 autologous patients. Demographic and treatment characteristics of the sample are presented in Table 1. The patients were from a larger sample of 405 allogeneic and autologous patients enrolled in a prospective, longitudinal study of HCT survivorship between April, 1987 and January, 1990. To be included in the study, patients had to be at least 18 years old and about to receive a first HCT (Syrjala et al., 2005). The study and all study materials were approved by the FHCRC IRB. The patient sample for the present study was limited to autologous HCT patients because autologous patients do not receive immunosuppressive medications for graft versus host disease prophylaxis. All patient stem cells were procured from bone marrow harvest. After bone marrow harvest, the patients received conditioning regimen of Cyclophosphamide and TBI (n=36), Cyclophosphamide, Busulfan and TBI (n=12), Cyclophosphamide and Busulfan (n=9), or other chemotherapy and TBI (n=3) or other chemotherapy without TBI (n=4) prior to transplant.
Table 1.
Demographic and Clinical Characteristics
| Pre-transplant Age (N=70) | ||
| Mean ± SD | 37.66 ± 11.60 | |
| Range | 18–62 | |
| Frequency (N=70) | Proportion | |
| Gender | ||
| Female | 32 | 45.71 |
| Male | 38 | 54.29 |
| Ethnicity/Race | ||
| Caucasian, non-Hispanic | 69 | 98.6 |
| Hispanic | 1 | 1.4 |
| Education | ||
| Less than high school | 3 | 4.6 |
| High school or GED | 22 | 33.8 |
| Some college, trade school, | 28 | 43.1 |
| Completed college | ||
| Graduate Degree | 12 | 18.46 |
| Marital Status | ||
| Single | 15 | 22.4 |
| Married or Cohabitating | 45 | 67.2 |
| Separated/Divorced/ | 7 | 10.5 |
| Widowed | ||
| Disease Status at Transplant | ||
| Relapse | 38 | 54.3 |
| Remission | 32 | 45.7 |
| Diagnosis | ||
| Lymphoma | 28 | 40 |
| Acute Leukemia | 28 | 40 |
| Hodgkin Disease | 8 | 11.4 |
| Other | 6 | 8.6 |
| TBI status | ||
| Yes | 51 | 72.9 |
| Mean Total Dose (Gy) ± SD | 9.31 ± 6.03 | |
| Range | 6.00–19.60 | |
| Stem cell dose (106 CD34+ cells/kg body weight) | ||
| Mean ± SD | 3.07 ± 1.69 | |
| Range | 0.35–9.22 | |
| GM-CSF post-transplant (yes) | 2 | 3 |
| Corticosteroids post-transplant (yes) | 44 | 63 |
| Mean ±SD days corticosteroid | 9.95 ± 5.42 | |
| Range days corticosteroid tx | 1–17 | |
| Any Infection day 5-day 21 (yes) | 48 | 76.2 |
| Pre-treatment Distress | ||
| Low (0.0 – 0.76) | 8 | 12.90 |
| Medium (0.77–1.81) | 43 | 69.35 |
| High (≥ 1.82) | 11 | 17.74 |
| Pre-treatment Anxiety | ||
| Low (0.0 – 0.34) | 24 | 34.29 |
| Medium(0.35 – 0.95) | 32 | 45.71 |
| High ( ≥ 0.96) | 14 | 20.00 |
| Pre-treatment Depression | ||
| Low (0.0 – 1.00) | 45 | 64.29 |
| Medium (1.01–1.73) | 18 | 25.71 |
| High (≥ 1.74) | 7 | 10.00 |
Self-Report Measures
All self-report measures, described below, were completed between 2 and 14 days before transplant at the transplant clinic. Assessment was scheduled as rapidly after arrival as possible, and within a week of arrival unless medical events interceded.
Cancer and treatment-related distress was measured with the Cancer and Treatment Distress (CTXD) scale. The CTXD scale is a 27-item measure of treatment-related distress specific to the HCT process. Items such as “nausea and vomiting”, “possibility of relapse”, and “being a burden to other people” are rated for the extent to which they cause distress or worry to the patient, regardless of whether they have actually occurred. Ratings are made on a 4-point scale from 0 (“no distress) to 3 (“severe distress”) and a mean score for the 27 items is computed (Syrjala et al., 2004). This measure has been shown to detect unique treatment-related distress and to predict pain better than measures of generalized anxiety or depression (Schulz-Kindermann et al., 2002; Syrjala and Chapko, 1995). The scale has good internal consistency with alphas ranging from .85 to .91 (Schulz-Kindermann et al., 2002; Syrjala and Chapko, 1995; Syrjala et al., 2004). The alpha in the present sample was .87.
Anxiety and depression were assessed using the anxiety and depression subscales of the Symptom Checklist 90-Revised (SCL-90-R). The SCL-90-R anxiety subscale (SCL-A) is comprised of 10 items such as “nervousness” or “shakiness inside”. The depression subscale (SCL-D) is comprised of 13 items such as “feeling blue” or “crying easily”. Participants are asked to indicate how much a problem has “distressed or bothered you during the past 7 days including today”. Items are rated using a scale from 0 (“not at all”) to 4 (“extremely”). Subscale scores are computed as the mean of subscale item scores, thus the scores could range from 0 to 4. The SCL-A and SCL-D are highly reliable, valid, and widely utilized in psychiatric studies (Derogatis et al., 1973). For the SCL-D, a cutoff score of 1.72 has been documented as having the highest positive predictive value for diagnosing major depression (Mulrow et al., 1995). Means and standard deviations from a community normative sample for the SCL-A are .30 (.37) and for the SCL-D are .36 (.44) (Derogatis, 1994). Internal consistencies range from .85 to .90, and test-retest reliabilities range from .68 to .86 (Derogatis and Cleary, 1977). The alpha for the SCL-A in the present sample was .89. The alpha for the SCL-D in the present sample was .86.
White blood cell (WBC) counts
Complete differential blood cell counts are performed every day by CLIA-certified laboratory during the inpatient period per the inpatient clinical protocol. Study patient total WBC counts were obtained from the research medical record database for days 5–22 of the post-transplant period as most patients have initial immune recovery by day 22 and counts before day 5 are near zero. All WBC values are the number of cells per microliter (cells/mcL) of blood.
Statistical Analysis
Because of the strong correlation between pre-transplant anxiety and depression, a composite variable was created by taking the mean of the anxiety and depression scale scores for each patient. In order to control for variables that we identified, a priori, to be important predictors of WBC count, evaluated age, sex, pre-transplant medical risk stats, stem cell dose, total body irradiation (TBI) dose, the presence of infection, if corticosteroids were given, and cytomegalovirus virus (CMV) status (positive or negative as potential covariates for the models. As we have done in previous studies, pre-transplant medical risk categories were assigned as either low or high depending upon the patient’s diagnosis and remission/relapse status (Syrjala et al., 2004). For the resultant distress model we included: age, pre-transplant medical risk status, if corticosteroids were given, and cytomegalovirus virus (CMV) status (positive or negative). For the resultant anxiety-depression composite models we included sex CMV, pre-transplant medical risk status, stem cell dose, total body irradiation (TBI) dose, and if corticosteroids were given. These covariates were chosen using the Wald test to compare with a model containing no covariates. All sets of covariates significantly improved the model at the p<0.05 level. To account for the expected nonlinear (quadratic) trend in the WBC count over time, all models included time, measured continuously by day and day squared, as a predictor. The models also included interactions of the predictors (pre-transplant treatment-related distress, anxiety, and depression) with time measured by day and day squared. All mixed effects regression analyses were conducted using continuous predictors with and without covariates.
A series of two sets of linear mixed model regression analyses were conducted to investigate the effects of pre-HCT treatment-related distress and an anxiety-depression composite predictor on change in WBC counts over days 5 through 22 post-HCT, using xtmixed (Stata SE 10.0 for Windows, Stata Corporation, College Station, TX). In an effort to estimate as few parameters as possible we used an exchangeable covariance structure for the two models. This a priori choice assumes that each pair is equally correlated. Because of the short time duration, we assume that observations are similarly correlated over time. We rejected the use of unstructured as it estimates many parameters and reduces the power of our models. We chose not to pursue autoregressive covariance structure because exponential decay is unlikely given the short time duration and influence of other factors on WBC recovery. We used mixed model methodology to adjust for the correlation of repeated measures, to allow for incomplete measurement, and to include covariates. Analyses began with day 5 post-transplant since a nadir in WBC continued from day 0 to day 5. The number of days post-transplant was added as a random effect that varied by patient. This method provides a more accurate estimate of error variability.
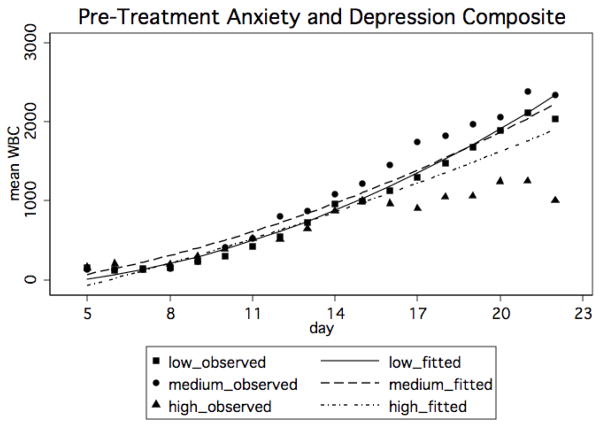
For the purposes of illustrating the statistically significant model (figure 1), categorical variables were created for the anxiety-depression composite score using clinically meaningful cut points to plot WBC curves over time. The SCL-A and SCL-D have published population norm cut scores (Derogatis et al., 1973). Pre-transplant anxiety can be defined as: low (an SCL-A score of 0.0–0.34), medium (a score between 0.35 and 0.95), and high (a score ≥ 0.96). Pre-transplant depression can be defined categorically as: low (an SCL-D score of 0.0–1.00), medium (a score between 1.01 and 1.73), and high (a score ≥ 1.74). In order to create cut scores for the composite, we averaged the anxiety and depression cut scores together, and so defined the categories as: low (0.0–0.67), medium (0.68–1.34) and high (≥1.35).
Figure 1.
Mean predicted WBC by day and pre-transplant anxiety and depression composite. The unadjusted means are also plotted for each group as a reference.
Results
Means for pre-transplant treatment-related distress, anxiety, and depression by diagnosis group are shown in Table 2. Treatment-related distress, anxiety, and depression scores did not differ by length of time between assessment and the start of treatment measured in days.
Table 2.
Means, standard deviations of distress, anxiety, and depression by diagnostic group.
| Distress (CTXD) | Anxiety (SCL-A) | Depression (SCL-D) | |
|---|---|---|---|
| Mean ± SD (n) | Mean ± SD (n) | Mean ± SD (n) | |
| Lymphoma | 2.17± 0.60 (25) | 0.66± 0.57 (28) | 0.91± 0.53 (28) |
| Acute Leukemia | 2.17± 0.51 (22) | 0.65± 0.67 (28) | 0.72± 0.67 (28 ) |
| Hodgkin | 2.32± 0.53 (7) | 0.48± 0.51 (8) | 0.82± 0.69 (8) |
| Other | 2.06± 0.23 (4) | 0.98± 0.92 (6) | 0.88± 0.64 (6) |
Associations among independent variables
While the correlation between pre-transplant anxiety and depression was 0.61 (n = 70, p < .001), the correlations of pre-transplant treatment-related distress with anxiety and depression were 0.37 (n = 62, p = .003) and 0.33 (n = 62, p = .008), respectively. When treatment-related distress, anxiety and depression were entered as continuous variables in one model predicting WBC over time, none emerged as significant predictors. For this reason and to increase parsimony, we created a composite variable by creating a mean of the SCL-Anxiety and SCL-Depression scores. We then created mixed-effects regression models for the treatment-related distress and the SCL anxiety-depression composite.
Results of mixed effects regression
Treatment-related distress
The mixed-effects regression model showing how pre-transplant treatment-related distress, as a continuous variable, was associated with change in WBC over day 5 through day 22 after transplant revealed no significant interactions between treatment-related distress and time or time squared using a Wald test of significance for both terms simultaneously (p = ns). There were significant effects of age (β = 12.23, p < .01), corticosteroid given (β = 25.91, p < .01) and CMV status (β = 180.47, p < .05). This indicated that the change in WBC over time was not different for different levels of treatment-related distress. Patients with higher treatment-related distress improved as fast as those with lower pre-treatment treatment-related distress.
SCL-Anxiety and Depression Composite variable
The mixed-effects regression model showing how the pre-transplant anxiety-depression composite, as a continuous variable was associated with change in WBC over day 5 through day 22 after transplant with and without treatment covariates are shown in tables 3 and 3a. There was no significant interaction between the anxiety-depression composite variable and time in the models with or without covariates. However, there was a significant interaction between the anxiety-depression composite variable and time squared (β = −2.80, p = .02) which declined slightly but remained significant after adding treatment covariates to the model (β = −2.36, p = .05). All of the covariates entered into the model were significant terms. In general, higher scores on the anxiety-depression composite were associated with slower change in WBC over time.
Table 3.
Results of mixed effects regression model for pre-transplant anxiety and depression composite predicting WBC
| Variable | Estimate | Std error | p-value |
|---|---|---|---|
| Day | −25.55 | 32.25 | 0.43 |
| Day squared | 6.07 | 1.08 | 0.00 |
| depression | −251.06 | 195.03 | 0.20 |
| Pre-depression by day | 56.64 | 35.42 | 0.09 |
| Pre-depression by day squared | −2.80 | 1.21 | 0.02 |
| Constant | 17.86 | 179.12 | 0.92 |
Table 3a.
Results of mixed effects regression model for pre-transplant anxiety and depression composite predicting WBC with treatment variable covariates
| Variable | Estimate | Std error | p-value |
|---|---|---|---|
| Day | −19.39 | 32.98 | 0.56 |
| Day squared | 5.85 | 1.08 | 0.00 |
| composite | −296.27 | 197.71 | 0.13 |
| composite by day | 50.92 | 36.49 | 0.16 |
| composite by day squared | −2.36 | 1.22 | 0.05 |
| Sex | −284.13 | 82.28 | 0.001 |
| Risk | −384.22 | 136.78 | 0.01 |
| Stem cell dose | −73.14 | 24.24 | 0.003 |
| TBI1 dose | −15.20 | 6.86 | 0.03 |
| Corticosteroid | 21.95 | 6.29 | 0.000 |
| CMV2 | 274.92 | 83.13 | 0.001 |
| constant | 670.49 | 275.66 | 0.02 |
Total body irradiation (TBI)
Cytomegalovirus virus (CMV) status (positive or negative)
Discussion
The present study sought to investigate the effects of pre-transplant self-reported treatment-related distress, anxiety, and depression symptoms on WBC counts among 70 adult autologous HCT patients during the first 3 weeks after their transplant. The immune recovery curves did not generally differentiate until after day 11, when the WBC counts began to rise. Pre-transplant anxiety-depression significantly predicted the pace of WBC recovery. In general, patients who reported higher levels of pre-transplant depression and anxiety had slower recovery of WBC counts compared to patients reporting lower levels of pre-transplant depression and anxiety.
We did not see the same association with pre-transplant reports of treatment-related distress. When treatment-related distress was used as a continuous predictor in the analyses, the interaction between time or time squared and treatment-related distress was not significant. While treatment-related distress, anxiety, and depression symptoms do tend to co-occur, the treatment-related distress variable is qualitatively different from the composite anxiety and depression variable, which was measured with a standardized instrument for the general population. While the anxiety and depression items of the SCL-90 measure clinical symptoms of anxiety and depression that patients bring to the transplant setting with them, the treatment-related distress instrument captures a distress response to an impending (but not current) threat, the HCT and all the possible treatment-related threats (e.g., mucositis, nausea and vomiting, hair loss, death). One reason the pre-transplant measure of treatment-related distress did not predict WBC recovery could be because the treatment-related distress instrument was measuring distress about something that had not occurred yet. However, in other HCT studies with both autologous and allogeneic transplant patients, pre-transplant treatment-related distress was a better predictor of pain and general distress during the first month of HCT than mood as measured with standardized measures (Schulz-Kindermann et al., 2002; Syrjala and Chapko, 1995). Perhaps the standardized anxiety and depression measures are capturing a construct that is a better predictor of biological functioning (i.e., WBC count) than the treatment-related distress measure. Also, the patients in the present study were all autologous and thus less vulnerable than allogeneic patients to many of the treatment-related threats assessed in the treatment-related distress measure (e.g. GVHD).
Overall, the results in these models indicate that pre-transplant anxiety and depression (general psychological distress), but not treatment-specific distress is associated with the pace of immune recovery in HCT patients, even after adding treatment-related covariates to the models. Higher levels of anxiety and depression are consistently associated with a slower WBC recovery.
These results, while the first to document such effects in the HCT setting, are similar to studies that have found high levels of psychological distress (including anxiety and depression) associated with general down regulation of immune function in humans (Kiecolt-Glaser et al., 2002; Segerstrom and Miller, 2004). Animal studies have shown that sympathetic innervation of the bone marrow modulates proliferation and mobilization of HSC in the bone marrow microenvironment (Katayama et al., 2006). For example, Katayama et al. (2006) showed that signals from the SNS suppress osteoblast function and control the attraction of stem cells to their niche in the bone marrow. Taken together, these data provide strong evidence that alterations in SNS tone (e.g., in response to a stressor) may influence proliferation and mobilization of hematopoietic stem cells (HSC).
The present data may have important clinical implications for HCT patients. The period of immune deficiency in the weeks after transplant, and its related morbidity and mortality are a critical time in the HCT process. Patients experience increased vulnerability to infection and usually need to be hospitalized during this phase of treatment. The period of immune deficiency immediately after transplant is also associated with chronic diarrhea, stomach upset, and severe mouth pain secondary to mucositis. Supportive therapies such as administration of exogenous immune growth factors have been developed to reduce this vulnerable time as much as possible. The present data suggest that reducing pre-transplant anxiety and depression could also contribute to reducing morbidity related to immune deficiency during this early period after transplant. If the models are replicated, and if the trends for pace of immune reconstitution continue, at day 90, the difference between WBC in a patient with low anxiety and depression and high anxiety and depression could be much greater. In the present study, most patients were discharged by day 22 and WBC counts were not collected daily. Future work should evaluate the effect of pre-transplant depressive symptoms and anxiety on infection rates, days of re-hospitalization, and WBC subset counts from day 5 post transplant to beyond day 22.
The relationship between pre-transplant depression symptoms and anxiety and recovery of WBC count is provocative for another reason. A number of studies have reported that pre-transplant depression is associated with decreased survival among HCT patients (e.g. Andrykowski et al., 1994; Grulke et al., 2008; Loberiza et al., 2002). Furthermore, pre-transplant depression was not associated with other clinical predictors, suggesting depressed mood pre-transplant was not due to clinical severity (Grulke et al., 2008). While some papers have not found this association, a recent review reported that the majority of prospective studies using reliable measures, with adequate sample sizes and robust statistical methods to control for medical and treatment confounds, did find a significant relationship between high levels of depressive symptoms and increased mortality (Hoodin et al., 2006). We did not test for survival advantages in the present sample because we felt the power was insufficient. But a more speedy return of immune function has been associated with better overall and disease-free survival in other studies (Porrata and Markovic, 2004). In fact, an absolute lymphocyte count of greater than 500 at 15 days post autologous transplant has been associated with lower recurrence rates and longer survival (Auletta and Lazarus, 2005; Porrata et al., 2008; Porrata and Markovic, 2004). While the dependent variable in the present study was total WBC, not absolute lymphocyte count, this helps put the present data into perspective. Future work should evaluate the effects of psychological functioning on recovery of WBC subsets, such as lymphocytes, neutrophils, etc.
The present secondary data analysis study has both limitations and strengths. Limitations of the present study include the age of the date. There have been major changes in HCT procedures since the present data were collected and with these changes have come improvements in immune recovery and survival. Thus, the findings from the present study cannot be generalized to current autologous HCT patients. We conducted the study with autologous patients to limit the confounding effect of immunosuppressive therapy given to allogeneic transplant recipients. Thus, the current findings cannot be generalized to allogeneic patients. Furthermore, WBC count is a fairly non-specific indicator of immune recovery. Future work should focus on recovery of leukocyte subsets in patients being treated with current regimens. Furthermore, it is not clear in the autologous setting whether the patient’s pre-transplant anxiety and depression is directly affecting the bone marrow micro-environment before harvesting or after infusion, or whether pre-transplant distress adversely affects the hematopoietic stem cell graft itself. It would also be valuable to study the effects of anxiety and depression on immune reconstitution through the later phases of treatment. Further research is needed to determine whether the course of anxiety and depression through acute treatment and long-term follow-up continues to influence immune function, morbidity and mortality. Other potential modulators of mood and WBC that could not be assessed in the present study include data on tricyclic anti-depressant use and data on nuetropenic fever during the post-transplant period. It is also not clear from the present study what relative role the SNS and HPA axis play in mediating the observed relationships. Strengths of the present study include the longitudinal design, the relatively homogeneous (i.e., all autologous) sample, the use of well-validated measures and sophisticated statistical methods.
In conclusion, this is the first study, to our knowledge, to document the effects of psychological distress on total WBC count among HCT patients. The present results are consistent with the work of Katayama et al. (2006) and suggest modulation of sympathetic outflow to the stem cell niche could be a unique mechanism by which psychological distress exerts influence on immune function in general, and specifically, total WBC count in HCT patients. Psychological intervention (e.g., stress management, relaxation training) has been shown to modulate sympathetic activity in other settings (Miller and Cohen, 2001). The present findings support future research to test the effects of psychological interventions to reduce distress, improve total WBC count, and improve health outcomes in HCT patients. Given that WBC recovery is essential to survival for HCT patients, these data provide a rationale for screening patients pre-transplant to determine those who would benefit from intervention to treat psychological distress in the HCT setting.
Research highlights.
Psychological modulation of total WBC reconstitution following HCT suggests a unique mechanism by which psychological distress can exert influence over the immune system.
Acknowledgments
Funded by grants from the National Cancer Institute (K07 CA107085-01(McGregor), CA 38522 (Syrjala), CA 78990 (Syrjala) and CA 112631 (Syrjala)).
Acronyms
- HCT
hematopoietic cell transplantation
- WBC
white blood cell
- SNS
sympathetic nervous system
- HPA
hypothalamic-pituitary-adrenal
- HSC
hematopoietic stem cells
- CTXD
Cancer and Treatment Distress
- SCL-90-R
Symptom-Checklist-90-Revised
- SCL-A
SCL-90-R anxiety subscale
- SCL-D
SCL-90-R depression subscale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- Afan AM, Broome CS, Nicholls SE, Whetton AD, Miyan JA. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br J Haematol. 1997;98:569–577. doi: 10.1046/j.1365-2141.1997.2733092.x. [DOI] [PubMed] [Google Scholar]
- Andorsky DJ, Loberiza FR, Lee SJ. Pre-transplantation physical and mental functioning is strongly associated with self-reported recovery from stem cell transplantation. Bone Marrow Transplant. 2006;37:889–895. doi: 10.1038/sj.bmt.1705347. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, Horowitz MM, Sobocinski KA, Rizzo JD, Wingard JR. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Brady MJ, Henslee-Downey PJ. Psychosocial factors predictive of survival after allogeneic bone marrow transplantation for leukemia. Psychosom Med. 1994;56:432–439. doi: 10.1097/00006842-199409000-00008. [DOI] [PubMed] [Google Scholar]
- Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone marrow transplantation. 2005;35:835–857. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Felten SY, Felten DL. Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol. 1992;14:329–344. doi: 10.1016/0192-0561(92)90162-e. [DOI] [PubMed] [Google Scholar]
- Broers S, Kaptein AA, Le Cessie S, Fibbe W, Hengeveld MW. Psychological functioning and quality of life following bone marrow transplantation: a 3-year follow-up study. J Psychosom Res. 2000;48:11–21. doi: 10.1016/s0022-3999(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Broome CS, Miyan JA. Neuropeptide Control of Bone Marrow Neutrophil Production: A Key Axis for Neuroimmunomodulation. Ann NY Acad Sci. 2000;917:424–434. doi: 10.1111/j.1749-6632.2000.tb05407.x. [DOI] [PubMed] [Google Scholar]
- Burns VE, Carroll D, Ring C, Drayson M. Antibody response to vaccination and psychosocial stress in humans: relationships and mechanisms. Vaccine. 2003;21:2523–2534. doi: 10.1016/s0264-410x(03)00041-0. [DOI] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Symptom Checklist-90-R: Administration, scoring, and procedures manual. 3. NCS Pearson, Inc; Minneapolis, MN: 1994. [Google Scholar]
- Derogatis LR, Cleary PA. Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br J Soc Clin Psychol. 1977;16:347–356. doi: 10.1111/j.2044-8260.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. The Journal of Experimental Medicine. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol. 2007;25:1223–1231. doi: 10.1200/JCO.2006.07.9079. [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- Felten SY, Felten DL. Neural-immune interactions. Prog Brain Res. 1994;100:157–162. [PubMed] [Google Scholar]
- Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18:1539–1549. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- Grulke N, Larbig W, Kachele H, Bailer H. Pre-transplant depression as risk factor for survival of patients undergoing allogeneic haematopoietic stem cell transplantation. Psycho-oncology. 2008;17:480–487. doi: 10.1002/pon.1261. [DOI] [PubMed] [Google Scholar]
- Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- Iversen PO, Hjeltnes N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W, Stanghelle J, Benestad HB. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood. 2000;96:2081–2083. [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and Psychosomatic Medicine: Back to the Future. Psychosom Med. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Tang Y, Sanders VM, Jones SB. Activation of Antigen-Specific CD4+ Th2 Cells and B Cells In Vivo Increases Norepinephrine Release in the Spleen and Bone Marrow. J Immunol. 2000;165:725–733. doi: 10.4049/jimmunol.165.2.725. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Fairclough D, Parsons SK, Soiffer RJ, Fisher DC, Schlossman RL, Antin JH, Weeks JC. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19:242–252. doi: 10.1200/JCO.2001.19.1.242. [DOI] [PubMed] [Google Scholar]
- Loberiza FR, Jr, Rizzo JD, Bredeson CN, Antin JH, Horowitz MM, Weeks JC, Lee SJ. Association of Depressive Syndrome and Early Deaths Among Patients After Stem-Cell Transplantation for Malignant Diseases. J Clin Oncol. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- Maestroni GJM. Neurohormones and Catecholamines as Functional Components of the Bone Marrow Microenvironment. Ann NY Acad Sci. 2000;917:29–37. doi: 10.1111/j.1749-6632.2000.tb05370.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S. Psychological interventions and the immune system: a meta-analytic review and critique. Health Psychol. 2001;20:47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Williams JW, Jr, Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Ann Intern Med. 1995;122:913–921. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Mackinnon S. Immune reconstitution following haematopoietic stem cell transplantation. Br J Haematol. 2004;124:407–420. doi: 10.1046/j.1365-2141.2003.04767.x. [DOI] [PubMed] [Google Scholar]
- Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Litzow MR, Winters JL, Markovic SN. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14:807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrata LF, Markovic SN. Timely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantation. Clinical and experimental medicine. 2004;4:78–85. doi: 10.1007/s10238-004-0041-4. [DOI] [PubMed] [Google Scholar]
- Prieto JM, Atala J, Blanch J, Carreras E, Rovira M, Cirera E, Espinal A, Gasto C. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23:6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- Schulz-Kindermann F, Hennings U, Ramm G, Zander AR, Hasenbring M. The role of biomedical and psychosocial factors for the prediction of pain and distress in patients undergoing high-dose therapy and BMT/PBSCT. Bone Marrow Transplant. 2002;29:341–351. doi: 10.1038/sj.bmt.1703385. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl U, Bork S, Schaub S, Selbach O, Seres J, Aivado M, Schroeder T, Rohr UP, Fenk R, Kliszewski S, Maercker C, Neubert P, Bornstein SR, Haas HL, Kobbe G, Tenen DG, Haas R, Kronenwett R. Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood. 2004;104:81–88. doi: 10.1182/blood-2004-01-0373. [DOI] [PubMed] [Google Scholar]
- Storek J, Viganego F, Dawson MA, Herremans MM, Boeckh M, Flowers ME, Storer B, Bensinger WI, Witherspoon RP, Maloney DG. Factors affecting antibody levels after allogeneic hematopoietic cell transplantation. Blood. 2003;101:3319–3324. doi: 10.1182/blood-2002-05-1376. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Chapko ME. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain. 1995;61:69–79. doi: 10.1016/0304-3959(94)00153-6. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, Martin PJ. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. Jama. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late Effects of Hematopoietic Cell Transplantation Among 10-Year Adult Survivors Compared With Case-Matched Controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo Makoto M, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann Cells Maintain Hematopoietic Stem Cell Hibernation in the Bone Marrow Niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]