Abstract
Purpose
To investigate pigment clumping in idiopathic macular telangiectasia type 2 (IMT2) for its incidence, development, and progression during the course of the disease.
Methods
Patients with a diagnosis of IMT2 and greater than 12 months of follow-up were reviewed retrospectively. Measurements of the area of pigment clumping were performed and correlated with visual acuity and findings on spectral domain optical coherence tomography (OCT) and microperimetry (MP1).
Results
Fifty-three eyes in 27 patients with a mean follow-up of 42.5±14.2 months (range 12–79 months) were included. At study baseline, 16 eyes (30%) had evidence of pigment clumping without associated neovascular changes. During follow-up, 8/33 (24%) additional study eyes without prior pigment clumping developed it in stage 3 (Gass-Blodi classification) disease. Pigment clumping increased in overall area as a function of follow-up time. Pigment clumping was associated with increased intraretinal reflectivity on OCT and development of scotomas on microperimetry.
Conclusions
Pigment clumping commonly develops in stage 3 IMT2 disease, enlarges in area continuously over time, and is associated with declining visual function. Longitudinal measurements of the total area of pigment clumping may be helpful in following disease progression and may constitute a useful outcome measure for interventional clinical studies.
Keywords: Imaging, Macular telangiectasia, Microperimetry, Ocular coherence tomography, Retinal pigment
Introduction
Idiopathic macular telangiectasia type 2 (IMT2) is an uncommon acquired macular disorder of unknown etiology characterized by the presence of bilateral perifoveal/perifoveolar telangiectasia1, 2. Presenting patients often report a gradual reduction in visual acuity associated with reading difficulty and metamorphopsia 3–6. The progressive retinal changes occuring in the natural history of IMT2 have been previously characterized, and staging schemes for disease progression have been proposed 4, 7. Typical clinical findings of IMT2 include a loss of transparency in the perifoveal retina, crystalline deposits, telangiectactic vessels, and in some cases, the development of exudative neovascularization 7 and the formation of macular holes8. Optical coherence tomography (OCT) evaluations have also revealed progressive disruption and degeneration of the outer neuroretinal layers with the formation of intraretinal cavities 9–11.
An additional anatomical hallmark of IMT2 is the presence of pigment clumping, clinically observed as intra- or sub-retinal clumps of gray or black pigment that is typically located in the temporal perifoveal region. Although the appearance of pigment clumping in IMT2 has been previously well described, its emergence, evolution, and functional consequences have not been closely examined in a longitudinal manner. In the current study, we sought to define the prevalence of pigment clumping in a cohort of patients with IMT2 and to characterize, in quantitative terms, the evolution of pigment clumping over time. We also examined clinical features associated with pigment clumping utilizing multimodal imaging, including fundus photography, OCT, and microperimetry, in order to investigate its tissue associations and functional consequences. This data on the nature and quantitative description of pigment clumping may be useful in (1) highlighting certain pathophysiologic mechanisms in IMT2 that drive disease progression and (2) deriving a potential quantitative outcome measure that is also associated with visual dysfunction for use in future interventional studies.
Methods
Patient population
Patient records, fundus photographs, and clinical data from two retina clinics were reviewed to identify patients (1) with a diagnosis of IMT2 in at least one eye and (2) who were followed for at least 12 months with clinical examinations and fundus photography. All eyes with clinical evidence of IMT2 and fundus photographs of adequate quality were included in the current analysis. This study was performed with informed patient consent and conducted under a protocol approved by the local institutional review board and as part of the international MacTel project. All protocols were in accordance with the ethical standards stated in the Declaration of Helsinki.
Phenotypic grading of fundus images
The initial visit for each identified study eye was designated as the baseline visit. Fundus photographs for each eye at all study visits were graded for: (1) pigment clumping within a circle of 6mm diameter centered on the fovea, with pigment clumping defined as clinically visible granules or clumps of gray or black pigment in or beneath the retina, and (2) other clinical features associated with various stages of IMT2, including perifoveal telangiectactic vessels, perifoveal graying of the retina, retinal crystals, and neovascular changes.
Quantitation of area of involvement of pigment clumping
The retinal area involved with pigment clumping was measured from color fundus photographs using computer-aided image processing software (Image J; NIH; Bethesda, MD). A region of interest (ROI) encompassing the entire macular region was delineated or “cropped” from each fundus image. Each resulting image was converted to a monochromatic image using the green channel to be analyzed in parallel with the original color image. In order that fundus images captured under different conditions could be more uniformly processed, fundus photographs were normalized to a similar range of grayscale intensity or color luminescence values based on the darkest and lightest pixels in the image. These images were then subjected to contrast enhancement under similar parameters. Pre- and postprocessing images were individually evaluated and compared to confirm the absence of processing artifacts and that relevant clinical features had not been altered or masked. The area of involvement of each pigment clump in fundus images was quantitated by precise planimetry using a graphics tablet and pen (Intuos 4; Wacom; Vancouver, WA) and the freehand selection tool in ImageJ. The total area of pigment clumping was obtained by summing up the areas of each individually circumscribed pigment clump. Planimetric measurements for each color and grayscale image were performed, and then averaged. The images were quantitatively graded by a single reader (BT) and qualitatively assented to by another reader (ADM). Circumscribed areas were converted to units of square micrometers using a uniform conversion scale derived for the set of images from each patient.
Optical coherence tomography (OCT) imaging
OCT imaging was performed in a subgroup of study eyes (n=14); Cirrus™ HD-OCT, Carl Zeiss Meditec, Jena, Germany). Scanning was performed with the 512 × 128 scan pattern centered on the fovea and covering a 6 × 6 mm area on the retina. En-face views of the OCT scans were aligned with corresponding fundus photographs to locate individual B-scans traversing the areas of pigment clumping.
Microperimetry
Microperimetry testing was performed on a small number of patients in this cohort (n=5) using the MP-1 device (NAVIS software version 1.7.1; Nidek, Padua, Italy). Retinal sensitivity in the macula was typically tested using a 68-loci circular grid centered on the center of the macula covering the central 20° of the macula (10-2 program) and a background luminance of 4 apostilibs (1.27 cd/m2). A testing stimulus of size Goldmann III (area of 4mm2, diameter 0.4°) and 200ms duration was used. A 4- -2 staircase strategy was employed, using testing intensities ranging from 127 to 2.54 cd/m2, which correspond to retinal sensitivities of 0 to 20 dB, respectively. Microperimetry was performed after pupillary dilation and prior to clinical examinations or fundus imaging.
Statistical analyses
The change in area of pigment growth was analyzed using both simple regression and a generalized linear mixed model using commercial statistical software (SAS version 9.2, SAS institute, Cary, NC). Correlative analyses of pigment clumping with visual acuity, OCT findings, and microperimetry findings were also performed.
Results
Baseline characteristics of study population
Clinical features of IMT2 were found bilaterally in 26 patients and unilaterally in only one patient (total of 53 eyes). The mean duration of follow-up was 42.5 months (range = 12–79 months). The majority of patients were Caucasian (77.8%) and in their 5th to 8th decade of life (mean age = 61 years, range = 40–79 years). About half (56%) were female. Demographic information for the study population at baseline is summarized in Table 1.
Table 1.
Patient demographics and characteristics of study eyes with idiopathic macular telangiectasia type 2 (IMT2) at study baseline (n = 53).
| Patient age, mean±SD (range), years | 61.1±10.1 (range = 40–79) |
|
| |
| Sex, female, % | 56% |
|
| |
| Race,% | |
| White | 21(77.8%) |
| African American | 4 (14.8%) |
| Asian | 2 (7.4%) |
|
| |
| Eyes with pigment clumping at baseline, n (%) | 20 (37.7%) |
| Proportion of eyes with pigment clumping and neovascular disease | 4/20 (20.0%) |
| Proportion of eyes with pigment clumping and without neovascular disease | 16/20 (80.0%) |
|
| |
| Eyes with neovascular changes at baseline, (n, %) | 6/61(11.3%) |
|
| |
| Mean baseline visual acuity of all eyes, logMAR (Snellen equivalent) | 0.39 (20/49) |
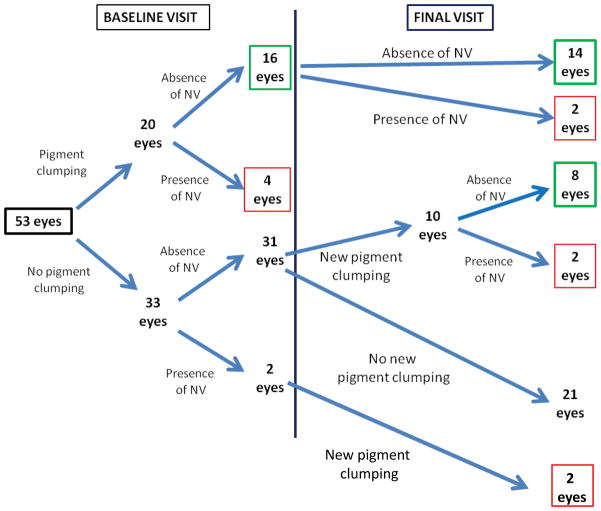
At study baseline, study eyes were categorized phenotypically according to the presence or absence of (1) pigment clumping and (2) neovascular changes (Fig. 1, Table 1). We chose to assess these two components separately due to the possibility that the presence of neovascularization would result in a fibrovascular or cellular response that may affect the presence and progression of intraretinal pigment clumping. We also evaluated whether the presence of neovascularization plays any role in the development of intraretinal pigment clumping. At the baseline visit, 20 out of 53 eyes (38%) had evidence of pigment clumping; of these, four eyes had evidence of concurrent neovascular changes, while 16 eyes did not. The remaining 33 eyes (62%) lacked pigment clumping; however, two of these also had evidence of neovascularization. From this cross-sectional sampling of eyes with IMT2 at various stages of severity, the overall prevalence of pigment clumping occurring without neovascular changes was approximately 30% (16/53).
Figure 1. Phenotypic characterization of study eyes (n=53) with idiopathic macular telangiectasia type 2 (IMT2) at study baseline and by final visit.
Schematic diagram showing the phenotypic scoring of study eyes according to (1) the presence or absence of pigment clumping, and (2) the presence or absence of neovascular (NV) changes at baseline visit (left) and by final visit (right). At baseline visit, 20 eyes were found to have evidence of pigment clumping; in 16 of these eyes, pigment clumping was not associated with neovascular changes. Of 33 eyes that lacked pigment clumping at baseline, 31 eyes had neither pigment clumping nor neovascular changes. By final visit, two of the 16 eyes that had pigment clumping and no neovascular changes at baseline developed neovascular changes. Also, of the 31 eyes that lacked both pigment clumping and neovascular changes at baseline, 10 eyes developed pigment clumping subsequently, eight of which did not develop neovascular changes. In summary, by the final visit, the total number of eyes with pigmentary changes not associated with neovascular changes was 22 eyes (green boxes), while the number of eyes with pigmentary changes associated with neovascular changes was 10 eyes (red boxes).
Emergence of pigment clumping de novo
Fundus photographs of study eyes were graded phenotypically for pigment clumping and neovascular changes at all follow-up visits. The mean duration of follow-up for all study eyes was 42.5±14.2 months (range = 12–79 months). During follow-up, 10 eyes that lacked pigment clumping at baseline developed new pigment clumping (Fig. 1). Of these, 8 eyes developed pigment clumping in the absence of neovascular changes. In the two remaining eyes, pigment clumping was preceded by the development of neovascular changes.
Of the former group of eight eyes, the development of new pigment clumping often occurred in the presence of other phenotypic changes associated with stage 3 IMT2, including telangiectactic vessels (8/8 eyes), perifoveolar retinal graying (8/8 eyes), abnormal retinal venules (7/8 eyes), and perifoveolar retinal crystals (4/8 eyes). An example of the emergence of new pigment clumping in one such eye is shown in Figure 2. We observed that pigment clumping tended to emerge as small, isolated “islands” often located in the temporal perifoveal region. These small “islands” were associated with dilated telangiectactic vessels and clustered in a perivascular distribution. All pigment clumps increased in size over time, and in some cases, separate pigment clumps were observed to coalesce and form a large, contiguous pigment clump.
Figure 2. Emergence of new pigment clumping in the absence of neovascular changes in an eye with idiopathic macular telangiectasia type 2 (IMT2).
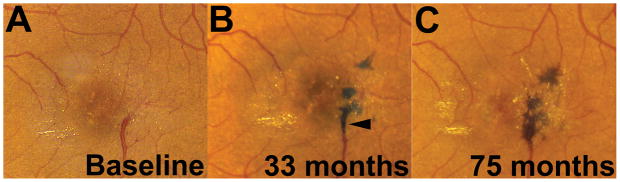
Color fundus photographs of a study eye during follow-up showing an example of pigment clumping emerging de novo in an eye with IMT2. At baseline visit (A), clinical features of IMT2 (telangiectasis, retinal crystals, abnormal venules, retinal graying) were present but without evidence of pigment clumping. At 33 months (B), separate clumps of pigment emerged de novo in the temporal perifoveal region, in some areas overlying perifoveal venules (arrowhead). At 75 months (C), these clumps had each increased in size and were partially coalescent.
By the final study follow-up visit, a total of 32 eyes (out of 53 eyes) demonstrated evidence of pigment clumping. In up to eight of these eyes, pigment clumping may have developed secondary to antecedent neovascular changes. A total of 22 eyes (out of 53 eyes) contained pigment clumping that was not associated with prior or concurrent neovascular changes. Visual acuity was assessed among eyes with pigment clumping and without neovascularization at baseline and final visits. Eyes with neovascularization were excluded to avoid potential confounding caused by sequelae of neovascularization such as the formation of subretinal fibrosis. Visual acuity at final visit among all eyes with pigment clumping without neovascularization (n = 22) was lower than in eyes without pigment clumping and without neovascularization (n = 21) (logMAR = 0.43 vs. 0.22, Snellen equivalent 20/53 vs. 20/33, p<0.05).
Dynamics of change of pigment clumping area
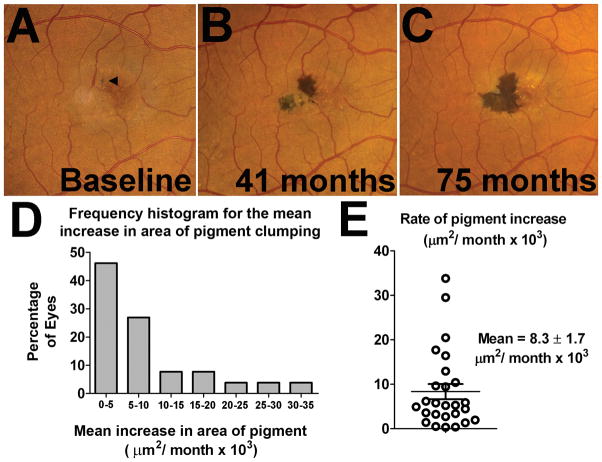
The areas associated with pigment clumping during study follow-up were evaluated quantitatively using planimetric methods. As pigment clumping arising after neovascular changes may have been induced secondarily from tissue disruption in a non-specific manner (commonly observed in other exudative retinal diseases12), study eyes with a longitudinal record of pigment clumping without neovascular changes were identified and specifically analyzed (n=22 of the 24 eyes with pigment clumping in the absence of neovascular changes). An example of a study eye with such a longitudinal record of progression is shown in Figure 3A–C. We observed that every eye in this subset (22 out of 22 eyes) demonstrated a monotonic increase in the total area of pigment clumping as a function of time. The rate of increase in total area was found to be variable between individual eyes (Fig. 3D), but most eyes demonstrated a linear rate of increase for the period examined (mean (±SD) r2 correlation for eyes with ≥3 time points = 0.93(±0.07), least-squares linear regression, n= 19 eyes). In cases where the increase in pigment area was monitored between fellow eyes of the same patient, a moderate correlation in the rate of pigment areal growth between fellow eyes was observed (Pearson’s r=0.50). The overall mean (±SEM) rate of pigment area increase was calculated to be 8.3 ± 1.7 × 103 μm2 per month (Figure 3E).
Figure 3. Natural history of pigment clumping changes in idiopathic macular telangiectasia type 2 (IMT2).
(A–C) An example of a study eye that demonstrated progressive increases in the total area of pigment clumping with time. The total area of pigment increased from 13 × 103 μm2 at baseline (A, arrowhead)) to 70 × 103 μm2 at 41 months (B), and to 127 × 103 μm2 at 75 months (C). Separate islands of pigment clumping were often observed to emerge in close proximity to one another and then coalesce as they enlarged with time. (D) Frequency histogram showing different rates of growth for study eyes. All study eyes for which longitudinal follow-up of pigment clumping was available (n = 22) demonstrated a monotonic increase in pigment area as a function of time. Most eyes demonstrated a linear rate of increase with time in the area of pigment clumping. (E) Graph showing the distribution of the average linear rate of expansion of pigment area, with a mean (±SEM) rate of growth of 8.3±1.7 × 103 μm2 per month.
Laminar location of pigment clumping within the retina
Spectral domain OCT imaging was obtained on a subset of study eyes (n=14 eyes). Cross-sectional examination of OCT images revealed that clinically visible pigment clumping was spatially correlated with areas of intraretinal hyperreflectivity whose dimensions in the horizontal plane were similar to those measured in fundus photographs for the pigment clumps. These hyperreflective signals were located in varying levels of the laminar structure comprising the retina, ranging from the retinal pigment epithelium (RPE)-photoreceptor interface (Fig. 4A) to the inner limiting membrane (Fig. 4C). These signals also tended to be adjacent to areas of retinal atrophy or degeneration, suggesting that pigment clumping may be a secondary process initiated in areas of primary extant outer retinal degeneration, resulting in RPE cell proliferation followed by intraretinal migration.
Figure 4. Cross-sectional localization of pigment clumping within the neuroretina using optical coherence tomography (OCT).
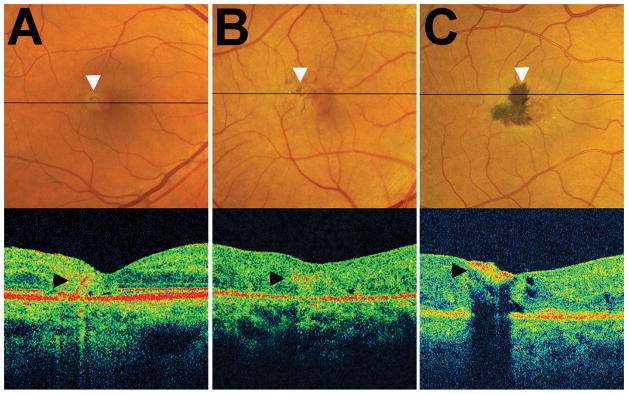
Horizontal OCT B-scans were obtained in the retinal plane traversing an area of pigment clumping (indicated by a black horizontal line) in individual study eyes. (A) Example of a study eye with a small isolated island of pigment in the temporal perifoveal region. OCT demonstrates a band of hyperreflectivity (arrowhead) that originated from the RPE layer and extended into the inner layers of the retina. (B) Example of a study eye with multiple small islands of pigment clumping which on OCT are observed as a horizontal area of reflectivity within the retina at the level of the outer plexiform layer or inner nuclear layer (arrowhead). (C) Example of a study eye with a large contiguous area of pigment clumping which appears on OCT as a hyperreflective plaque on the inner aspect of the retina (arrowhead).
Association of pigment clumping with retinal sensitivity
Microperimetry testing was performed on three study eyes containing pigment clumping in the absence of neovascularization. In these eyes, the location of pigment clumping was spatially correlated with areas of functional scotoma and decreased retinal sensitivity on microperimetry. As areas of pigment clumping expanded with time, the number of points on microperimetry with reduced retinal sensitivity was also observed to increase (Fig. 5).
Figure 5. Association of pigment clumping with functional scotomas on microperimetry testing.
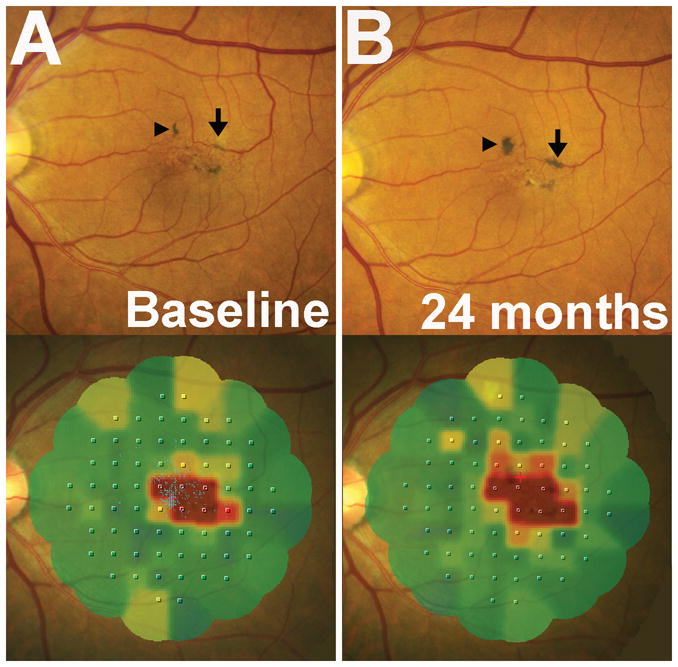
Longitudinal fundus photographs and microperimetric evaluations in a study eye with pigment clumping without neovascular changes. (A) The location of areas of pigment clumping observed on fundus photographs (top) are spatially correlated with the location of functional scotomas and decreased retinal sensitivity on microperimetry (MP1) testing (bottom). (B) Progression in the areas of pigment clumping over 24 months in the same eye (arrows in A, B) was also accompanied by an increase in the extent of retinal loci reporting decreased retinal sensitivity. Color coding in MP1 interpolated map output employs green and yellow to represent points of higher retinal sensitivity and orange and red to represent points of lower retinal sensitivity. Red areas on the interpolated map indicate areas of absolute scotoma.
Association of pigment clumping with neovascular disease
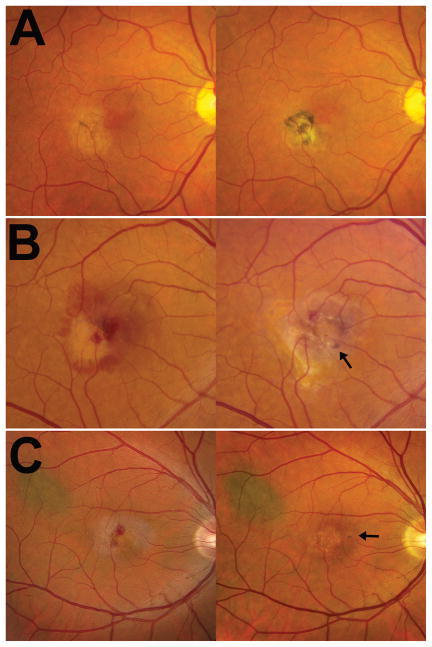
Among the 53 eyes in our cohort, six eyes had neovascular disease at baseline, with an additional four eyes developing neovascular disease by the end of the follow-up period (Fig. 1). The development of new neovascular changes was documented in eyes both with (n = 2) and without (n = 2) antecedent pigment clumping, indicating that pigment clumping was not a necessary precondition for the development of neovascular changes, nor did it conversely provide protection against the emergence of neovascularization (Fig. 6). The development of pigment clumping following neovascularization was also observed and likely represents a non-specific tissue reaction to exudative changes, in contrast to the emergence of pigment clumping de novo in the other study eyes.
Figure 6. Association of neovascular changes with pigmentary changes in idiopathic macular telangiectasia type 2 (IMT2).
(A) Example of a study eye in which pigment clumping (arrowhead) was present prior to neovascular changes (left). Neovascular changes developed subsequently with retinal fibrosis and an increase in pigment clumping (right). (B, C) Examples of study eyes in which neovascular changes developed in the absence of pigment clumping (left), with pigment clumping developing subsequent to neovascular changes (right, arrows). Pigment clumping was observed in study eyes to develop either de novo or following the development of retinal neovascularization which was accompanied by exudative changes and retinal fibrosis. Neovascular changes were also observed to emerge in study eyes with and without antecedent pigment clumping, which indicated that pigment clumping is unlikely to be a necessary pre-condition for the development of neovascularization.
Discussion
In this retrospective analysis of patients with a diagnosis of IMT2 of all stages of severity from two retina clinics, we examined the prevalence and longitudinal progression of pigment clumping in IMT2. We found pigment clumping without associated neovascularization in about one-third (30%) of all eyes with IMT2. When eyes with concurrent neovascular changes were included, this prevalence was about 38%, which corresponds well to that found in a larger natural history study of IMT2 (33%) 13. These features of pigment clumping and neovascular changes are part of a clinical staging classification proposed by Gass and Blodi7, in which the presence of pigment clumping without neovascularization constitutes stage 4 disease, and the presence of retinal neovascularization constitutes stage 5 disease.
In the current study, we had divided for separate analysis study eyes with pigment clumping occurring in the absence of antecedent neovascular changes and study eyes which developed pigment clumping subsequent to neovascular changes. We focused primarily on the former group of study eyes, reasoning that pigment clumping emerging in a non-neovascular context is more directly a result of disease events particular to IMT2. In comparison, pigment clumping that follows neovascular changes is also observed in exudative subretinal diseases such as neovascular age-related macular degeneration (AMD) and pathologic myopia. This may represent a more general response to outer retinal tissue disruption secondary to exudative changes, rather than a direct and specific consequence of IMT2 disease progression.
To our knowledge, a detailed quantitative study of the emergence and growth of pigment clumping in IMT2 has not been previously performed. In this longitudinal analysis, eight study eyes were observed to develop pigment clumping de novo during follow-up in the absence of antecedent neovascularization. In these cases, pigment clumping emerged in retinal areas containing telangiectactic vessels, retinal graying/loss of retinal transparency, and prominent right-angle retinal venules, which are features of stage 3 disease in the Gass-Blodi staging scheme7. These observations indicate that clinical changes associated with stage 3 disease precede the development of pigment clumping. The pathophysiology of pigment clumping, visible on OCT imaging as focal intraretinal hyperreflectivity in this and other studies 14, 15, has been thought to consist of reactive hyperplasia of the RPE4 and intraretinal RPE migration16. However, the nature of the cellular mechanisms initiating these RPE changes in IMT2 is obscure. Our OCT observations indicate that pigment clumping is initiated in areas of outer retinal thinning and disruption, suggesting that atrophic changes in the photoreceptor layer may create a permissive environment for RPE transformation, an interaction perhaps akin to the formation of “bone spicules” in the context of retinitis pigmentosa17–19 and intermediate AMD20. Also, we observed that the initial emergence of pigment clumping in affected eyes was localized in the temporal perifoveal region and was juxtaposed to telangiectactic venules. These observations suggest a possible inductive influence of telangiectactic vessels for RPE migration, which furthermore may provide a scaffold for migrating RPE cells to adhere. While the signals that guide intraretinal RPE migration in IMT2 are unknown, inflammatory cytokines and chemokines, such as those found in other retinal vascular diseases21, have been demonstrated to stimulate RPE migration 22, 23 and may be potentially relevant in IMT2.
In this study, we documented a consistent and monotonic increase in the total area of pigment clumping among all study eyes with non-neovascular IMT2. The growth in the area of pigment clumping was generally slow and tended to progress at a linear rate. The mean rate of growth was variable between eyes, ranging from 0.3 to 33.0 × 103 μm2 per month. For patients with pigment clumping in both eyes, the rate of increase in pigment area was found to be only moderately correlated between fellow eyes. Observing that pigment clumping, following its initial emergence, monotonically increased in area with time is suggestive of an ongoing disease process which continually drives the sequelae of RPE hyperplasia and migration in affected eyes. Thus, measurements of pigment clumping may serve as a quantitative marker of disease progression. Although variability in the growth rate of pigment clumping from case to case may complicate comparisons between treatment groups in an interventional trial, treatments that slow down, arrest, or reverse pigment clumping may signal an effect deserving further attention. Pigment clumping is likely to induce direct functional consequences in IMT2 that are in addition to those induced by foveal atrophy. For instance, pigment clumps map topographically on microperimetry testing to the location of functional scotomas 24, 25, and as shown here, are likely to produce enlarging functional scotomas in concert with continuous increases in the area of pigment clumping. This may also relate to reduced reading speeds of patients as the disease progresses6. In our analysis of eyes that did not have neovascularization, study eyes with pigment clumping demonstrated a lower mean visual acuity than eyes that did not have pigment clumping, indicating the possibility that pigment clumping may also contribute negatively to visual acuity. Whether this difference is also contributed to by a potentially larger amount of pre-existing photoreceptor dropout in eyes with pigment clumping is however unclear.
Although it has been suggested that pigment clumping may be a pre-condition for neovascularization in IMT216, we did not find this association. Out of 53 study eyes, we observed a total of 4 eyes in which neovascular changes were observed without concurrent evidence of pigment clumping. On the other hand, pigment clumping was also not “protective” against the development of neovascularization: out of 16 study eyes that had pigment clumping without neovascular changes at baseline, two eyes developed neovascularization de novo during study follow-up. On the whole, our observations indicate that the emergence of pigment clumping and development of neovascular changes are not strongly related in IMT2.
Although not present in all eyes with IMT2, our study indicates that pigment clumping, when present, increases progressively in all cases and is likely associated with decreased functional parameters. These observations raise the possibility that quantitative changes in pigment area may constitute a potentially useful outcome measure for interventional clinical studies of IMT2. The progressive increase in the area of pigment clumping also highlights unknown but ongoing pathogenic mechanisms that continually drive disease progression. Future clinical and basic science investigations into the phenomenon of pigment clumping in IMT2 may be helpful in defining useful disease outcome measures as well as uncovering potentially critical disease mechanisms with therapeutic significance.
Summary statement.
A retrospective case series examining the prevalence and progression of retinal pigment clumping in macular telangiectasia. Pigment clumping is a characteristic feature of macular telangiectasia which progresses over time, is associated with decreased visual function, and may reflect a reaction to underlying neurodegeneration.
Acknowledgments
This work has been supported by funding from the National Eye Institute Intramural Research Program and the Lowy Medical Foundation, Sydney, Australia.
Footnotes
Commercial relationships: none of the authors have relevant commercial financial relationships
References
- 1.Aung KZ, Wickremasinghe SS, Makeyeva G, Robman L, Guymer RH. The prevalence estimates of macular telangiectasia type 2: the Melbourne Collaborative Cohort Study. Retina. 2010;30:473–478. doi: 10.1097/IAE.0b013e3181bd2c71. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Blodi BA, Meuer SM, Myers CE, Chew EY, Klein BE. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol. 2010;150:55–62. e52. doi: 10.1016/j.ajo.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemons TE, Gillies MC, Chew EY, et al. The National Eye Institute Visual Function Questionnaire in the Macular Telangiectasia (MacTel) Project. Invest Ophthalmol Vis Sci. 2008;49:4340–4346. doi: 10.1167/iovs.08-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yannuzzi LA, Bardal AM, Freund KB, Chen KJ, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124:450–460. doi: 10.1001/archopht.124.4.450. [DOI] [PubMed] [Google Scholar]
- 5.Charbel Issa P, Holz FG, Scholl HP. Metamorphopsia in patients with macular telangiectasia type 2. Doc Ophthalmol. 2009;119:133–140. doi: 10.1007/s10633-009-9190-9. [DOI] [PubMed] [Google Scholar]
- 6.Finger RP, Charbel Issa P, Fimmers R, Holz FG, Rubin GS, Scholl HP. Reading performance is reduced by parafoveal scotomas in patients with macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2009;50:1366–1370. doi: 10.1167/iovs.08-2032. [DOI] [PubMed] [Google Scholar]
- 7.Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology. 1993;100:1536–1546. [PubMed] [Google Scholar]
- 8.Patel B, Duvall J, Tullo AB. Lamellar macular hole associated with idiopathic juxtafoveolar telangiectasia. Br J Ophthalmol. 1988;72:550–551. doi: 10.1136/bjo.72.7.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albini TA, Benz MS, Coffee RE, et al. Optical coherence tomography of idiopathic juxtafoveolar telangiectasia. Ophthalmic Surg Lasers Imaging. 2006;37:120–128. [PubMed] [Google Scholar]
- 10.Cohen SM, Cohen ML, El-Jabali F, Pautler SE. Optical coherence tomography findings in nonproliferative group 2a idiopathic juxtafoveal retinal telangiectasis. Retina. 2007;27:59–66. doi: 10.1097/01.iae.0000256663.94734.e1. [DOI] [PubMed] [Google Scholar]
- 11.Gaudric A, Ducos de Lahitte G, Cohen SY, Massin P, Haouchine B. Optical coherence tomography in group 2A idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 2006;124:1410–1419. doi: 10.1001/archopht.124.10.1410. [DOI] [PubMed] [Google Scholar]
- 12.Spaide R. Autofluorescence from the outer retina and subretinal space: hypothesis and review. Retina. 2008;28:5–35. doi: 10.1097/IAE.0b013e318158eca4. [DOI] [PubMed] [Google Scholar]
- 13.Clemons TE, Gillies MC, Chew EY, et al. Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel Project Report No. 2. Ophthalmic Epidemiol. 2010;17:66–73. doi: 10.3109/09286580903450361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surguch V, Gamulescu MA, Gabel VP. Optical coherence tomography findings in idiopathic juxtafoveal retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol. 2007;245:783–788. doi: 10.1007/s00417-006-0432-1. [DOI] [PubMed] [Google Scholar]
- 15.Wong WT, Forooghian F, Majumdar Z, Bonner RF, Cunningham D, Chew EY. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. Am J Ophthalmol. 2009;148:573–583. doi: 10.1016/j.ajo.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100:769–780. doi: 10.1001/archopht.1982.01030030773010. [DOI] [PubMed] [Google Scholar]
- 17.Flannery JG, Farber DB, Bird AC, Bok D. Degenerative changes in a retina affected with autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1989;30:191–211. [PubMed] [Google Scholar]
- 18.Jaissle GB, May CA, van de Pavert SA, et al. Bone spicule pigment formation in retinitis pigmentosa: insights from a mouse model. Graefes Arch Clin Exp Ophthalmol. 2010;248:1063–1070. doi: 10.1007/s00417-009-1253-9. [DOI] [PubMed] [Google Scholar]
- 19.Li ZY, Possin DE, Milam AH. Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology. 1995;102:805–816. doi: 10.1016/s0161-6420(95)30953-0. [DOI] [PubMed] [Google Scholar]
- 20.Ho J, Witkin AJ, Liu J, et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118:687–693. doi: 10.1016/j.ophtha.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meleth AD, Agron E, Chan CC, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:4295–4301. doi: 10.1167/iovs.04-1057. [DOI] [PubMed] [Google Scholar]
- 22.Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41:4324–4332. [PubMed] [Google Scholar]
- 23.Mitsuhiro MR, Eguchi S, Yamashita H. Regulation mechanisms of retinal pigment epithelial cell migration by the TGF-beta superfamily. Acta Ophthalmol Scand. 2003;81:630–638. doi: 10.1111/j.1395-3907.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 24.Charbel Issa P, Helb HM, Rohrschneider K, Holz FG, Scholl HP. Microperimetric assessment of patients with type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2007;48:3788–3795. doi: 10.1167/iovs.06-1272. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz-Valckenberg S, Ong EE, Rubin GS, et al. Structural and functional changes over time in MacTel patients. Retina. 2009;29:1314–1320. doi: 10.1097/IAE.0b013e3181a4d2f1. [DOI] [PubMed] [Google Scholar]