Abstract
Background
Glutathione S-transferase (GST) enzymes are involved in detoxifying chemotherapy and clearing reactive oxygen species formed by radiation. We explored the relationship between the host GSTP1 105 A>G polymorphism (rs1695), tumor GSTpi protein expression, and clinical outcomes in pediatric medulloblastoma. We hypothesized that the GSTP1 105 G-allele and increased tumor GSTpi expression would be associated with lower progression-free survival and fewer adverse events.
Procedure
The study included 106 medulloblastoma/primitive neuroectodermal tumor (PNET) patients seen at Texas Children’s Cancer Center. Genotyping was performed using an Illumina HumanOmni1-Quad BeadChip and GSTpi expression was assessed using immunohistochemistry. We used the Kaplan-Meier method for survival analyses and logistic regression for toxicity comparisons.
Results
Patients with a GSTP1 105 AG/GG genotype (vs. AA) or who had received high dose craniospinal radiation (≥ 34 Gy vs. <26 Gy) had a greater risk of requiring hearing aids than their counterparts (OR 4.0, 95%CI 1.2-13.6, and OR 3.1, 95%CI 1.1-8.8, respectively, n=69). Additionally, there was a statistically significant interaction between these variables. Compared with the lowest risk group (GSTP1 105 AA-low dose radiation), patients with a GSTP1 105 AG/GG genotype who received high dose radiation were 8.4 times more likely to require hearing aids (95%CI 1.4-49.9, p-trend=0.005, n=69). When adjusted for age, cumulative cisplatin dose, and amifostine use, the association remained.
Conclusions
The GSTP1 105 G-allele is associated with permanent ototoxicity in pediatric medulloblastoma/PNET and strongly interacts with radiation dose. Patients with this allele should be considered for clinical trials employing radiation dose modifications and cytoprotectant strategies.
Keywords: Glutathione S-Transferase, Polymorphism, Radiation, Ototoxicity, Medulloblastoma
Introduction
Medulloblastoma is the most common malignant brain tumor in pediatrics, accounting for 20% of central nervous system malignancies in the United States [1,2]. Treatment in children at least three years of age consists of surgical resection, craniospinal radiation (CSI), and chemotherapy [3,4]. This produces long-term overall survival of 60-80% [5]. Despite improving survival, many individuals experience therapy-related morbidities, including neurocognitive impairment, endocrinopathies, and hearing loss [5,6]. Well established prognostic criteria for survival include age and risk grouping - based on the initial disease extent and the degree of surgical resection [1,5]. More recently, the presence of distinctive disease subtypes characterized by molecular classification schemes has been proposed [7,8]. However, for toxicities, it is largely uncertain which patients will develop them and how severe they will be.
One group of enzymes that deserves more attention in medulloblastoma is the glutathione S-transferase (GST) family. GSTs detoxify electrophilic compounds via conjugation of reduced glutathione. These compounds include mutagenic and carcinogenic materials, chemotherapy agents and their metabolites, and free radicals [9]. Genetic variation in these enzymes, including GSTP1 105 A>G SNP (rs1695) and whole gene deletions in GSTM1 and GSTT1, have been associated with survival and toxicities in other brain tumors and other cancers treated with platinum agents and radiation [10-15]. Additionally, higher tumor GSTpi expression has been correlated with worse prognosis in adults with high grade glioma and other platinum-responsive malignancies [16-18]. Little is known about the role of GSTP1 polymorphisms and tumor GSTpi expression in medulloblastoma outcomes.
In this study, we assessed the relationship between the germline GSTP1 105 A>G polymorphism, tumor GSTpi expression, and survival and the occurrence of toxicities in patients with medulloblastoma/PNET seen at our institution. We hypothesized that the presence of a G-allele at the GSTP1 105 site and increased tumor GSTpi expression would be associated with lower progression-free survival and reduced therapy-related toxicities.
Methods
Study population
There were 106 patients with medulloblastoma or supratentorial primitive neuroectodermal tumor (PNET) seen at Texas Children’s Cancer Center included in this study. Peripheral blood samples were obtained for genotyping from 86 patients. Tumor samples were obtained for immunohistochemical (IHC) staining from 51 patients. Paired peripheral blood and tumor samples were available on 32 patients. The parents/legal guardians of all subjects provided informed consent for an IRB-approved institutional protocol for SNP-cancer outcome associations. Patients were diagnosed between March 1987 and July 2009 and were younger than 19 years at diagnosis. Seventy patients (66%) were treated on the SJMB96/03 or CCG-A9961 protocols with the remainder mostly treated with vincristine, cisplatin, and cyclophosphamide based regimens [3,4]. Medical record abstractions were performed for patient and therapy characteristics and outcomes including survival events and the occurrence of acute toxicities/late effects (i.e., present or absent). Acute toxicities included were myelosuppression, renal insufficiency, and neurotoxicity. They were deemed present if they led to at least one instance of chemotherapy dose reduction, delay, or omission. Late effects included were hypothyroidism, growth hormone deficiency, chronic renal insufficiency, and ototoxicity. These were defined as present if they were noted in the medical record greater than one year after the end of the initial therapy regimen and led to an intervention. These interventions were defined for hypothyroidism and growth hormone deficiency as hormone supplementation, for chronic renal insufficiency as ongoing nephrology care, and for ototoxicity as the need for hearing aids.
Laboratory methods
Peripheral blood samples were collected in EDTA vacutainers and genomic DNA was extracted from mononuclear cells using the QIAamp DNA blood mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. DNA concentrations were determined using the PICOgreen method and DNA quality was determined by gel electrophoresis of extracted products. GSTP1 105 A>G genotyping was performed as part of another study involving these subjects. For that study, the Illumina HumanOmni1-Quad BeadChip (Illumina, San Diego, CA) was used according to the manufacturer’s protocol.
Immunohistochemical staining of formalin-fixed, paraffin-embedded tumor samples was performed to assess GSTpi expression. Sections were rehydrated in PBS and then incubated with a polyclonal rabbit anti-human GSTpi antibody at a 1:500 dilution. The slides were then rinsed with PBS and incubated with an avidin-conjugated mouse anti-rabbit antibody. After further PBS rinsing, the slides were treated with a biotinylated peroxidase solution (Vector Laboratories, Burlingame, CA) and developed with 0.05% diaminobenzidine and 0.01% H2O2 in 50 mM Tris-HCL buffer (pH 7.5). GSTpi expression was quantified for staining intensity and proportion of positive cells. Intensity was assessed in 600 cells (200-fold magnification). Based on the cytoplasmic staining intensity of at least 70% of cells, the intensity was categorized in quartiles. In the same microscopic fields, the proportion of cells expressing GSTpi was evaluated and also categorized into quartiles. An IHC score was defined as the product of the category values for the staining intensity and proportion.
Statistical analyses
Descriptive analyses were performed for patient, disease, and therapy characteristics, the GSTP1 105 A>G genotype, and the GSTpi IHC staining variables. The consistency of the genotypes with Hardy-Weinberg equilibrium and published results was assessed. All SNP analyses were performed using co-dominant and dominant modeling. Spearman rank correlation methods were used to assess the correlation, rs, between the genotyping and IHC staining. Since radiation therapy is typically delayed, reduced, or omitted for patients younger than three years, separate survival analyses were performed for patients diagnosed before three years of age. Survival analyses were performed using the Kaplan-Meier method with log-rank comparisons. Progression-free survival (PFS) and overall survival (OS) were calculated from the date of diagnosis to progression/recurrence or death, respectively, or for survivors, the date of the last contact.
Patients of all ages were included in the toxicity analyses with some exceptions: 1) those having the outcome of interest as a pre-existing condition (e.g., pre-therapy hearing loss), 2) those not receiving toxicity-inducing therapies (e.g., CSI unexposed subjects were excluded from endocrinopathy analyses), 3) and those lacking adequate medical documentation for evaluation of the toxicity. Toxicity analyses were performed with Chi-Square and Fisher’s Exact tests, as appropriate. Odds ratios with 95% confidence intervals were calculated using univariate logistic regression; multivariable logistic regression models were constructed in a forward selection manner. For the ototoxicity outcome, exploratory interaction analyses were conducted by creating combined genotype-CSI dose groups and comparing them with the Chi-Square test of homogeneity and score test for trend. These analyses were performed unadjusted and adjusted for age group (divided at median), cumulative cisplatin dose group (divided at median=300 mg/m2), and amifostine use (yes or no). Cumulative cisplatin doses were standardized using a 1 mg/kg=30 mg/m2 conversion. Statistical significance was defined as a p-value less than 0.05 and all tests of significance performed were two-sided. No adjustments for multiple testing were implemented since only a single polymorphism was studied. For all analyses, the Stata 11 software package was used (StataCorp LP, College Station, TX).
Results
Patient, disease, and therapy characteristics
The patient, disease, and therapy characteristics are summarized in Table I. The median follow-up in survivors was 7.3 years (range 1.7-23.5 years, n=78). The mean and median ages at diagnosis were 7.0 and 6.0 years, respectively (range 0.5-18 years, n=106). Eighty-six patients (81%) were at least three years of age at diagnosis. Seventy-four patients (70%) were male and 47 (45%) were non-Hispanic white. In addition to 97 patients with medulloblastoma, 9 patients with supratentorial PNET were included. Fifty-two patients (61%) were classified as average-risk and 33 patients (38%) were high-risk, including the 5 patients with supratentorial PNET who were at least three years of age. All high-risk patients received high dose CSI (median 36 Gy, range 34.2-39.6 Gy), while 50/52 (96%) of the average-risk patients received low dose CSI (median 23.4 Gy, range 18-25.2 Gy).
Table I. Clinical Data for Study Patients.
| Variable | All Patients (n = 106) | Patients ≥ 3 years (n = 86) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Study subset | ||||
| Genotyping | 86 | 81 | 68 | 79 |
| IHC Staining | 51 | 48 | 46 | 53 |
| Overlap | 32 | 30 | 29 | 34 |
| Gender | ||||
| Male | 74 | 70 | 60 | 70 |
| Female | 32 | 30 | 26 | 30 |
| Race/Ethnicity | ||||
| Non-Hispanic white | 47 | 44 | 38 | 44 |
| Hispanic | 37 | 35 | 28 | 33 |
| Other | 22 | 21 | 20 | 23 |
| Disease | ||||
| Medulloblastoma | 97 | 92 | 81 | 94 |
| PNET | 9 | 8 | 5 | 6 |
| Risk | ||||
| High | NA | NA | 33 | 38 |
| Average | NA | NA | 52 | 61 |
| Unknown | NA | NA | 1 | 1 |
| Radiation Dose | ||||
| High | 42 | 39 | 35 | 41 |
| Low | 57 | 54 | 50 | 58 |
| Focal only | 3 | 3 | 0 | 0 |
| None | 3 | 3 | 0 | 0 |
| Unknown | 1 | 1 | 1 | 1 |
| Chemotherapy | ||||
| SJMB/CCG-A9961 | 70 | 66 | 68 | 79 |
| Other regimen | 34 | 32 | 16 | 19 |
| None | 2 | 2 | 2 | 2 |
IHC, immunohistochemistry; PNET, primitive neuroectodermal tumor; Craniospinal radiation dose: high, 34.2-39.6 Gy (median 36 Gy), low, 18-25.2 Gy (median 23.4 Gy); Chemotherapy (other regimen), non-SJMB96/03 and non-CCG-A9961 treatment regimen.
Genotyping and immunohistochemical staining
The frequencies of AA, AG, and GG were 0.37 (32/86), 0.41 (35/86), and 0.22 (19/86), respectively, yielding a minor allele frequency (MAF) for the G-allele of 0.42. These data are consistent with Hardy-Weinberg equilibrium and with allelic frequencies reported in public databases (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1695). For the IHC staining, samples were more likely to have absent/mild staining intensity (59%) and over 50% of the cells staining positive (55%) (n=51). The GSTP1 105 A>G genotype did not correlate with the GSTpi expression variables (−0.1<rs<0.05; p≥0.48).
Survival
Survival was assessed by age and risk grouping (Table II). Eighty-six patients were at least three years of age at diagnosis and 20 were less than three years. Those younger than three years had significantly worse 5-year PFS, but not 5-year OS (p=0.001 and 0.12, respectively). For risk grouping, the 5-year PFS and OS did not significantly differ between high-risk (n=33) and average-risk (n=52) categories (p=0.22 and 0.74, respectively).
Table II. 5-year Progression-Free Survival and 5-year Overall Survival for Known Prognostic Factors in Medulloblastoma.
| Group | N | Events | PFS | 95%CI | p | Events | OS | 95%CI | p |
|---|---|---|---|---|---|---|---|---|---|
| ≥ 3 years | 86 | 18 | 77.5 | 66.5 - 85.3 | 0.001 | 14 | 80.1 | 68.3 - 87.9 | 0.12 |
| < 3 years | 20 | 10 | 48.1 | 24.7 - 68.3 | 6 | 66.4 | 38.9 - 83.7 | ||
| High-risk | 33 | 9 | 70.9 | 51.1 - 83.8 | 0.22 | 6 | 79.2 | 59.1 - 90.1 | 0.74 |
| Average-risk | 52 | 9 | 81.3 | 67.0 - 89.8 | 8 | 79.5 | 61.8 - 89.6 |
PFS, 5-year progression-free survival; OS, 5-year overall survival.
In patients at least 3 years of age, there were no significant differences in PFS based on the GSTP1 105 A>G genotype (n=68) or GSTpi expression (n=46) in univariate and multivariable analyses. Among the 41 patients in the GSTP1 105 AG/GG group, there were 7 progressions (5-year PFS 80.7, 95%CI 63.3-90.4) and in the 27 patients with the AA genotype, there were 2 progressions (5-year PFS 92.2%, 95%CI 72.2-98.0), (p=0.38).
Toxicities
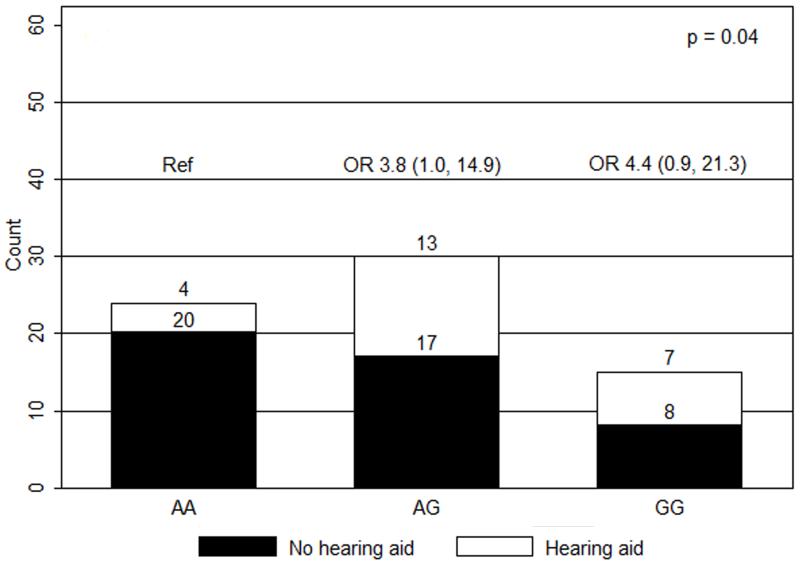
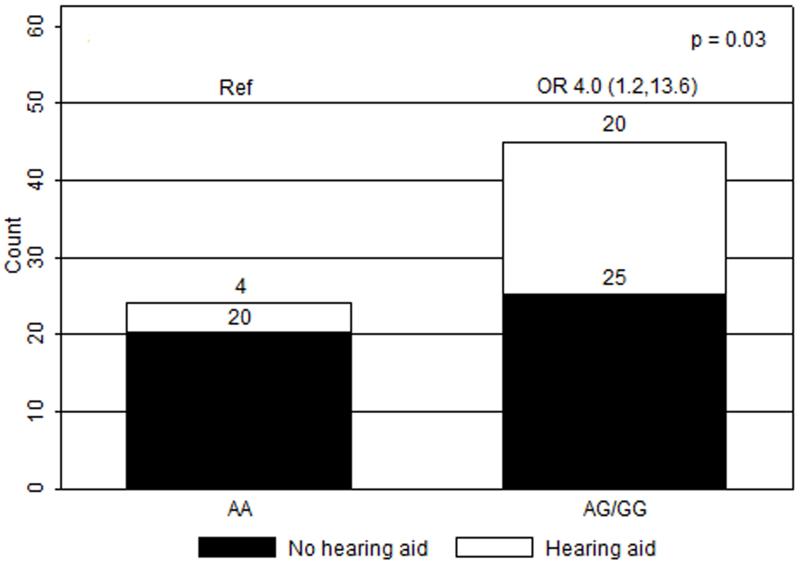
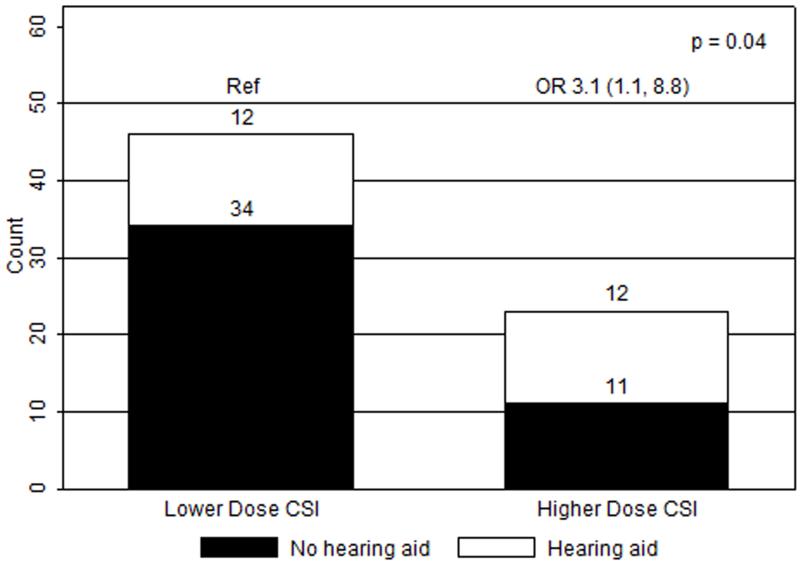
In univariate analyses, only the GSTP1 105 A>G genotype and craniospinal radiation dose were significantly associated with hearing loss requiring hearing aids (n=69). When compared to patients with the AA genotype (n=24), patients with the AG (n=30) and GG (n=15) genotypes had greater risks of severe ototoxicity (ORAG/AA 3.8, 95%CI 1.0-14.9; ORGG/AA 4.4, 95%CI 0.9-21.3, p=0.04) (Figure 1). With dominant modeling, patients in the AG/GG group had a greater risk of severe hearing impairment when compared to patients with the AA genotype (OR 4.0, 95%CI 1.2-13.6, p=0.03) (Figure 2). Compared to those receiving low dose CSI, patients who received high dose, were more likely to experience hearing loss requiring hearing aids (OR 3.1, 95%CI 1.1-8.8, p=0.04) (Figure 3). Ototoxicity did not differ by cumulative cisplatin dose (ORhigh/low 0.3, 95%CI 0.1-1.2, p=0.08; n=67) or amifostine use (ORyes/no 0.5, 95%CI 0.2-1.5, p=0.2, n=69). Multivariable analyses showed elevated ORs for GSTP1 105 A>G genotype and CSI dose without statistical significance (ORSNP 3.5, 95%CI 0.99-12.1, p=0.05; ORCSI 2.6, 95%CI 0.89-7.8, p=0.08; n=69).
Figure 1.
Ototoxicity by GSTP1 105 A>G genotype (co-dominant model). OR, odds ratios with 95% confidence intervals in parentheses; Ref, reference group.
Figure 2.
Ototoxicity by GSTP1 105 A>G genotype (dominant model). OR, odds ratios with 95% confidence intervals in parentheses; Ref, reference group.
Figure 3.
Ototoxicity by craniospinal radiation (CSI) dose group. Low dose CSI, 18 - 25.2 Gy (median 23.4 Gy); high dose CSI, 34.2 - 39.6 Gy (median 36 Gy); OR, odds ratios with 95% confidence intervals in parentheses; Ref, reference group.
To explore a potential interaction between the GSTP1 105 A>G genotype and CSI dose, patients were divided into four groups using all 2x2 combinations, ordered by a proposed risk for severe ototoxicity (n=69) (Table III). In the lowest risk group were individuals with the AA genotype who received low dose CSI and in the highest risk group were those in the AG/GG group who received high dose CSI. Using the lowest risk group as a reference, a significant dose-response pattern was observed (ORAA-High=1.3, 95%CI 0.1-17.4; ORAG/GG-Low=2.7, 95%CI 0.6-12.1; ORAG/GG-High=8.4, 95%CI 1.4-49.9, p-homogeneity=0.03, p-trend=0.005). The trend remained significant when adjusting for age group, cumulative cisplatin dose group, and amifostine use (p=0.02, n=67).
Table III. Risk of Severe Ototoxicity by Combination of GSTP1 105 A>G Genotype and Craniospinal Radiation Dose Group.
| Genotype - CSI dose group |
N (hearing aid) |
N (No hearing aid) |
OR | 95%CI |
|---|---|---|---|---|
| AA-Low | 3 | 16 | 1 (Ref) | NA |
| AA-High | 1 | 4 | 1.3 | 0.1 - 17.4 |
| AG/GG-Low | 9 | 18 | 2.7 | 0.6 - 12.1 |
| AG/GG-High | 11 | 7 | 8.4 | 1.4 - 49.9 |
CSI, craniospinal radiation dose: high 34.2-39.6 Gy (median 36 Gy), low, 18-25.2 Gy (median 23.4 Gy); Ref, reference group. In crude analyses (above), p-homogeneity=0.03 and p-trend=0.005. After adjusting for age group, cumulative cisplatin dose group, and amifostine use, p-trend=0.02.
Of the 86 patients genotyped, 17 patients were excluded from the ototoxicity analyses: 4 with pre-existing hearing loss, 9 who were lost to follow-up prior to one year post-primary therapy, and 4 who received focal radiation alone. Among the 69 patients in the ototoxicity analyses, 68 (99%) received cisplatin and 69 (100%) received CSI with 24 of 69 patients (35%) requiring hearing aids. In analyses involving the cumulative cisplatin dose, 2 children, for whom dose information was unavailable, were excluded. The median cumulative cisplatin dose was 300 mg/m2 (range 0-720 mg/m2). The mean cumulative cisplatin doses for those requiring hearing aids (302.6 mg/m2, 95%CI 263.1-342.1) and those not (328.5 mg/m2, 95%CI 294.4-362.1) were not statistically different (p=0.31).
There were no differences in other toxicities based on the GSTP1 105 A>G genotype. Additionally, no relationship between the toxicities and the GSTpi IHC score was demonstrated.
Discussion
We observed that patients in the GSTP1 105 AG/GG group were 4 times more likely to require hearing aids than those with the AA genotype. Moreover, exploratory analyses, based on combinations of the GSTP1 105 A>G genotype and CSI dose group, suggests a novel interaction, characterized by the risk allele conferring greater risk in individuals treated with high radiation doses than those treated with low doses.
The primary mechanism of platinum-induced ototoxicity occurs through the generation of free radicals, leading to apoptosis of the cochlear outer hair cells [19]. This produces sensorineural hearing loss (SNHL) with the high frequencies most likely affected. The hearing loss is typically permanent and may occur during therapy or in the months afterwards. Months to years later, radiation therapy may also cause SNHL, usually high-frequency, via cochlear injury mediated by reactive oxygen species [20,21]. Genetic variations in GSTs, a major enzyme family involved in clearing free radicals, are an ideal mechanism to explain the differences in ototoxicity susceptibility after medulloblastoma therapy. Supporting the role of GSTs in medulloblastoma toxicities is a prior study from our group. We demonstrated that children with medulloblastoma (n=42) having GSTM1 and GSTT1 deletions were at significantly greater risk for any acute toxicity (≥ grade 3) requiring therapy modification (≥1 null genotype: OR 6.4, 95% CI 1.2-34) and cognitive impairment (27.2 point post-radiation full-scale IQ drop, p=0.002) [22]. No association between ototoxicity and GSTM1 and GSTT1 deletions was detected.
While the influence of the GSTP1 105 A>G genotype on ototoxicity in medulloblastoma has not been previously explored, its role in ototoxicity in other malignancies has been studied. In 173 adults with testicular cancer treated with cisplatin-based regimens, hearing was evaluated with the threshold for impairment set at the 75th percentile, based on Norwegian men in their fifties in the general population [23]. The investigators found a greater risk of hearing loss in the AG/AA group when compared to those with the GG genotype (OR 4.3, 95%CI 1.3-14.4). The relationship between the GSTP1 105 A>G genotype and ototoxicity was evaluated in two other studies with no association discovered [24,25]. In both studies, children and young adults with cancer (e.g., osteosarcoma, neuroblastoma, brain tumors) treated with cisplatin-based regimens were assessed.
None of the above studies have compared the role of GSTP1 genotype between those who received radiation and those who did not. Moreover, few studies have assessed the role of the GSTP1 105 A>G genotype on radiation-associated toxicities. Two such studies were conducted in women with breast cancer who received adjuvant radiation [26,27]. A greater risk of acute skin toxicities was reported in women with the GSTP1 105 GG genotype (HR 2.3, 95%CI 1.04-5.0) and a slightly greater proportion of late pleural thickening was observed in women with the GG genotype (OR 1.1, p=0.004). More recently, in patients with esophageal adenocarcinoma treated with cisplatin-based radiochemotherapy, those in the GSTP1 105 AG/GG group were more likely to experience grade 3-4 radiation-attributable toxicities (e.g., dysphagia) than those with the AA genotype (31% vs. 0%, p=0.005) [28].
The GSTP1 105 A>G SNP is characterized by an adenine or guanine at position 313 resulting, respectively, in an isoleucine or valine at codon 105. Its functional significance varies by the exposure with the G-allele conferring increased GSTpi activity to platinum agents in vitro [29]. The variable metabolism of cisplatin by GSTP1 105 A>G genotype may not be mediated by the GSTpi free radical detoxification activity, but rather may depend on interactions with other pathways [30]. For example, JNK/SAPK pathway activity, which may be modulated by GSTpi expression, has been shown to influence cisplatin-mediated cell death. There are no data on the functional implications of the GSTP1 105 A>G genotype on radiation-associated reactive oxygen species, although it may be inferred from clinical studies that the G-allele leads to decreased GSTpi activity [26-28].
Our finding of the GSTP1 105 G-allele as a risk factor for severe ototoxicity in children with medulloblastoma/PNET may appear to contradict results observed by others [23,29]. However, previous clinical investigations included subjects on cisplatin-based regimens without radiation, a central component of medulloblastoma therapy [23-25]. Additionally, prior in vitro functional studies were performed with cisplatin alone, without radiation, which may interact with GSTpi [29,31]. We found that severe ototoxicity is associated with the combined exposure of the GSTP1 105 G-allele with high dose CSI. This is consistent with the clinical evidence showing that the G-allele is associated with greater radiation-related toxicities [26-28]. We did not find an association between severe hearing loss and the cumulative cisplatin dose. Several groups have suggested that the correlation between ototoxicity and the cumulative cisplatin dose is more pronounced above 400 mg/m2 [32,33]. The importance of CSI as opposed cisplatin in our study may be partly explained by the relatively lower cumulative cisplatin doses to which our population was exposed (median 300 mg/m2). Moreover, cisplatin dose modifications, which were widespread in our study, confounded the association between cumulative cisplatin dose and ototoxicity.
We also evaluated the relationship between tumor GSTpi expression and outcomes in medulloblastoma/PNET and found no associations. IHC staining may not be the best method of assessing GSTpi activity, particularly when using tumor tissue in which the activity may be disregulated. Instead, substrate-specific activity assays in normal tissue may be preferable.
Hearing loss remains a significant problem for patients with medulloblastoma. As many as half experience impaired hearing with roughly one-quarter of all patients needing hearing aids [34]. Hearing loss can substantially diminish quality of life. Cisplatin dose modifications for early ototoxicity, conformal radiation techniques, and amifostine prophylaxis have decreased this impairment, but have not eliminated it [35,36]. If our finding of a GSTP1 105 A>G SNP-ototoxicity link and its association with radiation dose can be validated, we can identify a group of patients at elevated risk for severe hearing loss. With this group, more sensitive screening methods for early ototoxicity may be applied, radiation dose reductions may be considered, or more targeted cytoprotectant strategies than are currently being employed with amifostine may be implemented.
This study is the first evaluating the relationship between the germline GSTP1 105 A>G genotype, tumor GSTpi expression, and ototoxicity in patients with medulloblastoma/PNET. Our population was largely uniformly treated with approximately 66% treated on the SJMB96/03 or CCG-A9961 protocols with the remainder mostly treated with regimens including the same chemotherapy. Additionally, with a median follow up in survivors of over 7 years, adequate time elapsed to assess the outcomes of interest. The analyses accounted for the potential confounders of age, cumulative cisplatin dose, and amifostine use. Although genetic admixture was not included in the analyses, the patients were almost exclusively non-Hispanic Caucasian, Hispanic, or African-American and the reported allelic frequencies for these three groups is very similar (MAF 39-42%) and was consistent with those observed in our study.
Our study had several limitations. This was a retrospective study spanning over two decades, during which time radiation techniques have progressed and toxicity monitoring and interventions have evolved. Also, radiation oncology reports specifying the cochlear doses were not available for a substantial proportion of the subjects. However, since most patients received conformal radiation, it was presumed that the radiation dose to the cochlea could be reasonably approximated by the minimum dose to which all tissues in the craniospinal axis were exposed (i.e., the CSI dose). The need for using the CSI dose as a surrogate for the cochlear dose will be avoided in our future prospective studies. Additionally, while patients with known pre-existing hearing loss were excluded from the analyses, it is possible that some patients with prior mild hearing loss were included due to lacking documentation. Lastly, the study sample size was small and limited the precision of our statistical estimates.
In the future, we plan to more extensively investigate the relationship between clinical outcomes in medulloblastoma and glutathione S-transferases and other detoxification/free radical clearance enzymes including glutathione peroxidases, glutathione reductase, superoxide dismutases, catalase, and metallothioneins. These studies will be performed with a larger, independent sample of patients and may provide a more comprehensive understanding of the role that detoxification and free radical clearance enzymes play in medulloblastoma outcomes and may ultimately influence future therapeutic strategies.
Acknowledgements
This work was supported by research grants from the National Institutes of Health (P50CA127001-04), the Gillson Longenbaugh Foundation, the John S. Dunn Research Foundation, the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation, and the American Brain Tumor Association.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
References
- 1.Pizzo PA, Poplack DG. Principles and Practice of Pediatric Oncology. 6th ed Lippincott Williams & Wilkins; Philadelphia: 2011. p. 772.p. 782. [Google Scholar]
- 2.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER Update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 3.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St. Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Gajjar A, Vezina G, et al. Phase III Study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 5.Pomeroy SL. [Accessed October 21, 2011];Epidemiology, treatment, and prognosis of medulloblastoma. UpToDate. http://www.uptodate.com/contents/epidemiology-treatment-and-prognosis-of-medulloblastoma.
- 6.Hoffman KE, Yock TI. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24:1387–1396. doi: 10.1177/0883073809342275. [DOI] [PubMed] [Google Scholar]
- 7.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilburn L, Okcu MF, Wang T, et al. Glutathione S-transferase polymorphisms are associated with survival in anaplastic glioma patients. Cancer. 2010;116:2242–2249. doi: 10.1002/cncr.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okcu MF, Selvan M, Wang LE, et al. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–2625. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong TS, Cao Y, Scheurer ME, et al. Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neuro Oncol. 2009;11:825–832. doi: 10.1215/15228517-2008-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielinska E, Zubowska M, Misiura K. Role of GSTM1, GSTP1, and GSTT1 gene polymorphism in ifosfamide metabolism affecting neurotoxicity and nephrotoxicity in children. J Pediatr Hematol Oncol. 2005;27:582–589. doi: 10.1097/01.mph.0000187429.52616.8a. [DOI] [PubMed] [Google Scholar]
- 14.Lo HW, Ali-Osman F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr Opin Pharmacol. 2007;7:367–374. doi: 10.1016/j.coph.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Zschenker O, Raabe A, Boeckelmann IK, et al. Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother Oncol. 2010;97:26–32. doi: 10.1016/j.radonc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Ali-Osman F, Brunner JM, Kutluk TM, Hess K. Prognostic significance of glutathione S-transferase pi expression and subcellular localization in human gliomas. Clin Cancer Res. 1997;3:2253–2261. [PubMed] [Google Scholar]
- 17.Joshi MB, Shirota Y, Danenberg KD, et al. High gene expression of TS1, GSTP1, and ERCC1 are risk factors for survival in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res. 2005;11:2215–2221. doi: 10.1158/1078-0432.CCR-04-1387. [DOI] [PubMed] [Google Scholar]
- 18.Surowiak P, Materna V, Kaplenko I, et al. Augmented expression of metallothionein and glutathione S-transferase pi as unfavorable prognostic factors in cisplatin-treated ovarian cancer patients. Virchows Arch. 2005:626–633. doi: 10.1007/s00428-005-1228-0. [DOI] [PubMed] [Google Scholar]
- 19.Tabuchi K, Nishimura B, Nakamagoe M, et al. Ototoxicity: Mechanisms of Cochlear Impairment and its Prevention. Curr Med Chem. 2011;18:4866–4871. doi: 10.2174/092986711797535254. [DOI] [PubMed] [Google Scholar]
- 20.Low WK, Tan MG, Chua AW, et al. 12th Yahya Cohen Memorial Lecture: The cellular and molecular basis of radiation-induced sensori-neural hearing loss. Ann Acad Med Singapore. 2009;38:91–94. [PubMed] [Google Scholar]
- 21.Jereczek-Fossa BA, Zarowski A, Milani F, Orecchia R. Radiotherapy-induced ear toxicity. Cancer Treat Rev. 2003;29:417–430. doi: 10.1016/s0305-7372(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 22.Barahmani N, Carpentieri S, Li XN, et al. Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma. Neuro Oncol. 2009;11:292–300. doi: 10.1215/15228517-2008-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenburg J, Kraggerud SM, Cvancarova M, et al. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 24.Peters U, Preisler-Adams S, Hebeisen A, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Ross CJ, Katzov-Eckert H, Dube MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosone CB, Tian C, Ahn J, et al. Genetic predictors of acute toxicities related to radiation therapy following lumpectomy for breast cancer: a case-series study. Breast Cancer Res. 2006;8:R40. doi: 10.1186/bcr1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edvardsen H, Kristensen VN, Grenaker Alnaes GI, et al. Germline glutathione S-transferase variants in breast cancer: relation to diagnosis and cutaneous long-term adverse effects after two fractionation patterns of radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1163–1171. doi: 10.1016/j.ijrobp.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Yoon HH, Catalano P, Gibson MK, et al. Genetic variation in radiation and platinum pathways predicts severe acute radiation toxicity in patients with esophageal adenocarcinoma treated with cisplatin-based preoperative radiochemotherapy: results from the Eastern Cooperative Oncology Group. Cancer Chemother Pharmacol. 2011;68:863–870. doi: 10.1007/s00280-011-1556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishimoto TM, Ali-Osman F. Allelic variants of the human glutathione S-transferase P1 gene confer differential cytoprotection against anticancer agents in Escherichia coli. Pharmacogenetics. 2002;12:543–553. doi: 10.1097/00008571-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Peklak-Scott C, Smitherman PK, Townsend AJ, Morrow CS. Role of glutathione S-transferase P1-1 in the cellular detoxification of cisplatin. Mol Cancer Ther. 2008;7:3247–3255. doi: 10.1158/1535-7163.MCT-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravard A, Luccioni C, Moustacchi E, Rigaud O. Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int J Radiat Biol. 1999;75:639–645. doi: 10.1080/095530099140285. [DOI] [PubMed] [Google Scholar]
- 32.Bertolini P, Mathilde Lassalle Mercier G, et al. Platinum Compound-Related Ototoxicity in Children: Long-Term Follow-Up Reveals Continuous Worsening of Hearing Loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–96. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 35.Paulino AC, Lobo M, Teh BS, et al. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;78:1445–1450. doi: 10.1016/j.ijrobp.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Fouladi M, Chintagumpala M, Ashley D, et al. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J Clin Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]