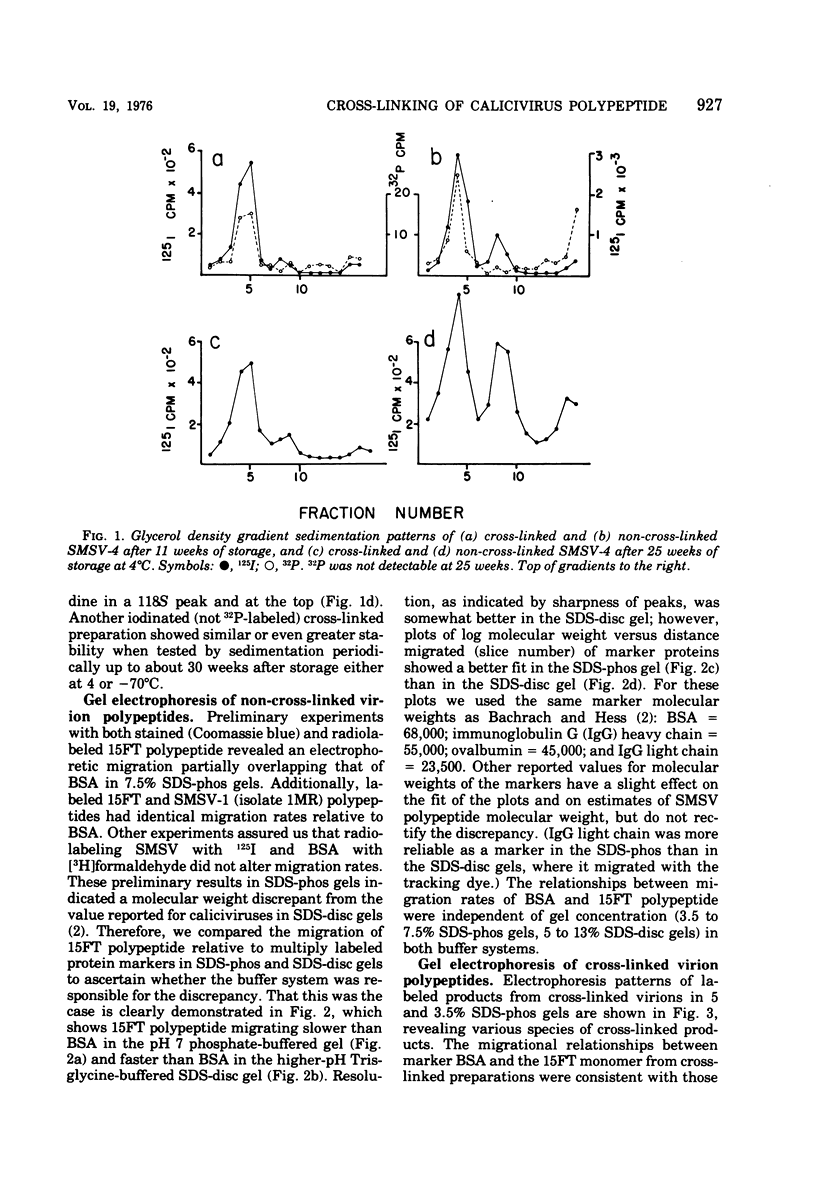
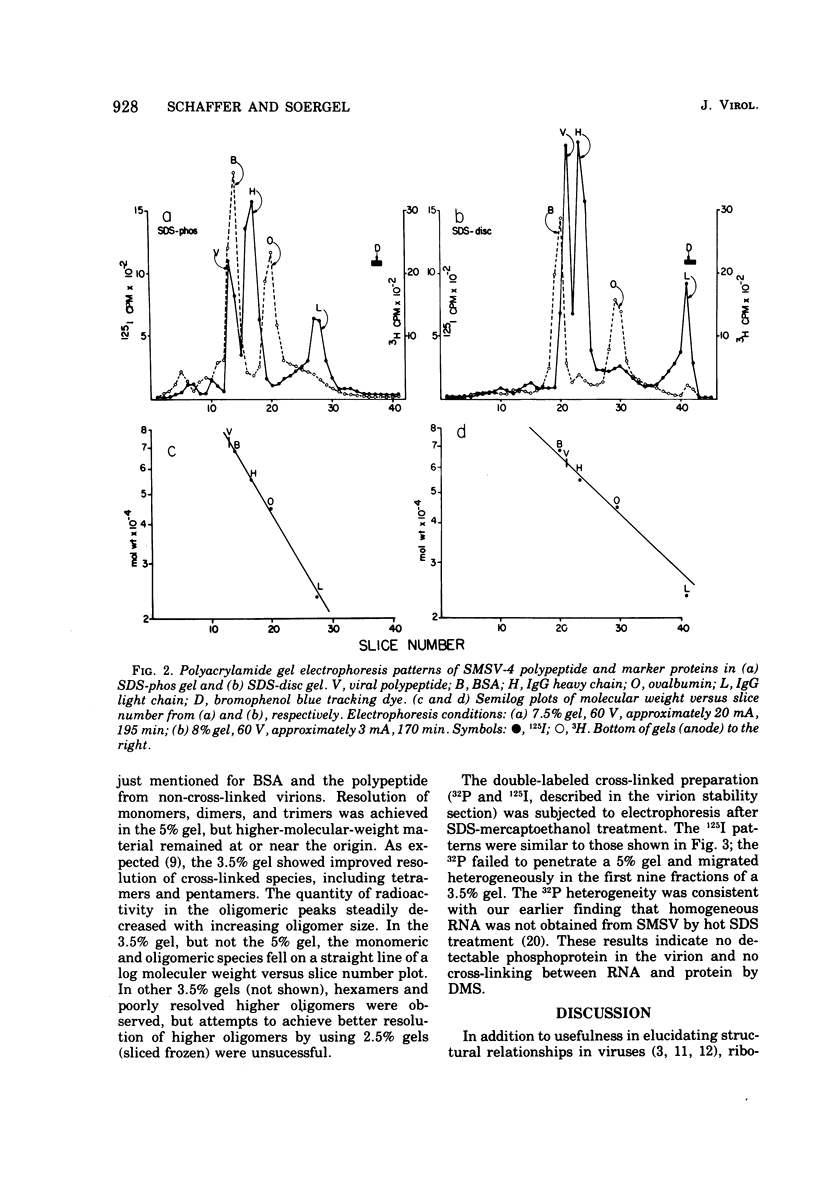
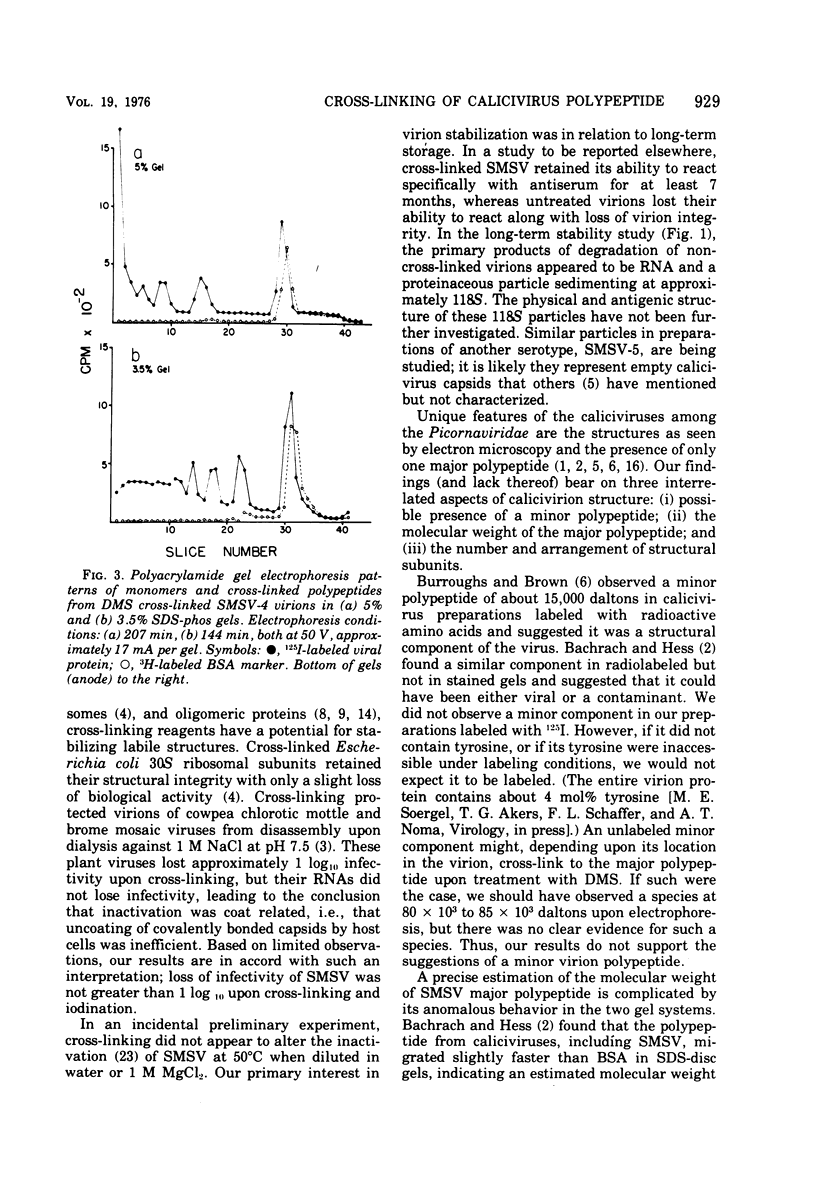
Abstract
A calicivirus, San Miguel sea lion virus serotype 4, isolate 15FT, externally labelled with 125I, was shown by gel electrophoresis to possess a single major polypeptide. The polypeptide migrated anomalously upon electrophoresis in two sodium dodecyl sulfate (SDS) systems: more slowly than bovine serum albumin in a continuous phosphate-buffered system and more rapidly than bovine serum albumin in a discontinuous system. Estimated molecular weights in the two systems were approximately 71,000 and 64,000, respectively. There was no clear evidence for a minor virion polypeptide. Treatment of purified San Miguel sea lion virions with dimethyl suberimidate, a cross-linking reagent, preserved virion integrity during long-term storage at 4 degrees C. Oligomeric species of the polypeptide were observed upon electrophoresis of products from cross-linked virions. Based upon a preferred polypeptide molecular weight estimate of 71,000 and distribution of oligomeric species, a calicivirion model with 120 monomeric protein units is proposed as an alternative to a 180-unit model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Waterson A. P., Prydie J., Fletcher E. W. The structure of a feline picornavirus and its relevance to cubic viruses in general. Arch Gesamte Virusforsch. 1968;25(1):105–114. doi: 10.1007/BF01243095. [DOI] [PubMed] [Google Scholar]
- Bachrach H. L., Hess W. R. Animal picornaviruses with a single major species of capsid protein. Biochem Biophys Res Commun. 1973 Nov 1;55(1):141–149. doi: 10.1016/s0006-291x(73)80070-1. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Hershey J. W., Traut R. R. Spatial arrangement of ribosomal proteins: reaction of the Escherichia coli 30S subunit with bis-imidoesters. Proc Natl Acad Sci U S A. 1972 May;69(5):1327–1331. doi: 10.1073/pnas.69.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Hull R. Comparative virology of the small RNA viruses. J Gen Virol. 1973 Jun;20(Suppl):43–60. doi: 10.1099/0022-1317-20-Supplement-43. [DOI] [PubMed] [Google Scholar]
- Burroughs J. N., Brown F. Physico-chemical evidence for the re-classification of the caliciviruses. J Gen Virol. 1974 Feb;22(2):281–286. doi: 10.1099/0022-1317-22-2-281. [DOI] [PubMed] [Google Scholar]
- Camacho A., Carrascosa J. L., Vinuela E., Salas M. Discrepancy in the mobility of a protein of phage phi29 in 2 different SDS polyacrylamide-gel systems. Anal Biochem. 1975 Dec;69(2):395–400. doi: 10.1016/0003-2697(75)90141-4. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Cross-linking of the spike glycoproteins in Semliki Forest virus with dimethylsuberimidate. Virology. 1974 Dec;62(2):385–392. doi: 10.1016/0042-6822(74)90400-0. [DOI] [PubMed] [Google Scholar]
- Hucho F., Janda M. Investigation of the quaternary structure of beef liver glutamate dehydrogenase with bifunctional reagents. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1080–1088. doi: 10.1016/0006-291x(74)90807-9. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Agol V. I., Bachrach H. L., Brown F., Cooper P. D., Fiers W., Gard S., Gear J. H., Ghendon Y., Kasza L. Picornaviridae. Intervirology. 1974;4(5):303–316. doi: 10.1159/000149863. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Prato C. M., Akers T. G., Smith A. W. Serological evidence of calcivirus transmission between marine and terrestrial mammals. Nature. 1974 May 17;249(454):255–256. doi: 10.1038/249255a0. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E. Biochemical and biophysical characterization of calicivirus isolates from pinnipeds. Intervirology. 1973;1(3):210–219. doi: 10.1159/000148848. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E. Liquid scintillation radioassay in disposable microcentrifuge tubes: radioimmune precipitates and other applications. Appl Microbiol. 1974 Aug;28(2):280–287. doi: 10.1128/am.28.2.280-287.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E., Straube D. C. Electrophoretic analysis of ribosomal and viral ribonucleic acids with a simple technique for slicing low-concentration polyacrylamide gels. Appl Microbiol. 1971 Oct;22(4):538–545. doi: 10.1128/am.22.4.538-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. W., Akers T. G., Madin S. H., Vedros N. A. San Miguel sea lion virus isolation, preliminary characterization and relationship to vesicular exanthema of swine virus. Nature. 1973 Jul 13;244(5411):108–110. doi: 10.1038/244108a0. [DOI] [PubMed] [Google Scholar]
- Soergel M. E., Smith A. W., Schaffer F. L. Biophysical comparisons of calicivirus serotypes isolated from pinnipeds. Intervirology. 1975;5(3-4):239–244. doi: 10.1159/000149920. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Vande Woude G. F., Bachrach H. L. Sodium dodecylsulfate-dependent anomalies in gel electrophoresis: alterations in the banding patterns of foot-and-mouth disease virus polypeptides. Anal Biochem. 1974 Apr;58(2):337–346. doi: 10.1016/0003-2697(74)90201-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



