Abstract
Rationale
Most cardiac ryanodine receptor (RyR2) mutations associated with Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) are postulated to cause one distinctive form of Ca2+ release dysfunction. Considering the spread distribution of CPVT mutations, we hypothesized that dysfunctional heterogeneity was also feasible.
Objective
To determine the molecular and cellular mechanism(s) by which a novel RyR2-V2475F mutation associated with CPVT in humans triggers Ca2+-dependent arrhythmias in whole hearts and intact mice.
Methods and Results
Recombinant channels harboring CPVT-linked RyR2 mutations were functionally characterized using [3H]ryanodine binding and single channel recordings. Homologous recombination was used to generate a knock-in mouse bearing the RyR2-V2475F mutation. Ventricular myocytes from mice heterozygous for the mutation (RyR2-V2475F+/−) and their wild-type (WT) littermates were Ca2+-imaged by confocal microscopy under conditions that mimic stress. The propensity of WT and RyR2-V2475F+/− mice to develop arrhythmias was tested at the whole heart level and in intact animals. Recombinant RyR2-V2475F channels displayed a) increased cytosolic Ca2+ activation, b) abnormal PKA phosphorylation, and c) increased activation by luminal Ca2+. The RyR2-V2475F mutation appears embryonic lethal in homozygous mice, but heterozygous mice have no alterations at baseline. Spontaneous Ca2+ release (SCR) events were more frequent and had shorter latency in isoproterenol-stimulated cardiomyocytes from RyR2-V2475F+/− hearts, but their threshold was unchanged with respect to WT. Adrenergically-triggered tachyarrhythmias were more frequent in RyR2-V2475F+/− mice.
Conclusions
The mutation RyR2-V2475F is phenotypically strong among other CPVT mutations and produces heterogeneous mechanisms of RyR2 dysfunction. In living mice, this mutation appears too severe to be harbored in all RyR2 channels, but remains undetected under basal conditions if expressed at relatively low levels. β-adrenergic stimulation breaks the delicate Ca2+ equilibrium of RyR2-V2475F+/− hearts and triggers life-threatening arrhythmias.
Keywords: CPVT, inherited arrhythmias, ryanodine receptor
INTRODUCTION
Type 2 ryanodine receptors (RyR2s) are the calcium release channels of sarcoplasmic reticulum (SR) that provide the majority of calcium ions (Ca2+) necessary to induce contraction of cardiac cells.1 In their intracellular environment, RyRs are regulated by a variety of cytosolic and luminal factors so that their output signal (Ca2+) induces finely-graded cell contraction without igniting cellular processes that may lead to aberrant electrical activity (ventricular arrhythmias).2 The importance of RyR2 dysfunction has been recently highlighted with the demonstration that point mutations in RYR2, the gene encoding RyR2 channels, are associated with Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), an arrhythmogenic syndrome characterized by the development of adrenergically-mediated ventricular tachycardia in individuals with an apparently normal heart.3 Equivalent mutations engineered in the murine RyR2 gene also result in the development of phenotypes that recapitulate the major clinical manifestations of CPVT. Hence, CPVT mice represent a bona fide experimental model where the role of deranged Ca2+ homeostasis in the triggering of arrhythmias (Ca2+-dependent arrhythmogenesis) may be integrally assessed. However, the molecular mechanisms that link a mutation in the RyR2 protein and the development of tachyarrhythmia remain incompletely understood.
To date, a considerable number of single-amino acid RyR2 mutations (>150) have been associated with the development of CPVT.4 and (http://www.fsm.it/cardmoc) Remarkably, the vast majority of these mutations3 fall within three loci (“hot spots”) termed CPVT-I, CPVT-II, and CPVT-III domains, of the RyR2 protein that are involved in several aspects of RyR2 regulation. Yet, most CPVT mutations characterized to date display a single mechanism of RyR2 dysfunction. 6-13 This stereotyped mechanism of dysfunction is puzzling given the multiple functional domains of the RyR2 protein potentially affected by each of the CPVT mutations. Moreover, CPVT episodes, by definition, occur after acute β-adrenergic stimulation but it is unknown whether phosphorylation of RyR2 channels is an obligatory step to elicit abnormal Ca2+ release in mutant channels or whether sympathetic stimulation only exacerbates the activity of a RyR2 channel already on the verge of ignition.
We performed an in-depth characterization of a novel CPVT-linked mutation, RyR2-V2475F, that was identified post mortem in a case of unexplained drowning of a young boy.14 The mutation falls within the canonical CPVT-II domain, but unlike other RyR2 mutants, RyR2-V2475F displays multiple mechanisms of RyR2 dysfunction, including increased cytosolic Ca2+ sensitivity, altered [Ca2+]luminal regulation and abnormal response to PKA phosphorylation. In vivo, the RyR2-V2475F mutation is highly arrhythmogenic. Thus, the RyR2-V2475F mutation produces a heterogeneous mechanism of RyR2 dysfunction.
METHODS
Recombinant mutant channels were generated by site-directed mutagenesis, expressed in HEK293 cells and purified as described before.6 Mice with the RyR2-V2475F mutation were generated by homologous recombination. RyR2-V2475F+/− mice and age-matched wild-type (WT) littermates were maintained and studied according to the protocol approved by the Institutional Animal Care and Use Committees of the University of Wisconsin-Madison and the University of Michigan-Ann Arbor, and by the Association for Assessment and Accreditation of Laboratory Care International.
An expanded Materials and Methods section is available in the online data supplement.
RESULTS
Expression and characterization of recombinant RyR2-V2475F channels
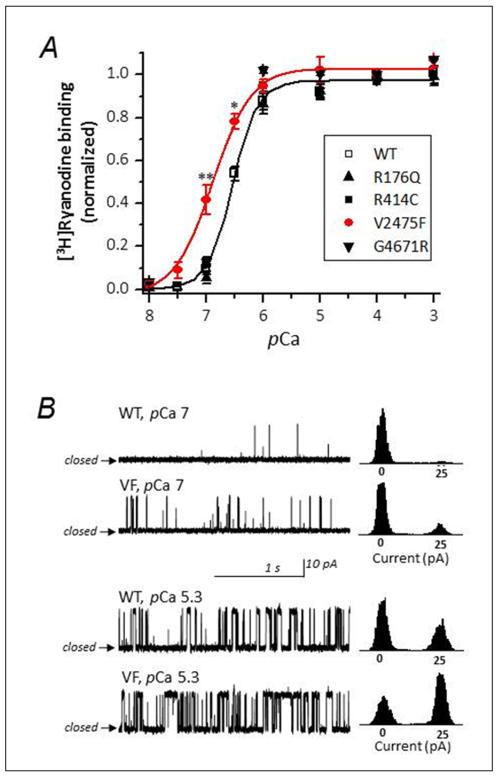
RyR2 mutations associated with CPVT cluster in three well defined domains that control different aspects of channel function. Because these canonical domains are well conserved among species, we used site-directed mutagenesis to introduce in the mouse RYR2 gene several mutations that are associated with CPVT in humans. The RyR2 mutations R176Q and R414C (pertaining to CPVT-I), V2475F (pertaining to CPVT-II), and G4671C (pertaining to CPVT-III) were expressed as described in Methods. We first tested their sensitivity to activation by Ca2+ using [3H]ryanodine binding assays. [3H]Ryanodine is a conformationally-sensitive ligand that binds to the open state of RyRs and may be used as a reliable index of the activity of the channel. Fig. 1A shows the Ca2+-dependence of [3H]ryanodine binding of purified WT (control) and mutant channels. For all channels, [Ca2+] threshold for activation was above pCa 8, and reached plateau at pCa ~5. The activation was sigmoidal and could be fitted with a Hill equation with an EC50 = pCa 6.3±0.12 for WT, R176Q, R414C and G4671 channels. Remarkably, only V2475F displayed significant difference with WT, with an EC50 = 6.8±0.15. At pCa 7, activation of WT channels was only ~10% of normalized value, whereas in V2475F, activation exceeded ~40%. Since previous work has demonstrated that Ca2+ in the range of 100 nM (pCa 7) to 10 μM (pCa 5) binds to a high affinity Ca2+-binding site in the cytosolic portion of the RyR protein,2,6,7 the results suggest that the V2475F mutation confers RyR2 channels hypersensitivity to cytosolic Ca2+. Mutations that alter RyR2 activity at pCa 7 are especially interesting since this is the basal [Ca2+] reached during diastole.
Figure 1. RyR2-V2475F channels display higher sensitivity to cytosolic Ca2+ than WT channels.
A, Ca2+-dependence of [3H]ryanodine binding curves performed in parallel using the solubilized and purified channels indicated in the inset. Asterisk indicates p<0.05. B, Single channel recordings of WT and V2475F channels reconstituted in lipid bilayers. Left panels show 2-sec segments of representative single channel activity at the indicated cis (cytosolic) Ca2+ and the right panels are current histograms of at least 5 min of cumulative recording performed in n=6 (WT) and n=4 (V2475F) channels
To directly test whether the V2475F mutation increased the sensitivity of the RyR2 channel to cytosolic Ca2+, we reconstituted purified WT and V2475F channels in planar lipid bilayers.15 Fig. 1B, left panels, shows single channel activity at the indicated cis (cytosolic) [Ca2+]; the right panels are current histograms of cumulative recording performed in n=6 (WT) and n=4 (V2475F) channels. To exclude effects of [Ca2+]luminal on the activity of the channel, [Ca2+] in the trans side of the channel was intentionally low (nominally free [Ca2+], ~ 3 μM) and current flowed from cytosolic to luminal sides of the channel. At cytosolic pCa 7, the probability of the channel being open (Po) was higher for V2475F compared to WT channels (Po = 0.081±0.022 and 0.023±0.006, respectively). At higher [Ca2+] (pCa 5.3), Po increased for both channels, as expected, but the activity of V2475F was still significantly higher than WT (Po = 0.683±0.192 and 0.422±0.124, respectively). Overall, these results confirmed that the mutation alone (in the absence of other cofactors) increases the sensitivity of RyR2 channels to cytosolic Ca2+ and confers a “gain-of-function” phenotype.
PKA phosphorylation exacerbates the hypersensitivity of V2475F channels to cytosolic Ca2+
Tachyarrhythmia in CPVT is triggered by physical exercise or anxiety, that is, during sympathetic stimulation.3,5 Sympathetic stimulation of the heart triggers the β-adrenergic cascade, which activates protein kinases.1,2 We tested the effect of PKA and CaMKII phosphorylation on recombinant WT and V2475F channels. Fig. 2A shows Western blots of WT and V2475F channels before (“Ctl.”) and after (“+PKA”) incubation with the catalytic subunit of PKA (1 μg/ml). Antibodies used were: anti-RyR2, which detected total protein, and the phosphorylation-sensitive anti-pS2808 (PKA and CaMKII site), anti-pS2030 (PKA site) and anti-pS2814 (CaMKII site).16 As expected, PKA phosphorylation of WT and V2475F channels did not change the band intensity of anti-RyR2 or anti-pS2814 and increased the phospho-signal of anti-pS2808 and anti-pS2030. However, V2475F channels were less sensitive to S2808 phosphorylation, and more sensitive to S2030 phosphorylation with respect to WT (Fig. 2B). We next tested whether the differential phosphorylation of V2475F channels had a functional correlate. We repeated the Ca2+-dependence of [3H]ryanodine binding experiments using the PKA-treated WT and V2475F channels. PKA phosphorylation did not change significantly the sensitivity of WT channels (EC50 = 6.4±0.14) but exaggerated the already abnormal sensitivity of V2475F for cytosolic Ca2+ (EC50 = 7.1±0.16) (Fig. 2C). By contrast, we did not detect differential CaMKII phosphorylation of S2808 or S2814 (S2030 was not responsive to CaMKII, as expected), or changes in [3H]ryanodine binding after CaMKII phosphorylation (Online Fig. I). These results suggest that PKA phosphorylation of V2475F channels, an almost obligatory condition during β-adrenergic stimulation of the heart, may be an exacerbating condition that favors uncontrollable Ca2+ release in CPVT episodes.
Figure 2. PKA phosphorylation increases the sensitivity of V2475F channels to cytosolic Ca2+.
A, Western blots of WT and V2475F channels before (“Ctl.”) and after (“+PKA”) incubation with the catalytic subunit of PKA. Antibodies used were: anti-RyR2 (1:3000), anti-pS2809 (1:10000), anti-pS2030 (1:10000) and anti-pS2814 (1:10000). B, Average band intensity (normalized to total RyR2 protein) of n=4 independent determinations with the indicated antibodies. C, Ca2+-dependence of [3H]ryanodine binding using PKA-treated solubilized WT and V2475F channels. PKA phosphorylation did not change significantly the sensitivity of WT channels (EC50 = 6.4±0.14) but increased the already abnormal sensitivity of V2475F for cytosolic calcium (EC50 = 7.1±0.16, n=5). Asterisk indicates p<0.05.
V2475F channels display abnormal response to luminal Ca2+
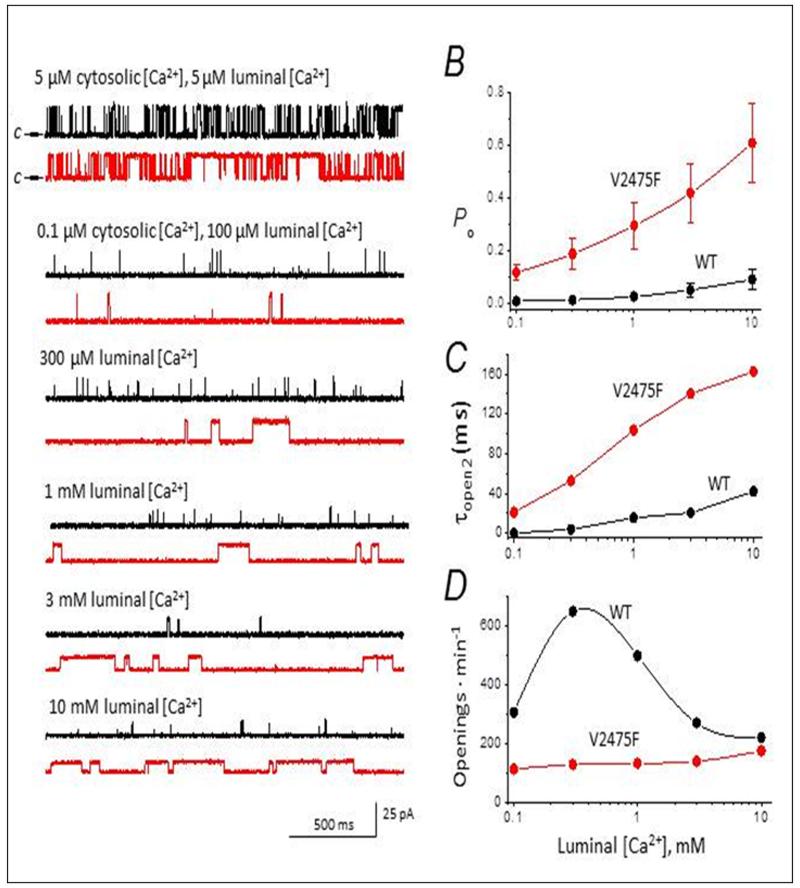
A prevalent mechanism believed to trigger CPVT episodes is increased channel sensitivity to luminal Ca2+.3 Recombinant WT and V2475F channels were reconstituted separately in planar lipid bilayers and the sensitivity of the channel to luminal Ca2+ was determined by clamping cis (cytosolic) Ca2+ to low levels (pCa 7) and increasing Ca2+ gradually in the trans (luminal) side of the channel. Fig. 3A describes the sequential steps followed to generate the Po vs. [Ca2+]luminal plot of Fig. 3B. For each [Ca2+]luminal tested, we recorded at least 2 min of continuous activity per channel. In WT and V2475F channels, an increase in [Ca2+]luminal produced an increase in Po, but the effect was more dramatic in the mutant channels (Fig. 3B). Mean open time was the main factor driving the increase in Po for both groups of channels (Fig. 3C). For example, increasing [Ca2+]luminal from 0.1 mM to 1 mM increased mean open time (τopen) from 2.6±1.1 to 15.7±3.8 ms in WT channels, and from 21±6.4 to 103.5±14 in V2475F channels. Mean close time (τclose) at the physiologically-relevant [Ca2+] of 1, 3 and 10 mM was only ~1.2-fold shorter for RyR2-V2475F. The frequency of openings was not directly related to [Ca2+]luminal, increasing only modestly for V2475F channels, and producing a biphasic response in WT channels (Fig. 3D). Thus, the negligible modification of τclose and the extraordinary prolongation of τopen by [Ca2+]luminal are outstanding characteristics of the V2475F mutation.
Figure 3. Increased sensitivity of V2475F channels to luminal calcium.
A, Representative single channel recordings (2-s traces) of WT (black) and V2475F (red) recombinant RyR2 channels, in the presence of the indicated calcium concentrations in the cis (cytosolic) and trans (luminal) sides of the channel. “c→” in the top traces corresponds to closed state of the channel, and this polarity is the same for all traces. Average Po (B), topen (C), and rate of openings (D) are plotted for WT and V2475F channels (n=6 and 5 channels, respectively) at the indicated luminal [Ca2+]. Cytosolic [Ca2+] was kept constant at 0.1 μmol/L throughout the titration.
Generation of RyR2-V2475F knock-in mice
We generated a knock-in mouse line harboring the RyR2-V2475F mutation. Fig. 4A shows the strategy used to generate the targeting vector and the resultant targeted allele. Fig. 4B and C show Southern blots and PCR that yield the expected amplification products. Fig. 4D shows that mice heterozygous for the mutation (RyR2-V2475F−/+) have no gross anatomical or histological cardiac alterations, as expected from the clinical presentation of CPVT in humans. Furthermore, functional echocardiographic data (heart rate, ejection fraction, LVDP, etc) is normal for RyR2-V2475F−/+ mice, resembling the clinical data (Fig. 4E and 4F, and Online Table I). However, heterozygotes propagate in a non-Mendelian fashion, generating offspring that is ~20% WT, ~80% heterozygotes (Fig. 4G). We were unable to detect homozygous embryos as early as 9 days of gestation. Therefore, although mice heterozygous for the mutation have no gross functional or structural alterations under basal conditions, the V2475F mutation appears too severe to be harbored in both alleles. Correspondingly, there are no known cases of CPVT patients homozygous for a given mutation and the heterozygous mice thus serve as excellent models to study this syndrome.
Figure 4. Generation of the RyR2-V2475F mouse.
A, Strategy for generation of the transgenic mice by homologous recombination. A, (first line) The region of the WT RYR2 gene containing exons 47, 48, 49 and 51 (numbered boxes). (Second line) the RyR2-V2475F targeting vector containing the V2475F substitution (*), a translationally silent Mlu I restriction site (M), the loxP flanked pGK promoter/EM7 promoter-NEO-pGHpA cassette (NEO), the location of the EcoRI restriction sites (R1) used in genotyping and the MC1-HSV-Tk cassette (TK). (Third line) Homologous recombination between the endogenous RyR2 locus and the V2475F targeting vector resulted in a chromosome carrying the V2475F substitution and the loxP flanked Neo cassette. (Last line): the V2475F allele after Cre excision of the Neo cassette. B, The 5′ and 3′ Southern blot probes used in genotyping the NeoR ES cells. C, PCR confirmation of WT and heterozygous V2475F mice. The expected 1Kb band is seen in the heterozygotes. The targeting vector was used as positive control. D, H&E-stained hearts indicate no structural alterations in V2475F heterozygous mice compared to WT. E, heart rate, and F, fractional shortening, were not significantly different between WT and V2475F heterozygous mice. G, Non-Mendelian propagation of V2475F heterozygous mice yields ~20% WT, ~80% heterozygotes, and 0% homozygotes.
Variable Po in RyR2 channels from RyR2-V2475F−/+ heterozygous mice
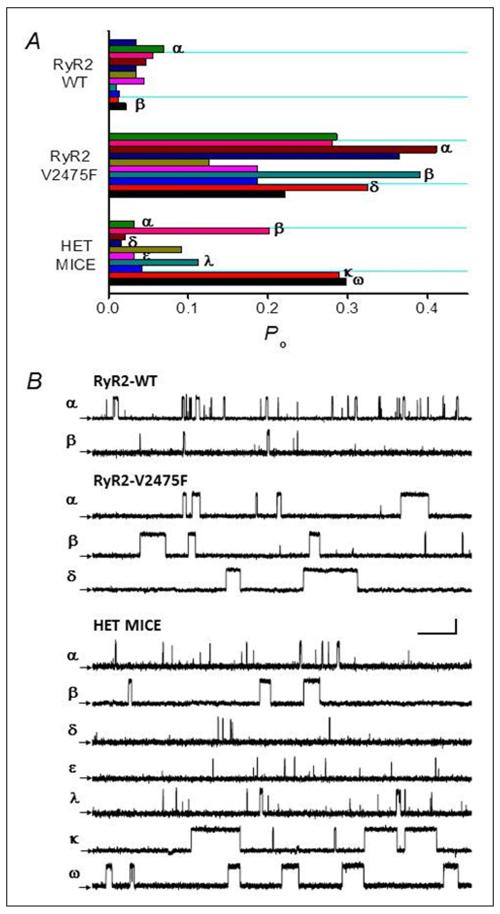
The generation of the RyR2-V2475F−/+ mice provided an excellent opportunity to study the effect of the mutation in a native environment. Fig. 5A shows aggregate data (Po) from single channel recordings using purified recombinant WT channels (“RyR2-WT”), recombinant V2475F (“RyR2-V2475F”), and RyR2 channels obtained from cardiac SR of RyR2-V2475F−/+ mice (“Het Mice”). Each bar represents an independent channel and all channels were recorded in the presence of 100 nM cytosolic [Ca2+] and 1 mM [Ca2+]luminal to maximize the phenotypic differences between WT and V2475F channels in their response to luminal Ca2+. As expected from the results of Figs. 1 and 3, WT channels displayed a modest response to luminal Ca2+ which yielded low Po (mean Po = 0.06±0.02, n=11) (Fig. 5). V2475F channels, on the other hand, displayed a dramatic increase in Po mainly due to an increase in mean open time, which yielded a Po = 0.29±0.08 (n=10). By contrast, RyR2 channels from RyR2-V2475F−/+ mice displayed widely variable Po (Fig. 5B). Of the 10 recorded channels, 3 had the “signature effect” of the V2475F mutation (prolonged mean open time), which accounted for their high Po. Thus, there is variability in channel activity that we suggest is attributed to the heterogeneous nature of the channel subunits contributing to the tetrameric RyR2 channel (Online Fig. V).
Figure 5. Variable Po in RyR2 channels from V2475F heterozygous mice.
Purified WT (“RyR2 WT”) and V2475F (“RyR2 V2475F”) recombinant RyR2 channels show distinctive Po when recorded in the presence of 0.1 μmol/L cis (cytosolic) Ca2+ and 1 mmol/L trans (luminal) Ca2+. By contrast, RyR2 channels obtained from V2475F+/− heterozygous mice (“HET MICE”) display variable Po under the same recording conditions. Each bar represents the average Po of independently-recorded individual channels. Mean Po for V2475F+/− channels were: 0.034, 0.016, 0.033 and 0.112 (α, δ, ε, and λ, respectively) and 0.214, 0.289, and 0.297 (β, κ, and ω, respectively). B, Representative 2-s recordings from 2 min of continuous activity of the channels correspondingly labeled with the same Greek character in A. Scale bars represent 15 pA (vertical) and 250 ms (horizontal).
Pro-arrhythmic behavior in ventricular myocytes from V2475F−/+ mice
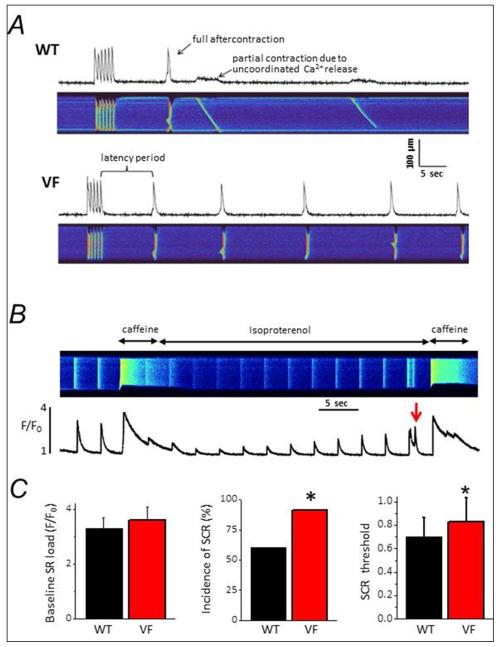
With the molecular phenotype displayed by V2475F−/+ mice in single channel experiments (Fig. 5), it was of interest to test whether their ventricular myocytes showed alterations in their intracellular Ca2+ signaling. Cells were subjected to a “stress” test to assess their capacity to handle SR Ca2+ load. Fluo-4 loaded ventricular myocytes were constantly perfused with Tyrode solution supplemented with Isoproterenol and then field-stimulated at 1 Hz during 5 sec to load the SR with Ca2+. After stimulation, cells were “rested” and constantly monitored to measure spontaneous Ca2+ release (SCR) events. Fig. 6A shows representative examples of line-scan images (length vs. time) of WT and V2475F−/+ cells subjected to the “stress” protocol. V2475F+/−cells subjected to this protocol had more SCRs, either partial or fully propagated (Fig. 6A). To determine the SR load at which SCR first occurs, WT or V2475F−/+ cardiomyocytes were perfused with Tyrode solution and paced at 0.3 Hz for 30s. Caffeine (10 mM) was then applied to measure “basal SR load”. Cells were then perfused with 100 nM Isoproterenol and continuously paced to gradually re-load the SR. This gradual re-loading of the SR with Ca2+ is reflected in the amplitude of the [Ca2+]i transient (Fig. 6B), which is low immediately after caffeine and then increases as Ca2+ entry through L-type Ca2+ channels (ICa) fills the SR. To determine the SR load at which SCR occurs, a second caffeine pulse was applied immediately after the appearance of the first SCR. Under these conditions, the amplitude of this second caffeine-induced Ca2+ transient closely approximated the SR load threshold for SCR. The aggregate data for 25 cells is shown in Fig. 6C. “Basal” SR load was not significantly different between WT and V2475F−/+ cells. On the other hand, the incidence (frequency) of SCR is higher in V2475F−/+ cells (Fig. 6C, middle bars). Finally, the normalized SR load at which SCR first occurs is marginally higher in V2475F−/+ cells (0.74 for WT and 0.89 for V2475F −/+ of the caffeine-induced Ca2+ release; p=0.05).
Figure 6. Pro-arrhythmic behavior in ventricular myocytes from V2475F heterozygous mice.
A, Confocal line-scan images of [Ca2+]i dynamics in ventricular myocytes of WT and V2475F+/− hearts. Cells were constantly perfused with Tyrode solution supplemented with Isoproterenol and then stimulated at 1 Hz during 5 sec to load the SR with Ca2+. Cells were then “rested” and the latency and number of spontaneous calcium release (SCR) events measured (Fig. S6). B, Protocol to measure SR load threshold for SCR in WT and V2475F cells. Please see text for details. C, Combined data from at least n= 25 cells/genotype. Asterisk (*) indicates p≤0.05.
Due to the possibility that residual Ca2+ and/or caffeine from the first Caffeine-evoked Ca2+ transient affected the estimation of the threshold for SCR, we performed an independent test using HEK293 cells transfected with WT and V2475F channels. In this system, the main determinant of SOICR is assumed to be the intrinsic channel’s sensitivity for luminal Ca2+.6 Online Fig. III shows a similar threshold for store overload-induced Ca2+ release (SOICR) between WT- and V2475F-expressing cells. These results suggest that increased sensitivity to [Ca2+]luminal in single RyR2-V2475F channels does not manifest as a decrease in the threshold for SCR.
Arrhythmic behavior in whole hearts and in intact V2475F−/+ mice
Having demonstrated that V2475F−/+ cardiomyocytes display a pro-arrhythmic substrate in the form of higher frequency of SCR, we next tested whether whole hearts from the same mice showed propensity to develop arrhythmias under β-adrenergic stimulation. We performed experiments in freshly explanted hearts from V2475F−/+ mice using the Langendorff system and with simultaneous recording of electrograms, left ventricular (LV) pressure, temperature, and coronary flow. Fig. 7A (WT) and 7B (V2475F+/−) show electrogram (top panel) and LV pressure (middle panel), expanded from the bottom panel. Hearts were paced at 400-600 bpm with an electrode placed in the apex. In the WT group, perfusion with Tyrode solution supplemented with 1 μM isoproterenol increased LV pressure as expected and had no significant arrhythmic behavior. In contrast, the same maneuver performed in V2475F−/+ hearts frequently produced arrhythmic episodes characterized by premature ventricular complexes (PVC) (Fig. 7) and ventricular bigeminy (not shown). In extreme cases we observed ventricular fibrillation (Fig. 7B). The type of arrhythmia, incidence, and duration were also quantified by electrocardiography in anesthetized WT and V2475F−/+ mice after intraperitoneal injection of Epinephrine (2 mg/kg) and caffeine (120 mg/kg) (Fig. 7C). This cocktail had minimal effect on WT mice (6.5±2.4 and 2.2±0.6 non-sustained and sustained arrhythmias, respectively, during a 30-min recording), but was highly arrhythmogenic in V2475F−/+ mice (59±28 and 14.8±6.0 non-sustained and sustained arrhythmias, respectively, during a 30-min recording period). Most of the arrhythmias were identified as Premature Ventricular Complexes (PVC), bigeminy or tachyarrhytmias (Fig. 7C). Bidirectional ventricular tachycardia (BVT), was not present in WT mice but present in 2 out of 6 V2475F−/+ mice (Fig. 7C “VF-1” and 7D right panel).
Figure 7. Arrhythmic behavior in Langendorff-perfused whole hearts and in intact V2475F−/+ heterozygous mice.
A and B, Electrogram (red trace) and left ventricular pressure (blue trace) from WT and V2475F hearts. Freshly-explanted hearts were Langendorff-perfused with Tyrode solution without and then with 300 nM Isoproterenol, as indicated in the bottom panel of A. Top two traces were taken at the time indicated by the rectangular box in the bottom two traces. C, Surface electrocardiograms (1s duration) in anesthetized WT and V2475F mice after intraperitoneal injection of Epinephrine (2 mg/kg) and caffeine (120 mg/kg). D, Average data obtained in n=6 WT and n=6 V2475F mice over a constantly monitored period of 30 min. Sustained arrhythmias are arrhythmias lasting more than 5s. BDVT = bidirectional ventricular tachycardia.
Optical mapping reveals multiple ventricular foci of arrhythmia
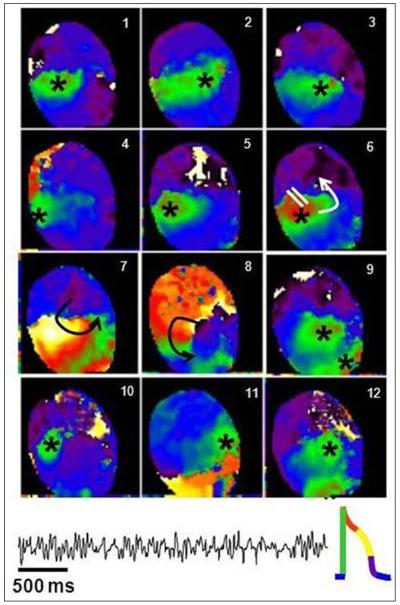
We mapped the anterior ventricular epicardium of V2475F−/+ and WT hearts. Two mutant and 2 WT hearts were perfused with 200 nM isoproterenol (0.2 mg/ml) for 10 minutes. Afterwards, the Ca2+ concentration was doubled (from 1.8 to 3.6 mM). A bolus of isoproterenol (0.2 mg/ml) plus caffeine (100 mM) was applied 10 and 20 min later. Long lasting arrhythmias (> 1 minute) were elicited in both mutant hearts. One WT heart had no arrhythmias, and the second heart underwent a short episode of VT (about 10 seconds). The spontaneous arrhythmias that occurred in the mutant hearts included bigeminy, short bursts of non-sustained VT and sustained polymorphic VT. Impulse propagation during polymorphic VT is described in more detail in the legend of Fig. 8.
Figure 8. Focal origin of polymorphic ventricular tachycardia.
Snapshots from a phase movie showing 12 consecutive beats in which breakthrough patterns emerged at different locations (asterisk), demonstrating the multifocal origin of polymorphic VT in a heterozygous V2475F heart. The volume-conducted ECG of the arrhythmia episode is shown below the snapshots. The colors denote the phases of the action potential, with green as upstroke (action potential insert at the bottom of the figure). The asterisks show the breakthrough sites, where the waves of depolarization (green) originated. During beat 6 a breakthrough occurs, however, unlike the other beats, unidirectional propagation block develops (white parallel lines), leading to the generation of a short-lived reentry (panels 7 and 8), after which, a pattern of focal tachycardia resumed.
DISCUSSION
In this study, we used a multidisciplinary approach encompassing molecular, cellular, whole heart and intact animal experiments to elucidate the mechanisms by which a novel RyR2 mutation, V2475F, causes RyR2 dysfunction, abnormal [Ca2+]i handling, and ventricular tachyarrhythmias. The mutation distinguished itself among other normally “silent” CPVT mutations by displaying a strong phenotype even in the absence of exacerbating factors (Fig. 1). Heterozygous mice (RyR2-V2475+/−) faithfully recapitulate the cardinal signs of CPVT, including normal functional echocardiogram and electrocardiogram at rest, as well as absence of structural cardiac abnormalities (Fig. 4). However, when challenged with an arrhythmogenic cocktail that activates the β-adrenergic cascade, RyR2-V2475F+/− mice display a highly arrhythmogenic phenotype (Figs. 7, 8).
Recombinant RyR2-V2474F channels exhibit three different functional defects
To date, most CPVT mutations have been functionally segregated into distinct groups affecting either luminal Ca2+ sensitivity,3,6,7 FKBP12.6 association,8,9 interdomain interactions,10,11 or Ca2+-dependent inactivation.12,13 This categorization provides a “clean” operational sorting of CPVT mutations but it appears counterintuitive based on the structural complexity of the RyR2 protein. Actually, it would appear logical that a given CPVT mutation affected more than one aspect of RyR2 regulation since multiple functional domains have been mapped to regions where each mutation falls.27 We present evidence here that the V2475F mutation affects cytosolic Ca2+ sensitivity (Fig. 1), PKA phosphorylation (Fig. 2), and luminal Ca2+ modulation (Fig. 3), three modes of RyR2 regulation highly relevant during a CPVT episode. Multiple modes of dysfunctional Ca2+ release for a single CPVT mutation has been suggested,5,13 but it has not been demonstrated. This is the first documented case of heterogeneous RyR2 dysfunction caused by a CPVT-associated mutation.
In simplified solutions containing Ca2+ as the only relevant agonist of RyRs, recombinant RyR2-V2475F channels displayed an increased sensitivity to activation by cytosolic [Ca2+] that was most conspicuous at pCa 7 (100 nM [Ca2+], Fig. 1). The increased Ca2+ sensitivity of V2475F was noted in a range of [Ca2+] sufficiently low (pCa 8 – pCa 6) to safely ascertain the exclusive participation of cytosolic activation sites, inasmuch as luminal Ca2+ sites require greater [Ca2+] for activation (tens to hundreds μmol/L, Fig. 2 and refs. 2,6,7). Furthermore, V2475F channels activated by cytosolic pCa 7 without [Ca2+]luminal displayed an increased frequency of openings without significant alteration of their mean close time (Fig. 1), suggesting that the mutation does not destabilize the channel’s close state as proposed for other CPVT mutations.8-11 Although other CPVT mutations have been shown to alter cytosolic Ca2+sensitivity, this phenotype becomes apparent only after PKA dissociation of FKBP12.6.8,9 In the case of V2475F, this is a defect intrinsic to the channel protein. At face value, this alteration portends significant diastolic Ca2+ leak in intact cardiomyocytes and is conceivable that it contributed to the embryonic lethality of the RyR2-V2475F homozygous mice.
Still, the most dramatic effect of the V2475F mutation was its remarkable hypersensitivity to luminal Ca2+ activation (Fig. 3A). The exaggerated response of V2475F channels to [Ca2+]luminal essentially dwarfed the otherwise modest response displayed by WT channels (Fig. 3B). Since the number of openings was actually decreased in the V2475F channels with respect to WT in the luminal Ca2+ titration curve (Fig. 3D), this dysfunction is at variance with altered cytosolic Ca2+ activation (Fig. 1) and strongly suggests the participation of luminal Ca2+ sites as determinants of this molecular phenotype. Also, we used purified RyR2 channels and thus, the easiest explanation for this effect is that Ca2+ acted directly on the RyR2 channel and not on accessory proteins, as postulated for some forms of luminal Ca modulation.21 Therefore, an extraordinary luminal Ca2+-induced increase in mean open time by direct interaction of Ca2+ ions with the channel is the most distinctive phenotype of this mutation (Fig. 4A). This “signature effect” was later used to estimate the approximate proportion of dysfunctional channels in heterozygous RyR2-V2475F+/− hearts.
By definition, arrhythmic episodes in CPVT are triggered by sympathetic stimulation. Others and we have previously shown that RyR2 channels are among the first proteins to undergo metabolic phosphorylation in hearts perfused with β-adrenergic agonists.15,22 A relevant question, therefore, is whether phosphorylation worsens the molecular phenotype of RyR2 channels harboring CPVT mutations. We found that PKA phosphorylation modestly but significantly increased the cytosolic Ca2+-dependence of activation of V2475F, but not WT channels (Fig. 2C). Specific phospho-sites were differentially phosphorylated in V2475F channels, resulting in decreased Ser2808 phosphorylation and increased Ser2030 phosphorylation (both PKA sites). The CaMKII site Ser2814 was not differentially affected. Thus, although the functional role of each of these sites is still controversial,22,23 there was uneven efficacy of PKA on the distinct phospho-sites, and this likely contributed to intensify the molecular phenotype of RyR2-V2475F channels. This is a novel aspect of RyR2 modulation in CPVT mutations. By contrast, CaMKII phosphorylation was not differentially affected by the V2475F mutation (Online Fig. I).
Severe arrhythmic phenotype in mice harboring the V2475F mutation
Given the strong molecular phenotype of V2475F channels, we expected a correspondingly severe phenotype in mice harboring this mutation. Indeed, the mutation appears embryonic lethal in homozygous mice (Fig. 4G), and triggers life-threatening arrhythmias in heterozygous mice (RyR2-V2475F+/−). However, given that the mutation confers RyR2 channels a gain of function at baseline (i.e, in the absence of sympathetic stimulation, Figs. 1 and 3), it is surprising that RyR2-V2475F+/− hearts are functionally and structurally indistinguishable from WT under basal conditions. We believe that at least two factors restrict the effects of the mutation at baseline. First, if we assume that mutant and WT alleles contribute equally and randomly to the formation of a tetrameric channel, then up to ~94% of RyR2s channels would be phenotypically altered in RyR2-V2475F+/− mice if only one subunit was necessary to confer the gain of function (dominant-positive mutation, please see Online Fig. V). However, only ~30% of RyR2-V2475F+/− channels reconstituted in lipid bilayers exhibited dramatically prolonged mean open time in the presence of 1 mM [Ca2+]luminal (Fig. 5). This fact alone strongly suggests that multiple subunits are necessary to confer the phenotype, thus decreasing the incidence of abnormal channels in the heterozygous population. Second, ventricular myocytes have powerful auto-regulatory mechanisms that control the activity of “leaky” RyR2s in the long run: Eisner’s group has shown that increasing RyR2 activity (by Ca2+-sensitization, phosphorylation, etc) induces more Ca2+ release in the first few beats, but this drives greater Ca2+ extrusion by the NCX and progressively decreases SR load, reducing the Ca2+ available for release in subsequent beats. Despite the reduced SR Ca2+ content, intracellular [Ca2+] transients remain relatively constant due to higher fractional release by the sensitized RyR2s, and a new steady-state is established in which Ca2+ influx and efflux are re-balanced.24 Thus, auto-regulatory mechanisms can effectively preclude tonic hyperactivity of RyR2s, at least within a defined range of SR loads. In the RyR2-V2475F+/− mouse, the basal hyperactivity of the relatively small pool of abnormal channels may be controlled by this mechanism.
In β-adrenergic stimulated ventricular myocytes, dysfunctional RyR2-V2475F+/− channels clearly contribute to increased propensity for spontaneous Ca2+ release (SCR) (Fig. 6A, 6C) despite the auto-regulatory mechanisms mentioned above. Interestingly, the [Ca2+]luminal necessary to provoke SCR is similar for V2475F+/− and WT cardiomyocytes (Fig. 6B), and in HEK293 cells, the threshold for SOICR was also similar for WT and RyR2-V2475F channels (Online Fig. I). These findings may appear counterintuitive given the increased Po of RyR2-V2475F as a function of [Ca2+]luminal (Fig. 3B), but it is possible that other processes such as cytosolic Ca2+ sensitivity and phosphorylation (both of which are altered in V2475F channels) take precedence over luminal [Ca2+] to determine the likelihood of SCR. Also, while most CPVT-linked mutations that display decreased threshold for SOICR also display dramatic decrease of their mean close time (τclose = 7- to 20-fold faster than WT),6,30 the V2475F mutation displays a τclose that is only ~1.2-fold faster than WT at physiologically-relevant [Ca2+] (Online Fig. II), clearly, a marginal alteration in the context of other CPVT mutations. If we make the reasonable assumptions that SOICR is a good surrogate for SCR, and that τclose is a critical determinant of the stability of the channel’s closed state, we would expect then that mutations that decrease τclose also lower the threshold for SCR. This is not the case for the V2475F mutation. Thus, we find it unlikely that the arrhythmogenic Ca2+ release in the RyR2-V2475F+/− cardiomyocytes is solely determined by their threshold for SCR. Instead, we believe that the increased sensitivity to luminal Ca2+, concomitantly with elevated cytosolic Ca2+ sensitivity and RyR2 phosphorylation, all converge to fasten RyR2 restitution, thus shortening Ca2+ release refractoriness and increasing the incidence of SCR. In support of this hypothesis, the latency for SCR, a direct index of RyR2 restitution, was substantially faster in RyR2-V2475F−/+ than WT cardiomyocytes (Online Fig. VI). The dissociation of [Ca2+]luminal from SCR likelihood is not unprecedented: in a canine model of ventricular fibrillation, the increased rate of arrhythmogenic SCR was not determined by [Ca2+]luminal; instead, RyR2 oxidation and phosphorylation shortened RyR2 refractoriness.31
In Langendorff-perfused hearts and in intact animals, β-adrenergic stimulation brings about discrete arrhythmic behavior in RyR2-V2475F+/− mice. The demonstration of tachyarrhythmias in normally arrhythmia-resilient mice26 is especially remarkable and underscores the importance of RyR2 dysfunction as arrhythmogenic trigger. Optical mapping revealed the multi-focal origin of such events (Fig. 8), compatible with triggered activity at various myocardial layers. All these events suggest active participation of ventricular cells as generators of arrhythmias, however, bidirectional ventricular tachycardia (BVT), a “pathognomonic” event of digitalis intoxication19 and CPVT17 that reflects alternating firing by the His-Purkinje system18 was also observed in intact mice. The remarkable electrocardiographic similarity across species caused by Ca2+-triggered arrhythmias suggests common pathways regardless of the molecular mechanisms. Induction of DADs is possibly the converging point.
CONCLUSIONS
Experiments in vitro showed that RyR2 mutations linked to CPVT, ARVD, or hypertrophic cardiomyopathy each exhibit distinct molecular phenotypes.13,25,28 Here, we demonstrate for the first time that a CPVT-linked mutation can simultaneously induce multiple molecular alterations in the RyR2 channel, yet retain the distinctive traits of the disease at the whole heart and intact animal levels. Given that various regulatory regions may potentially be affected by a point mutation in the RyR2, we find it surprising that this variegated molecular dysfunction had not been detected before. We hypothesize that multidisciplinary screening strategies in a larger number of mutations will show even more complex mechanisms of CPVT. Unveiling these mechanisms is not merely an academic exercise but a task with relevant clinical implications. For example, it is plausible that the location and the mechanisms of arrhythmia determine the severity of the phenotype or condition the efficacy of antiarrhythmic drugs, especially those that directly target the RyR2. Thus, identifying the mutations and their mechanisms could serve as a valuable tool for risk stratification or therapy individualization in CPVT patients.
Supplementary Material
Novelty and Significance.
What Is Known?
Mutations in RYR2, the gene encoding the cardiac Ca2+ release channel/ryanodine receptor (RyR2), are associated with Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), a syndrome characterized by exercise- or stress-induced tachyarrhythmias.
In murine models of CPVT, most RyR2 mutations associated with CPVT are postulated to cause one distinct defect in RyR2 function.
Since CPVT point mutations often map to regions of the channel that have been implicated in multiple regulatory interactions, categorization of the molecular phenotype into distinct functional groups may be counterintuitive.
What New Information Does This Article Contribute?
RyR2-V2475F, a previously uncharacterized mutation associated with CPVT in humans, produces not a single, but multiple, forms of RyR2 dysfunction.
Despite the heterogeneity of RyR2 dysfunction generated by the RyR2-V2475F mutation, the murine model retains the distinctive electrocardiographic traits of the disease, i.e., adrenergically-triggered premature ventricular complexes and bidirectional ventricular tachycardia.
CPVT is a rare, but highly malignant, arrhythmogenic syndrome characterized by exercise- or stress-induced tachyarrythmias in the absence of structural cardiac alterations. Mutations in RYR2 underlie a great portion of CPVT cases in humans, but the functional alterations caused by the mutations remain incompletely understood. Untimely Ca2+ release (i.e., diastolic or spontaneous sarcoplasmic reticulum Ca2+ release) by mutant RyR2 seems to be the primary event that generates arrhythmias in CPVT. Each CPVT-linked RyR2 mutation characterized to date was thought to cause spontaneous Ca2+ release by altering a single mechanism of RyR2 function, i.e., regulation of channel activity by luminal Ca2+, association with the accessory protein FKBP12.6, or channel inter-domain interactions. The novel CPVT-linked mutation RyR2-V2475F displays multiple mechanisms of RyR2 dysfunction, including increased cytosolic Ca2+ sensitivity, altered regulation by luminal [Ca2+], and abnormal PKA phosphorylation, three modes of channel regulation highly relevant in CPVT episodes. Given that various regulatory regions may potentially be affected by a point mutation in the RyR2, a variegated molecular dysfunction may be the norm in all CPVT mutations. Multidisciplinary screening strategies in a larger number of mutations may show even more complex mechanisms of CPVT and serve as a valuable tool for therapy individualization in CPVT patients.
Acknowledgments
SOURCES OF FUNDING
This work was supported by National Institutes of Health grants PO1-HL094291 and RO1-HL055438 (to HHV), by National Institutes of Health grants P01-HL039707 and P01-HL087226 (to JJ), by the Leducq Foundation and Centro Nacional de Investigaciones Cardiovasculares, Spain (to JJ), and by National Institutes of Health grant K99-HL105574 to (SNJ). RL was supported by an AHA pre-doctoral fellowship.
Non-Standard Abbreviations
- BDVT
Bidirectional Ventricular Tachycardia
- CaMKII
Calcium/Calmodulin-dependent Protein Kinase II
- CPVT
Catecholaminergic Polymorphic Ventricular Tachycardia
- LV
Left Ventricle
- LVDP
Left Ventricle Developed Pressure
- PKA
Protein Kinase A
- Po
Open Probability
- PVC
Premature Ventricular Complex
- RyR2
Cardiac Ryanodine Receptor
- RyR2-V2475F+/−
Heterozygous mutant RyR2 carrying the V2475F substitution
- SCR
Spontaneous Calcium Release
- SOICR
Store Overload-Induced Calcium Release
- τclose
Mean Close Time
- τopen
Mean Open Time
- VT
Ventricular Tachycardia
- V2475F
substitution of Valine to Phenylalanine in the position 2475 of RyR2 linear sequence
- WT
Wild Type
- [Ca2+]
Calcium concentration
- [Ca2+]luminal
Sarcoplasmic Reticulum Calcium Concentration
- [3H]ryanodine
Tritiated Ryanodine
Footnotes
DISCLOSURES
None.
Subject codes:
[5] Arrhythmias
[130] Animal models of human disease
[132] Arrhythmias-basic studies
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Ann Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Capes EM, Loaiza R, Valdivia HH. Ryanodine receptors. Skelet Muscle. 2011;1:18. doi: 10.1186/2044-5040-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JP, Mannens MM, Wilde AA, Ackerman MJ. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009;54:2065–74. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 6.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 9.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 10.George CH, Jundi H, Walters N, Thomas NL, West RR, Lai FA. Arrhythmogenic mutation-linked defects in ryanodine receptor autoregulation reveal a novel mechanism of Ca2+ release channel dysfunction. Circ Res. 2006;98:88–97. doi: 10.1161/01.RES.0000199296.70534.7c. [DOI] [PubMed] [Google Scholar]
- 11.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas NL, Lai FA, George CH. Differential Ca2+ sensitivity of RyR2 mutations reveals distinct mechanisms of channel dysfunction in sudden cardiac death. Biochem Biophys Res Commun. 2005;331:231–238. doi: 10.1016/j.bbrc.2005.02.194. [DOI] [PubMed] [Google Scholar]
- 13.Thomas NL, George CH, Lai FA. Functional heterogeneity of ryanodine receptor mutations associated with sudden cardiac death. Cardiovasc Res. 2004;64:52–60. doi: 10.1016/j.cardiores.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Tester DJ, Kopplin LJ, Creighton W, Burke AP, Ackerman MJ. Pathogenesis of unexplained drowning: new insights from a molecular autopsy. Mayo Clin Proc. 2005;80:596–600. doi: 10.4065/80.5.596. [DOI] [PubMed] [Google Scholar]
- 15.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase a phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 16.Huke S, Bers DM. Ryanodine receptor phosphorylation at serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 18.Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O’Connell R, Berenfeld O, Anumonwo J, Pandit SV, Vikstrom K, Napolitano C, Priori SG, Jalife J. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101:1039–48. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valent S, Kelly P. Digoxin-induced bidirectional ventricular tachycardia. N Engl J Med. 1997;336:550. doi: 10.1056/NEJM199702203360805. [DOI] [PubMed] [Google Scholar]
- 20.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–8. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrero P, Said M, Sánchez G, Vittone L, Valverde C, Donoso P, Mattiazzi A, Mundiña-Weilenmann C. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation. J Mol Cell Cardiol. 2007;43:281–91. doi: 10.1016/j.yjmcc.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res. 2012;110:796–9. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 23.Valdivia HH. Ryanodine Receptor Phosphorylation and Heart Failure. Phasing Out S2808 and “Criminalizing” S2814. Circ Res. 2012;110:1398–1402. doi: 10.1161/CIRCRESAHA.112.270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisner DA, Kashimura T, O’Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46:474–81. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Velasco M, Rueda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, Priori SG, Richard S, Gomez AM. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009;104:201–209. doi: 10.1161/CIRCRESAHA.108.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nerbonne JM. Studying cardiac arrhythmias in the mouse-a reasonable model for probing mechanisms? Trends Cardiovasc Med. 2004;14:83–93. doi: 10.1016/j.tcm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 27.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: Emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Wang R, Fill M, Chen SR. Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ Res. 2012;11:968–977. doi: 10.1161/CIRCRESAHA.111.256560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollman BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, Ventucci LA. In the RyR2R4496C Mouse Model of CPVT, β-Adrenergic Stimulation Induces Ca Waves by Increasing SR Ca2+ Content and Not by Decreasing the Threshold for Ca Waves. Circ Res. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 31.Belevych AE, Terentyev D, Terentyeva R, Ho HT, Gyorke I, Bonilla IM, Carnes CA, Billman GE, Györke S. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–77. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.