Abstract
Mounting evidence suggests a potential link between cocaine abuse, disruptions in hypothalamic-pituitary-thyroid axis signaling, and neuroplasticity, but molecular mechanisms remain unknown. Neurogranin (Ng) is a gene containing a thyroid hormone-responsive element within its first intron that is involved in synaptic plasticity. Transcriptional activation requires heterodimerization of thyroid hormone receptor (TR) and retinoid X receptor (RXR) bound by their respective ligands, tri-iodothryonine and 9-cis-retinoic acid (9-cis-RA), and subsequent binding of this complex to the thyroid hormone-responsive element of the Ng gene. In this study, the effects of chronic cocaine abuse on Ng expression in euthyroid and hypothyroid mice were assessed. In cocaine-treated mice, decreased Ng expression was observed in the absence of changes in levels of thyroid hormones or other hypothalamic-pituitary-thyroid signaling factors. Therefore, we hypothesized that cocaine decreases Ng expression via alterations in 9-cis-RA availability and TR/RXR signaling. In support of this hypothesis, RXR-γ was significantly decreased in brains of cocaine-treated mice while CYP26A1, the main enzyme responsible for neuronal RA degradation, was significantly increased. Results from this study provide the first evidence for a direct effect of cocaine abuse on TR/RXR signaling, RA metabolism, and transcriptional regulation of Ng, a gene essential for adult neuroplasticity.
Keywords: cocaine, hypothalamus, neurogranin, neuroplasticity, retinoic acid, thyroid
The hypothalamic-pituitary-thyroid (HPT) axis is comprised of a tightly regulated signaling cascade that ultimately controls the release of the thyroid hormones tri-iodothryonine (T3) and thyroxine (T4) from the thyroid gland and subsequent regulation of thyroid hormone (TH)-responsive genes in the brain. Processing of these hormones in the CNS occurs via crosstalk between astrocytes and neurons, as deiodinase-2 (D2), the enzyme responsible for conversion of T4 into its more potent T3 form, is expressed in astrocytes (Alkemade et al. 2005; Lechan and Fekete 2007). T3 is then transported to neurons where it can activate T3-responsive target genes or be inactivated by the deiodinase-3 (D3) enzyme (Alkemade et al. 2005; Zoeller et al. 2007). Both D2 and D3 are sensitive to circulating levels of TH, and serve as important regulatory elements.
Hypothyroidism, characterized by insufficient levels of circulating T3 and T4, contributes to cognitive impairments, decreased hippocampal neurogenesis, and even dementia (Bauer and Whybrow 2001; Desouza et al. 2005; Dong et al. 2005; Gong et al. 2010). Additionally, both overt and subclinical hypothyroidism (SCH) are implicated as leading risk factors for major depressive disorder (Fornaro et al. 2010; Mowla et al. 2011); in itself a risk factor for the development of a substance abuse disorder (Deykin et al. 1987; Christie et al. 1988). In fact, results from a large study of the association between the use of cocaine and depression indicated that the odds ratio of cocaine use for those with depression was 1.28 in 1981 (n = 3,006) and 3.53 in 1993–1994 (n = 1,679; Bohnert and Miech 2010). In this context, the association between cocaine abuse and HPT dysfunction represents a valid and under-represented field of research, as a number of cocaine abusers may suffer from underlying TH abnormalities. Additionally, TH deficits may be exacerbated with continued cocaine use/abuse, further contributing to cognitive dysfunction and hindering effective treatment.
A small number of studies have been conducted to examine the effects of cocaine abuse on TH levels and HPT functioning, both in clinical settings and in experimental animal models. However, few conclusions have been reached and numerous discrepancies exist among the findings. For instance, Dhopesh et al. (1991) reported that heavy cocaine abuse does not affect thyroid functioning in humans, and Budziszewska et al. (1996) substantiated this evidence in rats. However, other studies reported that cocaine abusers exhibit an impaired thyrotropin (TSH) response to thyroid-releasing hormone (Teoh et al. 1993; Vescovi and Pezzarossa 1999). Furthermore, dopamine, the neurotransmitter through which cocaine exerts a majority of its CNS effects by inhibition of its reuptake, is a known inhibitor of TSH secretion from the pituitary, and dopamine agonists induce hypothyroidism in humans (Shupnik et al. 1986; Haugen 2009). Importantly, none of these studies examined the expression of TH-responsive genes following cocaine abuse. Additional research is needed to increase understanding of the molecular interactions between cocaine abuse and HPT disruption.
Although conflicting evidence exists regarding the degree to which cocaine is directly neurotoxic, numerous studies have shown that chronic abuse can lead to cognitive dysfunction, mood disturbances, and vulnerability to the development of co-morbid psychiatric disorders (Ardila et al. 1991; Bolla et al. 1998; Ford et al. 2009). Microarray studies from post-mortem human brain tissue from cocaine abusers have provided valuable insight into some of the transcriptional changes occurring in response to cocaine. Changes in genes related to the maintenance of the extracellular matrix, myelination, signal transduction, and synaptic plasticity have been consistently observed (Albertson et al. 2004; Lehrmann et al. 2006; Lull et al. 2008). Similarly, microarray studies involving hypothyroid human patients and animals also show changes in neural plasticity (Dong et al. 2005; Royland et al. 2008).
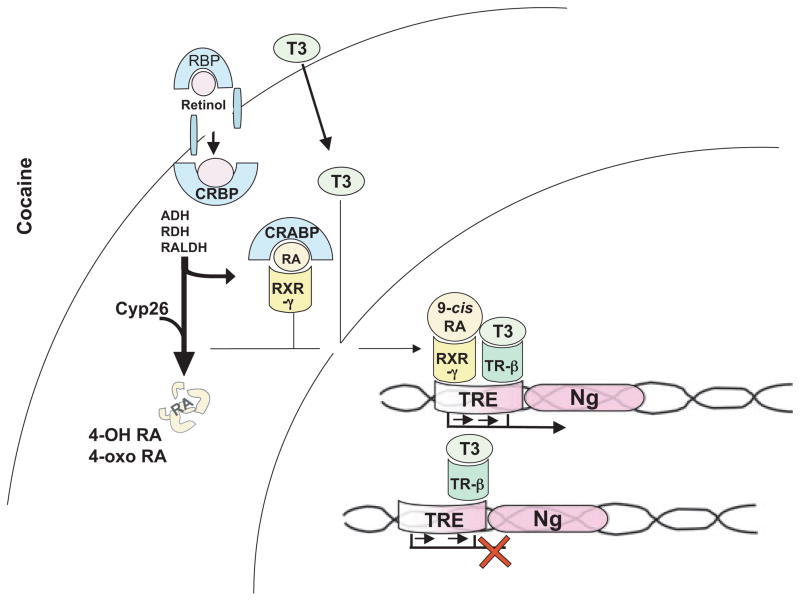
One gene that is directly regulated by TH levels and that is implicated in synaptic plasticity is neurogranin (Ng), also called RC3, which contains a thyroid hormone receptor (TR)/retinoid X receptor (RXR) response element within its first intron (Fig. 1) (Martinez de Arrieta et al. 1999). It is well documented that hypothyroidism results in decreased Ng transcription and expression, particularly in the cerebral cortex and hippocampus (Iniguez et al. 1992; Enderlin et al. 2004). Transcriptional activation of the Ng gene requires both TH and 9-cis-retinoic acid (9-cis RA), an isomer of all-trans retinoic acid, which is present in the brain (McCaffery and Drager 1994; Kastner et al. 1995; Connor and Sidell 1997; Krezel et al. 1999; Zetterstrom et al. 1999; Husson et al. 2003; Enderlin et al. 2004). Retinoic acid (RA) binds to cellular retinol-binding protein (CRBP) in the cytoplasm, during which time it can either be degraded by the enzyme CYP26A1, a member of the cytochrome p450 family, or transferred to RXR for transport into the nucleus. Retinoic acid is required for transcriptional activation of Ng, as decreased levels of Ng comparable to those observed in hypothyroidism are also evident during vitamin A deficiency (Husson et al. 2003).
Fig. 1.
Retinoic acid signaling pathway for neurogranin expression. Retinoic acid is transported into the cell by CRBP and cellular levels are controlled by CYP26A1 enzyme that degrades RA. RA is processed by a series of dehydrogenase enzymes (ADH, ROLDH, RALDH) into its 9-cis RA form that can interact with RXR-γ. The RXR-γ/9-cis RA complex binds the TRE, along with the T3/TR-β to activate neurogranin expression. Without both ligands (9-cis RA and T3) binding to RXR-γ and TR-β, respectively, neurogranin expression is not initiated (red ‘X’). Tri-iodothryonine, T3; cellular retinol-binding protein, CRBP; CYP26, CYP26A1 enzyme; aldehyde dehydrogenase, ADH; retinol dehydrogenase, ROLDH, retinaldehyde dehydrogenase, RALDH; 9-cis-retinoic acid, 9-cis RA; retinoid-X-receptor-γ, RXR-γ; thyroid hormone nuclear receptor-β, TR-β; Thyroid hormone responsive element, TRE; Neurogranin, Ng.
In the present study, we assessed the effects of cocaine on HPT signaling in the CNS with particular focus on the expression of Ng in a murine model of chronic abuse. Cocaine was administered to both euthyroid and propylthiouracil (PTU)-induced hypothyroid mice to determine if cocaine abuse may exacerbate deficits observed in hypothyroidism. Consistent with previous studies, PTU treatment resulted in the depletion of serum T4 and decreased expression of Ng (Enderlin et al. 2004; Vallortigara et al. 2008). While chronic cocaine treatment did not alter serum TH levels, it significantly decreased Ng mRNA and protein levels in euthyroid mice, leading to the hypothesis that regulation may be occurring at the level of the TR/RXR nuclear receptors. In support of this hypothesis, mRNA and protein levels of RXR-γ, an RXR isoform predominantly expressed in adult hypothalamus, pituitary, and hippocampus, were significantly decreased in the brains of cocaine-treated animals. Increased protein levels of CYP26A1 were also observed in the brains of mice exposed to cocaine, suggesting that induction of RA metabolizing enzymes following cocaine exposure could lead to a down-regulation in RA signaling. Overall, our data demonstrate that cocaine increases CYP26A1 levels leading to dysregulation of RA signaling and RXR expression and function, potentially contributing to altered transcription of a number of RXR-responsive genes, including Ng.
Materials and methods
Animals
Ten-week old C57BL/6 male mice (Charles River) were utilized for the study. All procedures were approved and conducted in accordance with Temple University IACUC guidelines. Mice were singly housed in an animal facility with constant airflow, controlled temperature (21–23°C) on a 12-h light/dark cycle and supplied with food and water ad libitum. Food and water intake was measured every 2 days. Mice were divided into seven groups with 6–10 mice per group: (i) euthyroid control, (ii) cocaine without withdrawal, (iii) cocaine with 48 h withdrawal + challenge, (iv) hypothyroid, (v) subclinical hypothyroid, (vi) hypothyroid + cocaine with 48 h withdrawal + challenge and (vii) subclinical hypothyroid + cocaine with 48 h withdrawal + challenge (Table 1). Mice in groups 1, 2, and 3 received normal chow (Harlan Teklan, Madison, WI, USA). Mice in the hypothyroid groups 4 and 6 were fed a low iodine diet supplemented with 0.15% PTU (6-n-propyl-2-thiouracyl-P) (Harlan Teklan). Mice in groups 5 and 7 received a low iodine diet with 0.075% PTU to induce a SCH state. Mice in cocaine groups 2, 3, 6, and 7 were injected intraperitoneally (i.p.) with 15 mg/kg cocaine (Sigma, St Louis, MO, USA) dissolved in sterile saline once per day for 14 days. Mice from group 2 were euthanized immediately following the final cocaine injection, while the rest of the groups underwent 48 h of withdrawal as described above. Mice in groups 1, 4, and 5 were given equivalent volumes of saline injections on the same schedule as those receiving cocaine. Ocular blood draws were conducted from each mouse from alternating eyes once per week for the duration of the study for plasma thyroid hormone measurements (Chiang et al. 1998; Hoff 2000).
Table 1.
Treatment groups
| Group | Treatment |
|---|---|
| 1 | Euthyroid |
| 2 | Cocaine no WD |
| 3 | Cocaine + WD + challenge |
| 4 | Hypo |
| 5 | SCH |
| 6 | Hypo + cocaine + WD + challenge |
| 7 | SCH + cocaine + WD + challenge |
Euthyroid, control; Hypo, Hypothyroid, PTU-fed; Cocaine, 14 days, 15 mg/kg; WD, 48 h withdrawal; challenge, 15 mg/kg challenge dose cocaine; SCH, subclinical hypothyroid (0.075% dose PTU).
N = 6–10 mice per group.
Stereotypic behavior testing
Stereotypic behavioral testing was conducted on each mouse. Mice in groups 1 and 3–7 were injected for 14 days with either cocaine or saline and allowed a 48-h withdrawal period prior to final challenge injections of either cocaine (groups 3, 6, and 7) or saline (groups 1, 4, and 5). During the 48 h period of cocaine withdrawal, all mice were singly housed for 24 h in the testing room for acclimation. On the test day, mice were observed for 1 min at 15, 30, 45, and 60 min after cocaine challenge or saline injections. A ‘0’ time reading served as the baseline measure taken 1 min before cocaine or saline injection. Stereotypical behavior was scored based on a modified rating scale as previously described (Creese and Iversen 1974; Daunais and McGinty 1995; Schlussman et al. 1998). The scoring scale consisted of the following rating system: (i) sleeping, inactive (ii) alert, actively grooming, (iii) increased sniffing, (iv) intermittent rearing and sniffing, (v) increased locomotion, (vi) intense sniffing in one location, (vii) continuous sniffing and pivoting, (viii) continuous rearing and sniffing, (ix) maintained rearing and sniffing, and (x) splayed hind limbs. The scores for all time points were plotted and stereotypic behavior was assessed by taking the area under the curve and by the average total activity at each time point.
Tissue harvest
Animals were heavily anesthetized with isoflurane and decapitated. Brains were removed and placed into ice-cold phosphate-buffered saline (PBS). Brains from four to seven mice per group were sectioned longitudinally and half of each brain was used for protein analysis and the other half for RNA analysis. Each half of the brain was further sectioned into the following regions: anterior frontal cortex (1.32 mm anterior of Bregma), temporal lobe (~30 mg of tissue taken from ventral-lateral posterior frontal cortex section), posterior frontal cortex (post-FC) (1.32 mm anterior of Bregma to 2.92 mm posterior of Bregma), and hindbrain/cerebellum (2.92 mm posterior of Bregma). Tissues for protein analysis were stored at −80°C, and those for RNA analysis were placed in RNAlater® stabilization solution (Qiagen, Valencia, CA, USA) and stored at −20°C. For immunohistochemical analysis, whole brains from 1–3 mice per group were fixed in 10% buffered formalin for 24 h and processed for immunolabeling by standard paraffin embedding and sectioning.
ELISA – serum thyroid hormone levels
Triiodothyronine (T3) and thyroxine (T4) levels were measured by enzyme-linked immunosorbent assay (ELISA) according to manufacturers’ instructions (Calbiotech, Spring Valley, CA, USA; and Alpha Diagnostic International, San Antonio, TX, USA; respectively). Twenty-five microliters of each serum sample was measured in duplicate from each mouse and T3 and T4 levels were assessed by absorbance at 450 nm.
Homogenization of brain tissue and protein extraction
Each brain region was homogenized by mechanical dounce disruption on ice in TNN buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% NP40, 1 : 200 protease inhibitor cocktail; Calbiochem, San Diego, CA, USA). Once completely homogenized, samples were centrifuged at 13 000 × g at 4°C for 5 min. Supernatant containing the protein was collected and protein concentrations were determined via the Bradford assay.
Western blot
Equal amounts of protein were loaded (20 μg per lane unless otherwise indicated) onto pre-cast midi-gels (4–12% Bis–Tris; Invitrogen, Carlsbad, CA, USA) and separated by electrophoresis for approximately 1 h at 160 V and transferred onto nitrocellulose. Membranes were blocked in 5% non-fat milk in Tris-buffered saline, 1% Tween-20 for 30 min before incubation with primary antibodies. Primary antibodies included: D2 (1 : 500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), D3 (1 : 1000; Novus, Littleton, CO, USA), Neurogranin (1 : 10000; Upstate Biotechnology, Lake Placid, NY, USA), RXR-γ (1 : 1000; Abcam, Cambridge, MA, USA), TR1β (1 : 1000; Santa Cruz), CYP26A1 (1 : 1000; Abcam) or loading control GAPDH (1 : 2000; Santa Cruz). Membranes were incubated for either 2 h at 23°C or overnight at 4°C, washed in 1× TBST, incubated with appropriate secondary anti-mouse or -rabbit antibodies (1 : 5000; Thermo Scientific, Waltham, MA, USA) for 1 h, and developed with ECL or ECL PLUS (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Band intensities were calculated using ImageJ software and normalized to a loading control (Rasband 1997–2009).
RNA extraction and quantitative RT-PCR
Approximately 10 mg of tissue was removed from each sample section for RNA extraction. Total RNA was extracted using the Qiagen RNeasy® kit according to manufacturer’s instructions (Qiagen). cDNA was generated using the iScript™ cDNA synthesis kit following the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA, USA). Generation of cDNA product was verified using primers for the housekeeping gene riboprotein. The PCR reaction was performed using a 96-well thermocycler and a standard amplification program. PCR products were separated through 1% agarose gel and visualized under UV light. Once a quality PCR product was confirmed for all samples, quantitative RT-PCR (qRT-PCR) analyses of TRα, TRβ, Ng, and RXR-γ were performed. Table 2 lists primer sequences utilized. Reaction mixtures for qRT-PCR consisted of LightCycler® 480 SYBR Green I Master Mix (Bio-Rad), forward and reverse primers for TRα, TRβ, Ng, RXR-γ, or riboprotein (housekeeping) (Table 2), 2 μL of cDNA (diluted 1 : 20) and H2O to bring the reaction volume up to 20 μL. Samples were placed in a 96-well plate and the qRT-PCR was performed on a Roche LightCycler® 480. All reactions were performed in triplicate and amplification curves and Cp values were obtained for analysis. Results were normalized against riboprotein using the ΔΔCt quantification method and expressed as mean ± SEM.
Table 2.
qRT-PCR primers utilized
| Accession no. | Gene name | Forward primer | Reverse primer |
|---|---|---|---|
| NM_009107 | Retinoid X receptor γ | 5-AGGCAGGTTTGCCAAGCTTCTG-3 | 5-GGAGTGTCTCCAATGAGCTTGA-3 |
| NM_022029.2 | Neurogranin | 5-CACCCAGCATCGACAAA-3 | 5-CGCTCTTTATCTTCTTCCTC-3 |
| NM_178060.3 | TRα1 | 5-GACTGACCTCCGCATGATCG-3 | 5-CCTGATCCTCAAAGACCTCC-3 |
| NM_001113417.1 | TRβ | 5-ATCAAGACAGTCACTGAGGC-3 | 5-GGGCATTCACAATGGGTGCTT-3 |
| NM_009438 | Riboprotein | 5-CCTGCTGCTCTCAAGGTTGTT-3 | 5-CGATAGTGCATCTTGGCCTTT-3 |
Immunofluorescence labeling
Four μm coronal sections of formalin-fixed, paraffin-embedded tissues were placed on electromagnetically charged glass slides. Slides were deparaffinized in xylene and rehydrated through descending grades of ethanol up to water. After non-enzymatic antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 30 min at 97°C in a vacuum oven, slides were washed with 1× PBS and placed in blocking solution (5% normal goat or horse serum; Vector Laboratories, Burlingame, CA, USA) for 2 h. Primary antibodies consisted of Ng (1 : 200; Upstate), RXR-γ (1 : 100; Abcam), or CRBPI (1 : 200; Santa Cruz). Sections were incubated with primary antibody overnight in a dark, humidified chamber at 23°C, rinsed 3× with PBS, and incubated with fluorescein isothiocyanate (FITC) (1 : 500; Vector Laboratories) or Texas Red (1 : 500; Vector Laboratories)-conjugated secondary antibodies for 1 h at 23°C in the dark. Sections were again washed 3× with PBS, cover-slipped with an aqueous based mounting medium containing DAPI for nuclear labeling (Vectashield; Vector Laboratories), visualized with a Nikon ultraviolet inverted microscope, and processed with deconvolution software (Slidebook 4.0; Intelligent Imaging, Denver, CO, USA). Deconvolution was performed using SlideBook4 software, allowing acquisition of multiple 0.25 μm thick digital sections and 3-D reconstruction of the image.
Statistical analysis
All data were analyzed using one-way analysis of variance (ANOVA) with post hoc testing where appropriate using GraphPad Prizm® (GraphPad Software Inc., San Diego, CA, USA) and results were expressed as mean ± SEM, n ≥ 3. Values of p ≤ 0.05 were considered statistically significant.
Results
Cocaine does not alter serum levels of thyroid hormones
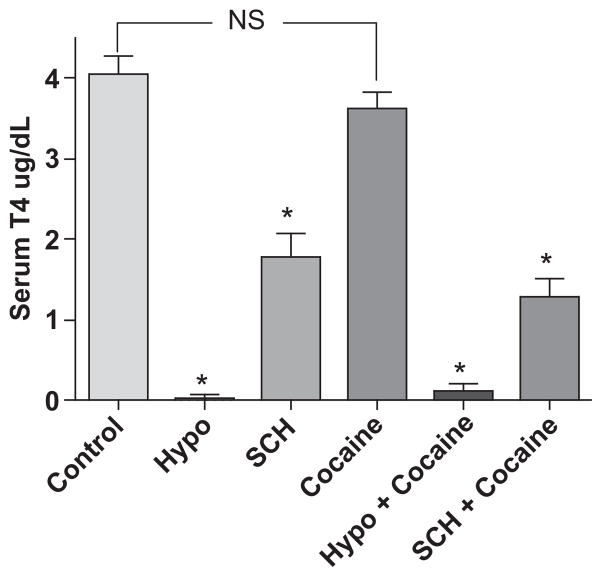
Hypothyroidism and subclinical hypothyroidism were confirmed by measuring serum levels of T4 from weekly blood draws in all groups of mice (Calikoglu et al. 1996; Elder et al. 2000; Tong et al. 2007; Vallortigara et al. 2008). T4 levels in euthyroid control mice ranged from 3.3 to 4.98 μg/dL (mean = 4.05 μg/dL); whereas, levels of T4 in mice fed 0.15% PTU for 15 days ranged from 0 (undetectable) – 0.22 μg/dL (mean = 0.03 μg/dL) (Fig. 2). Mice fed 0.075% PTU-containing chow for 15 days had T4 levels ranging from 0.37 to 2.4 μg/dL (mean = 1.87 μg/dL). Mice given cocaine for 14 days had normal T4 levels ranging from 2.8 to 4.1 μg/dL (mean = 3.62). Cocaine administration in combination with half or full dose PTU had no significant effects on T4 levels compared with PTU alone (Fig. 2). These results suggest that during PTU-induced overt or SCH in mice, as measured by free T4 levels, concurrent cocaine administration does not alter free T4 levels. As expected and as previously reported, levels of T3 were not affected by PTU. (data not shown) (Zoeller et al. 2007). Additionally, cocaine had no effect on T3 levels (data not shown).
Fig. 2.
Chronic cocaine administration has no effect on serum levels of free thyroxine (T4) in mice. Levels of T4 were significantly lower in PTU-fed mice with or without cocaine administration (hypothyroid mice and subclinical hypothyroid mice) compared with euthyroid control (*p ≤ 0.01). Cocaine had no effect on T4 levels. Hypo, hypothyroid; SCH, subclinical hypothyroid; n.s., not statistically significant. Data were analyzed by one-way ANOVA with Tukey’s multiple comparison post hoc test. n ≥ 6 mice per group.
Cocaine-induced stereotypic behavior is diminished in hypothyroid mice
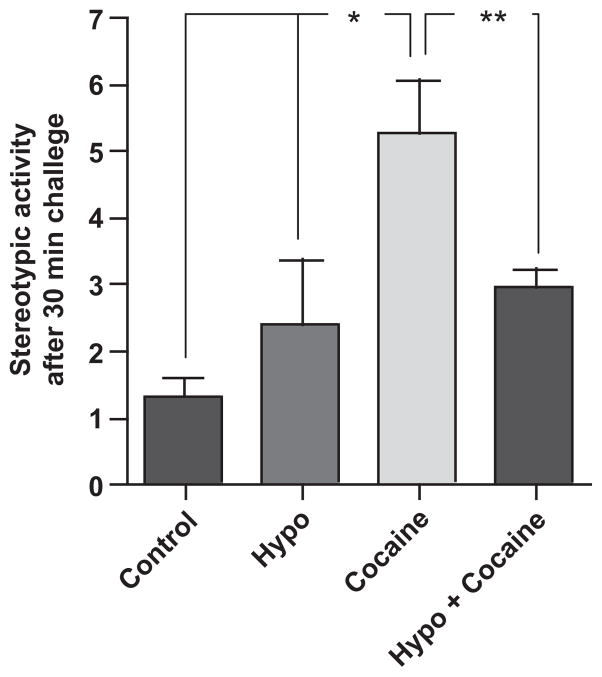
The effects of cocaine and hypothyroidism on stereotypic behavior were assessed in all groups of mice. As expected, cocaine-treated mice displayed significantly increased stereotypic behavior after receiving a challenge dose of cocaine following 48 h of withdrawal, indicative of sensitization (*p < 0.01) (Fig. 3). Stereotypic behavior in hypothyroid cocaine-treated mice was significantly decreased compared with euthyroid cocaine-treated mice (**p < 0.05) (Fig. 3). Stereotypic behavior in vehicle-treated hypothyroid (Fig. 3) and SCH (data not shown) mice was similar to behavior observed in euthyroid mice that did not receive cocaine. Likewise, behavior observed in SCH mice that received cocaine (data not shown) was similar to that of euthyroid mice that received cocaine injections. These results suggest that in the mouse model of chronic cocaine abuse, overt hypothyroidism diminishes typical stereotypic behavior associated with cocaine withdrawal and challenge. As cocaine alone did not alter T4 levels, but cocaine-induced stereotypic behavior was diminished in hypothyroid mice, we next addressed changes in the signaling that may be responsible, at least in part, for these observations.
Fig. 3.
Hypothyroidism diminished stereotypic behavior in cocaine-administered mice. After 14 days of once per day cocaine injection (15 mg/kg), mice were withdrawn from cocaine for 48 h followed by a challenge injection (15 mg/kg). Stereotypic behavior was assessed and cocaine-treated mice displayed significantly greater activity at 30 min post-challenge than control mice (*p < 0.01). Stereotypic behavior in hypothyroid mice administered cocaine was significantly diminished compared with euthyroid cocaine-treated mice (**p < 0.05). Data were analyzed by one-way ANOVA with Newman–Keuls multiple comparison post hoc test. n ≥ 6 mice per group.
Neurogranin is differentially expressed in the adult mouse brain and is regulated by TH in discrete brain regions
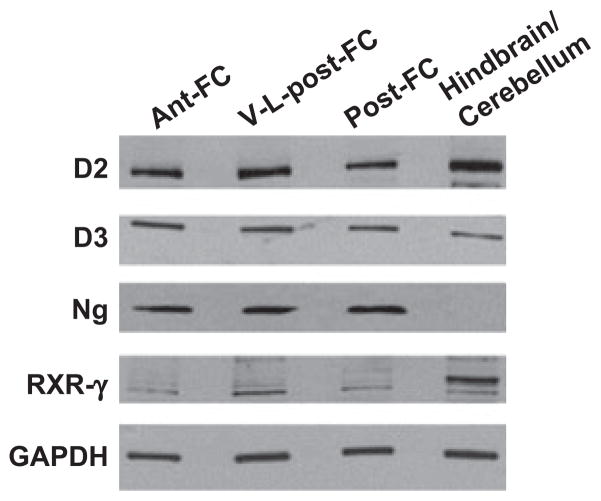
To assess baseline levels of D2, D3, Ng, and RXR in the four brain regions considered, levels were assessed by western analyses in euthyroid control tissues. While D2, D3 and RXR-γ were expressed in all brain regions assessed, Ng was not observed in the hindbrain/cerebellum (Fig. 4) in agreement with previous studies (Krezel et al. 1999; Zoeller et al. 2007; Royland et al. 2008). Following cocaine and/or PTU administration, differences in Ng expression were not observed in the anterior frontal cortex or ventral–lateral posterior frontal cortex (data not shown). However, analysis of posterior frontal cortex (Post-FC) sections, which include the hypothalamus and hippocampus, revealed significant changes in Ng expression in cocaine and PTU treatment groups. Given the importance of the structures in this brain region in HPT signaling, it is not surprising that the greatest effects on TH-responsive genes were observed within this region. Furthermore, these results are consistent with previous studies demonstrating that Ng is regulated by TH in discrete brain areas that include the hypothalamus and hippocampus (Martinez de Arrieta et al. 1999; Zoeller et al. 2007; Vallortigara et al. 2008). Thus, Post-FC tissues were assessed in the remainder of the experiments.
Fig. 4.
Differential expression of Ng in murine brains regions. Levels of D2, D3, Ng and RXR-γ were assessed by western analyses from 20 μg of protein from each of the four brain regions in euthyroid control mice (n = 6): anterior frontal cortex (Ant-FC), ventral-lateral posterior frontal cortex (V-L-post-FC), posterior frontal cortex (post-FC), and hindbrain/cerebellum. Deiodinase 2, D2; deiodinase 3, D3; neurogranin, Ng; retinoid X receptor-γ, RXR-γ; GAPDH, loading control.
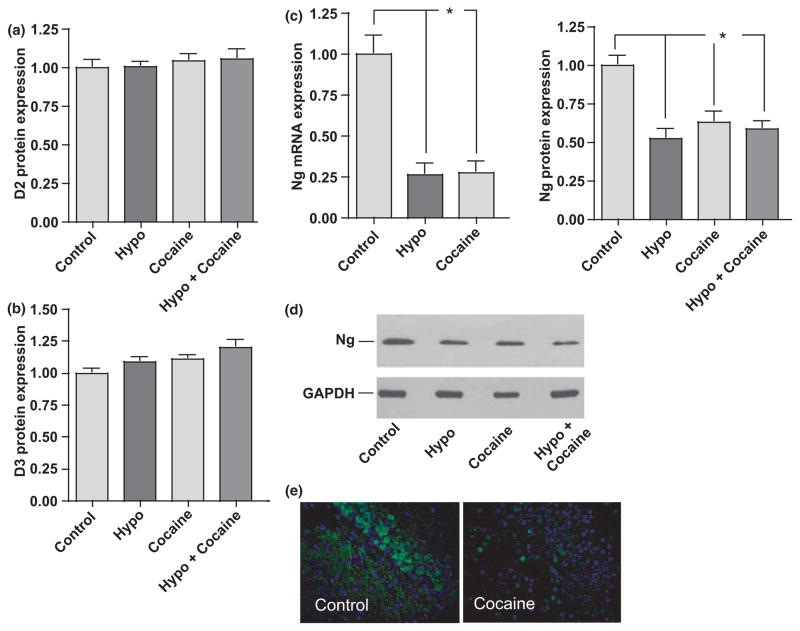
Cocaine decreases neurogranin levels but has no effect on levels of deiodinases 2 and 3
While the thyroid gland produces both T3 and T4, the vast majority of TH produced is in the form of T4 that is subsequently converted to the more bioactive T3 form by deiodinase 2 (D2) (Tong et al. 2007). Our data from PTU-fed mice showed that less circulating T4 was available for conversion to T3 while serum levels of T3 were not changed (Fig. 2). A second enzyme, deiodinase 3 (D3), present in neurons processes T3 to inactive forms to prevent over-stimulation of TH-responsive signaling pathways. Changes in levels of either D2 or D3 may result in alterations in T3 levels, thereby affecting expression of TH-regulated genes. Consistent with our previous findings that TH levels were not affected by cocaine, western analyses revealed no significant changes in either D2 or D3 protein levels among groups (Fig. 5a and b). Therefore, changes in levels of TH-responsive genes are not likely due to altered D2 and/or D3 levels in this model.
Fig. 5.
Cocaine significantly decreases Neurogranin expression levels. Western analyses of protein levels of (a) D2, (b) D3 and (c) Ng were assessed after 14 days cocaine administration. No significant changes in D2 or D3 were observed among groups. (c, left panel) Ng mRNA and (c, right panel and d) protein levels were significantly decreased in cocaine-treated mice compared with control. (e) Neurogranin (green) immunofluorescent labeling of hippocampal tissue from representative control and cocaine-treated mice. Nuclei are labeled with DAPI (blue). (e) Magnification 400×. D2, deiodinase 2 enzyme; D3, deiodinase 3 enzyme; Ng, neurogranin; Hypo, hypothyroid. Data were analyzed by one-way ANOVA with Tukey’s multiple comparison post hoc test. n ≥ 6 mice per group. *p < 0.05.
Changes in levels of the TH-responsive gene Ng were assessed in Post-FC brain sections of euthyroid, hypothyroid and cocaine-treated mice. Decreased Ng mRNA and protein expression were observed in both hypothyroid (mRNA 35% and protein 50% of euthyroid controls) and cocaine-treated animals (mRNA 28% and protein 67% of euthyroid, controls) (Fig. 5c and d). Neither additive nor synergistic effects on Ng protein levels were observed in mice receiving both PTU and cocaine (59% of euthyroid controls) (Fig. 5c and d). In SCH mice and SCH mice receiving cocaine, levels of Ng were not significantly different from control values (82% and 84% of controls, respectively) (data not shown). Interestingly, cocaine had less of an effect on Ng levels in the SCH group than in euthyroid controls. Immunofluorescence labeling of representative hippocampal sections from control (euthyroid) and cocaine-treated mice (Fig. 5e) reveals that Ng levels (green) were significantly decreased by cocaine within this brain region. To rule out the possibility that the decreased expression of Ng was due to withdrawal from cocaine and not to the 14 day cocaine administration, one group of mice (n = 6) was killed immediately following the final cocaine injection of the 14-day paradigm and not subjected to the 48 h cocaine withdrawal and challenge. Messenger RNA and protein levels of Ng in brain sections from not withdrawn mice did not significantly differ from those of the cocaine 48 h withdrawal group (data not shown), confirming that the observed alterations in Ng levels were the result of chronic cocaine administration.
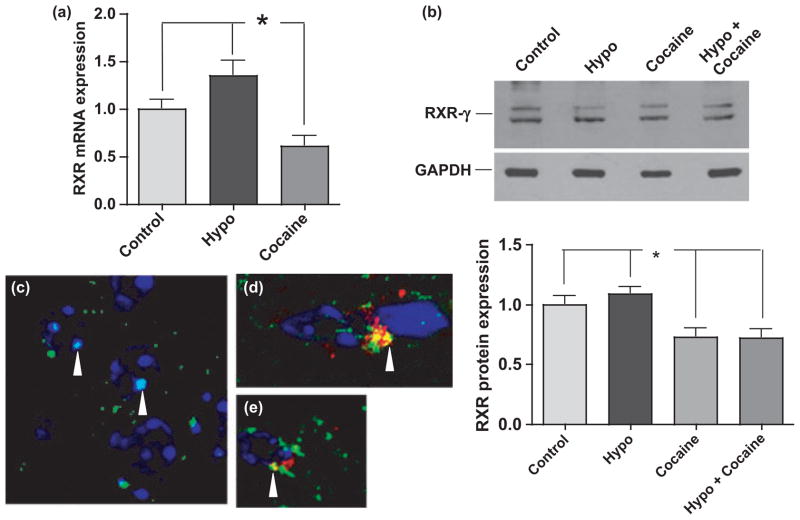
RXR-γ is decreased in cocaine-treated mice
In addition to T3 availability, thyroid hormone receptor (TR) is dependent upon heterodimerization with RXR for displacement of transcriptional co-repressors, recruitment of co-activators, and subsequent transcriptional activation of TH-responsive genes. Because no changes were observed in T4 levels in response to cocaine, yet significantly decreased expression of the TH-responsive gene Ng was observed, we hypothesized that dysregulation may be occurring at the level of the nuclear receptor complex. Two types of TH nuclear receptors exist: TR-α and -β, however, TR-β is the main regulator of the HPT axis and levels of this receptor type have been shown to fluctuate in direct response to thyroid status (Esaki et al. 2003; Vallortigara et al. 2008). Analyses of both mRNA and protein levels of TR-α and -β in euthyroid control versus cocaine-treated mice showed that cocaine had no significant effects on the levels of TH nuclear receptors (data not shown).
Transcriptional activation of Ng is controlled by the thyroid responsive element (TRE) in its promoter region. To induce transcription, RXR-γ must bind to 9-cis-RA and dimerize with the T3-TR-β complex and together these factors interact with the TRE (Fig. 1). Therefore, even though levels of TR are not changed in response to cocaine, alterations in RXR-γ levels could affect Ng transcription and expression. Analyses of RXR-γ in cocaine treated mice indicated that both the message and protein levels were significantly decreased (*p < 0.001) (Fig. 6a and b). Message levels were decreased by 40%, and protein levels by 26% in cocaine-treated euthyroid mice compared with euthyroid control and to hypothyroid mice. In euthyroid mice, abundant RXR-γ is observed in the neuronal nuclei (arrowheads) (Fig. 6c). Likewise, co-localization of RXR-γ and CRBP is observed in the cytoplasm of euthyroid control mouse nuclei (arrowheads) with no nuclear labeling (Fig. 6d and e). In contrast, very low immunoreactivity of RXR-γ could be detected in the cocaine treated mice (not shown). These results indicate that cocaine may be acting on the RA-RXR-γ signaling pathway, rather than on the thyroid hormone pathway.
Fig. 6.
Cocaine significantly decreases RXR-γ. (a) qRT-PCR and (b) western analyses revealed significantly decreased RXR-γ in posterior forebrain regions of mice treated with cocaine. (c) RXR-γ immuno-fluorescence labeling (green) of a representative euthyroid control mouse brain showing co-localization of RXR-γ with nuclei labeled with DAPI (blue) (arrowheads). (d, e) CRBP-1 (red) and RXR-γ (green) double immunofluorescence labeling of a representative euthyroid control mouse brain showing co-localization (arrowheads, yellow). (c–e) Magnification 1000×. RXR-γ, retinoid-X receptor-γ; Hypo, hypothyroid. *p ≤ 0.001. Data were analyzed by one-way ANOVA with Tukey’s multiple comparison post hoc test. n ≥ 6 mice per group.
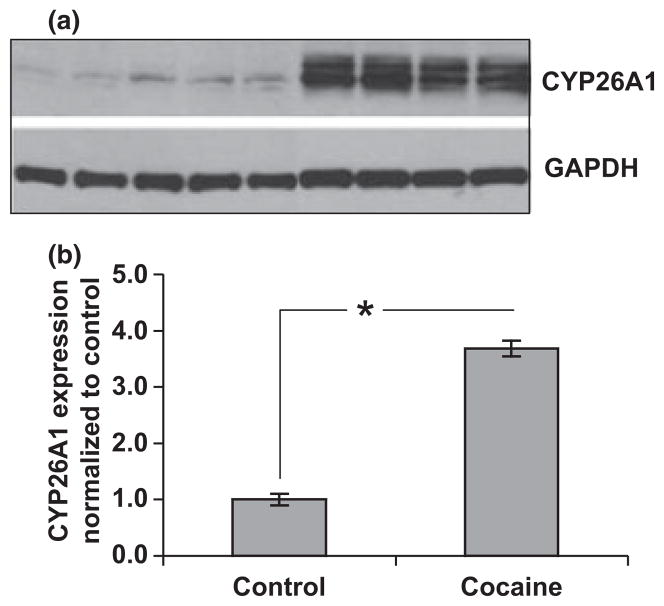
CYP26A1 is increased in cocaine-treated mice
CYP26A1 is a member of the cytochrome p450 enzyme family and represents a key RA metabolizing enzyme in the brain. Previous studies have demonstrated that cocaine increases cytochrome p450 expression in liver and cardiac tissues, but its effects on this family of enzymes in the brain has not been previously explored in depth (Wang et al. 2002; Lane and Bailey 2005). Here, we show that chronic cocaine administration results in a significant increase in CYP26A1 protein levels in brain tissue (*p < 0.001, Fig. 7). Taken together, our data suggest that cocaine contributes to increased metabolism of RA by induction of CYP26A1 and, subsequently, a reduction in the level of 9-cis-RA available to potentiate RXR signaling. This finding may account for the observed decrease in RXR-γ levels, as receptor levels are regulated by RA levels in a positive feedback system, as well as for the decreased transcription of Ng, which requires 9-cis-RA for activation.
Fig. 7.
Cocaine significantly increases level of CYP26A1. (a) Western analyses of euthyroid control mice (n = 5) and cocaine-treated mice (n = 4) indicated that cocaine significantly increased levels of CYP26A1 in posterior frontal cortex. (b) densitometric quantification, *p < 0.001 Data were analyzed by one-way ANOVA with Tukey’s multiple comparison post hoc test.
Discussion
In the present study, we evaluated the effects of chronic cocaine abuse on HPT signaling in euthyroid and PTU-induced hypothyroid and subclinical hypothyroid mice. Previous studies have suggested cocaine may contribute to thyroid dysfunction but molecular mechanisms remain unknown. Furthermore, literature regarding the specific effects of cocaine on thyroid hormone responsive genes is lacking. Both chronic cocaine abuse and hypothyroidism have been associated with similar co-morbidities, including major depressive disorder and cognitive dysfunction; specifically, in areas related to memory and that are reliant on neuroplasticity (Gold et al. 1981; Regier et al. 1990; Haggerty et al. 1993; Baldini et al. 1997; Ferris et al. 2008). Ng is one of the only brain-specific genes involved in synaptic plasticity and its expression is TH-dependent in many brain regions (Vallortigara et al. 2008). Because of its sensitivity to T3 levels, Ng is decreased in hypothyroidism, particularly in the hippocampus (Dong et al. 2005; Vallortigara et al. 2008; Gong et al. 2010). Given that cocaine abuse can also result in deficiencies in hippocampal-related functions, we investigated the effects of chronic cocaine treatment on Ng levels and on several aspects of HPT signaling that could explain alterations in Ng expression. All male mice were used in this study to avoid confounding variables involving the effects of the female estrous cycle on TH processing. Furthermore, hypothyroidism has previously been shown to exert a greater effect on gene expression in male mice pups than in females (Dong et al. 2005; Royland et al. 2008). Gender-specific differences in the effects of cocaine on RA and Ng signaling are unknown and represent an area for future investigations.
Results from this study demonstrate that PTU-induced hypothyroidism and chronic cocaine abuse similarly reduce Ng protein levels (to 52% and 67% of control, respectively) as well as mRNA levels (to 35% and 28% of control, respectively) in posterior forebrain sections (Fig. 5). We also chose to examine a subclinical hypothyroid state, induced by administering ½ dose of PTU, because this condition is relatively common in the human population and produces a number of cognitive and mood disturbances, yet few studies have used methods of manipulating thyroid status to mimic this state (Wang et al. 2002). Although we observed a downward trend in Ng levels in SCH groups compared with control levels, results did not achieve statistical significance. These findings do not rule out the possibility that SCH could disrupt neuroplasticity in human patients, and may be the subject of future studies. Previously, it has been demonstrated that Ng is decreased in the hippocampus of hypothyroid animals (Vallortigara et al. 2008). Upon immunohistochemical analysis in this study, the greatest differences in Ng expression between control and cocaine treatment groups were observed within the hippocampus, as well (Fig. 5e). Surprisingly, cocaine treatment did not alter serum T3 or T4 levels, or the expression levels of D2 or D3, indicating that the observed decrease in Ng was not a result of insufficient T3 levels, as is the case in hypothyroidism. However, it is possible that cocaine may alter the actual amount of T3 that reaches neurons via the disruption of normal astrocyte-neuron intercellular communication. Additionally, because SCH in humans is characterized by normal T3 and T4 levels but an abnormal TSH response, the results observed in cocaine-treated mice do not rule out the possibility that cocaine may have an effect on TSH that may ultimately lead to HPT alterations without directly impacting TH levels (Wang et al. 2002). However, the lack of significant findings in TH levels and other components of the HPT signaling pathway in cocaine-treated animals prompted us to evaluate the effects of cocaine at the level of nuclear receptor signaling.
Transcriptional activation of Ng requires the heterodimerization of ligand-activated TR and RXR-γ and binding of this complex to the TRE of the Ng gene. RXR belongs to a superfamily of nuclear receptors that bind the vitamin A derivative 9-cis-retinoic acid, as well as certain long-chain fatty acids such as docosahexaenoic acid (Wang et al. 2002). Because vitamin A deficiency has previously been shown to decrease Ng expression at the level of the RXR, we evaluated whether cocaine abuse influences RXR signaling to similarly reduce Ng levels. Three RXRs exist – RXR-α, RXR-β, and RXR-γ – which are encoded by separate genes and differentially expressed. RXR-γ is primarily expressed in the hypothalamus, pituitary, hippocampus, and striatum and therefore serves as a likely target for involvement in Ng regulation (Hoopes et al. 1992; Kliewer et al. 1992; Sugawara et al. 1995; Kumar et al. 2005; van Neerven et al. 2008). Furthermore, RXR-γ is known to be involved in dopaminergic signaling, as it is highly expressed in dopaminergic neurons in the striatum and RXR-γ mutant mice exhibit impaired locomotion and a blunted locomotor response to cocaine (Krezel et al. 1998; Zetterstrom et al. 1999). Additionally, mice subjected to vitamin A deficiency or targeted disruptions of RXR exhibit impaired long-term-potentiation and long-term-depression, reduced dopaminergic function, and depressive-like symptoms (Chiang et al. 1998; Husson et al. 2003; Krezel et al. 1998). Results from this study reveal for the first time that chronic exposure to cocaine decreases RXR-γ mRNA and protein levels in mouse posterior forebrain sections.
The mechanism(s) by which cocaine regulates RXR levels and function will continue to be examined in future studies. A number of possibilities exist for these observations. For instance, as RXR is biologically regulated by RA levels in a positive feedback system, cocaine exposure could result in increased metabolism of vitamin A or its derivatives, resulting in reduced bio-availability for retinoic acid receptor and RXR-dependent signaling. In support of this view, previous studies have shown that cocaine treatment in mice induces elevated levels of cytochrome p450 family members (Wang et al. 2002; Lane and Bailey 2005). We have expanded upon these studies by demonstrating that chronic cocaine administration significantly increases levels of CYP26A1, the main enzyme responsible for metabolism of cellular retinoic acid, in brain tissue (Figs. 1 and 7). Additionally, cocaine may alter the levels or function of CRBP1 or cellular retinoic acid binding protein I/II so that less retinol is taken up into cells, converted to RA, isomerized to 9-cis-RA, bound to RXR, and transported successfully to the nucleus to initiate transcription of responsive genes. As RXR has been demonstrated to shuttle between the cytosol and nucleus, subcellular localization studies following cocaine treatment could yield valuable insight into the intra-cellular response to cocaine.
Another point of potential regulation exists at the level of formation of the nuclear receptor complex. The TR/RXR complex unbound to its ligands is normally bound to the TRE of regulated genes where it associates with transcriptional repressors, such as SMRT and N-CoR, to promote histone deacetylase activity and inhibition of active transcription (Ferris et al. 2008). Binding of T3 to the TR results in displacement of these repressors and results in recruitment of co-activators that possess histone acetylase activity to initiate and promote transcription of target genes (Ferris et al. 2008). Thus, TH-responsive genes may also be sensitive to levels of these co-factors, and down-regulation of these genes could result from increased repression or decreased activation factors. Cocaine abuse has previously been associated with chromatin remodeling and changes in specific histone modifications in the striatum, resulting in the regulation of gene transcription, and therefore may alter Ng transcription in a similar manner (Kumar et al. 2005).
While RA signaling has recently been implicated in a number of physiological processes and its dysfunction may contribute to several neurodegenerative diseases, the effects of cocaine abuse on RA signaling have not been previously investigated. Our findings may shed light on a number of effects cocaine exerts for which no mechanisms have yet been elucidated. For instance, it has been well-documented that chronic cocaine abuse decreases dopamine-2-receptor (D2R) expression in a number of brain regions contributing to impaired dopaminergic signaling and a number of locomotor and psychological impairments attributed to cocaine abuse (Krezel et al. 1998; Lull et al. 2008). Importantly, the D2R gene contains an RXR-response element within its promoter region (Samad et al. 1997). Therefore, a cocaine-induced reduction in RXR signaling may account for altered D2R levels and function.
In conclusion, data presented in this study highlight an effect of cocaine abuse that has not been previously reported, as well as a novel mechanism for the regulation of TH-responsive genes following cocaine exposure. In light of these findings, we demonstrate how separate conditions, chronic cocaine abuse and HPT dysfunction, converge on a single signaling pathway. These results contribute to the understanding of the underlying complexity of nuclear receptor signaling, and suggest that a delicate balance between signaling pathways in the heterodimeric nuclear receptor complex must be struck in order to transduce proper signaling and maintain a healthy neural environment.
Acknowledgments
This study was supported by R21DA029523 to TDL. We are grateful to Jessica Otte and Ahmet Yunus Ozdemir for technical assistance and to Dr. Dianne Soprano for guidance regarding retinoid pathways.
Abbreviations used
- 9-cis-RA
9-cis-retinoic acid
- CRBP
cellular retinol-binding protein
- D2
deiodinase-2
- D2R
dopamine-2-receptor
- D3
deiodinase-3
- HPT
hypothalamic-pituitary-thyroid
- PBS
phosphate-buffered saline
- Post-FC
posterior frontal cortex
- PTU
propyl-thiouracil
- RXR
retinoid X receptor
- SCH
subclinical hypothyroidism
- T3
tri-iodothryonine
- T4
thyroxine
- TH
thyroid hormone
- TR
thyroid hormone receptor
- TRE
thyroid-responsive element
- TSH
thyrotropin
Footnotes
The authors have no conflicts of interest.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkemade A, Friesema EC, Unmehopa UA, Fabriek BO, Kuiper GG, Leonard JL, Wiersinga WM, Swaab DF, Visser TJ, Fliers E. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metabol. 2005;90:4322–4334. doi: 10.1210/jc.2004-2567. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Baldini IM, Vita A, Mauri MC, Amodei V, Carrisi M, Bravin S, Cantalamessa L. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:925–935. doi: 10.1016/s0278-5846(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Bauer M, Whybrow PC. Thyroid hormone, neural tissue and mood modulation. World J Biol Psychiatry. 2001;2:59–69. doi: 10.3109/15622970109027495. [DOI] [PubMed] [Google Scholar]
- Bohnert AS, Miech RA. Changes in the association of drug use with depressive disorders in recent decades: the case of cocaine. Subst Use Misuse. 2010;45:1452–1462. doi: 10.3109/10826081003777550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Budziszewska B, Jaworska-Feil L, Lason W. The effect of repeated amphetamine and cocaine administration on adrenal, gonadal and thyroid hormone levels in the rat plasma. Exp Clin Endocrinol Diabetes. 1996;104:334–338. doi: 10.1055/s-0029-1211463. [DOI] [PubMed] [Google Scholar]
- Calikoglu AS, Gutierrez-Ospina G, D’Ercole AJ. Congenital hypothyroidism delays the formation and retards the growth of the mouse primary somatic sensory cortex (S1) Neurosci Lett. 1996;213:132–136. doi: 10.1016/0304-3940(96)12836-6. [DOI] [PubMed] [Google Scholar]
- Chiang MY, Misner D, Kempermann G, Schikorski T, Giguere V, Sucov HM, Gage FH, Stevens CF, Evans RM. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron. 1998;21:1353–1361. doi: 10.1016/s0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Christie KA, Burke JD, Jr, Regier DA, Rae DS, Boyd JH, Locke BZ. Epidemiologic evidence for early onset of mental disorders and higher risk of drug abuse in young adults. Am J Psychiatry. 1988;145:971–975. doi: 10.1176/ajp.145.8.971. [DOI] [PubMed] [Google Scholar]
- Connor MJ, Sidell N. Retinoic acid synthesis in normal and Alzheimer diseased brain and human neural cells. Mol Chem Neuropathol. 1997;30:239–252. doi: 10.1007/BF02815101. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39:345–357. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty J. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Mol Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Deykin EY, Levy JC, Wells V. Adolescent depression, alcohol and drug abuse. Am J Public Health. 1987;77:178–182. doi: 10.2105/ajph.77.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhopesh VP, Burke WM, Maany I, Ravi NV. Effect of cocaine on thyroid functions. Am J Drug Alcohol Abuse. 1991;17:423–427. doi: 10.3109/00952999109001601. [DOI] [PubMed] [Google Scholar]
- Dong H, Wade M, Williams A, Lee A, Douglas GR, Yauk C. Molecular insight into the effects of hypothyroidism on the developing cerebellum. Biochem Biophys Res Commun. 2005;330:1182–1193. doi: 10.1016/j.bbrc.2005.03.099. [DOI] [PubMed] [Google Scholar]
- Elder DA, Karayal AF, D’Ercole AJ, Calikoglu AS. Effects of hypothyroidism on insulin-like growth factor-I expression during brain development in mice. Neurosci Lett. 2000;293:99–102. doi: 10.1016/s0304-3940(00)01508-1. [DOI] [PubMed] [Google Scholar]
- Enderlin V, Vallortigara J, Alfos S, Feart C, Pallet V, Higueret P. Retinoic acid reverses the PTU related decrease in neurogranin level in mice brain. J Physiol Biochem. 2004;60:191–198. doi: 10.1007/BF03167028. [DOI] [PubMed] [Google Scholar]
- Esaki T, Suzuki H, Cook M, Shimoji K, Cheng SY, Sokoloff L, Nunez J. Functional activation of cerebral metabolism in mice with mutated thyroid hormone nuclear receptors. Endocrinology. 2003;144:4117–4122. doi: 10.1210/en.2003-0414. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JD, Gelernter J, DeVoe JS, Zhang W, Weiss RD, Brady K, Farrer L, Kranzler HR. Association of psychiatric and substance use disorder comorbidity with cocaine dependence severity and treatment utilization in cocaine-dependent individuals. Drug Alcohol Depend. 2009;99:193–203. doi: 10.1016/j.drugalcdep.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Iovieno N, Clementi N, Boscaro M, Paggi F, Balercia G, Fava M, Papakostas GI. Diagnosis of co-morbid axis-I psychiatric disorders among women with newly diagnosed, untreated endocrine disorders. World J Biol Psychiatry. 2010;11:991–996. doi: 10.3109/15622975.2010.491126. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AL, Extein I. Hypothyroidism and depression. Evidence from complete thyroid function evaluation. JAMA. 1981;245:1919–1922. doi: 10.1001/jama.245.19.1919. [DOI] [PubMed] [Google Scholar]
- Gong J, Liu W, Dong J, Wang Y, Xu H, Wei W, Zhong J, Xi Q, Chen J. Developmental iodine deficiency and hypothyroidism impair neural development in rat hippocampus: involvement of doublecortin and NCAM-180. BMC Neurosci. 2010;11:50. doi: 10.1186/1471-2202-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty JJ, Jr, Stern RA, Mason GA, Beckwith J, Morey CE, Prange AJ., Jr Subclinical hypothyroidism: a modifiable risk factor for depression? Am J Psychiatry. 1993;150:508–510. doi: 10.1176/ajp.150.3.508. [DOI] [PubMed] [Google Scholar]
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res. 2009;23:793–800. doi: 10.1016/j.beem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J. Methods of blood collection in the mouse. Lab Animal Techniques. 2000;29:47–53. [Google Scholar]
- Hoopes CW, Taketo M, Ozato K, Liu Q, Howard TA, Linney E, Seldin MF. Mapping of the mouse Rxr loci encoding nuclear retinoid X receptors RXR alpha, RXR beta, and RXR gamma. Genomics. 1992;14:611–617. doi: 10.1016/s0888-7543(05)80159-4. [DOI] [PubMed] [Google Scholar]
- Husson M, Enderlin V, Alfos S, Feart C, Higueret P, Pallet V. Triiodothyronine administration reverses vitamin A deficiency-related hypo-expression of retinoic acid and triiodothyronine nuclear receptors and of neurogranin in rat brain. Br J Nutr. 2003;90:191–198. doi: 10.1079/bjn2003877. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Rodriguez-Pena A, Ibarrola N, Morreale de Escobar G, Bernal J. Adult rat brain is sensitive to thyroid hormone. Regulation of RC3/neurogranin mRNA. J Clin Invest. 1992;90:554–558. doi: 10.1172/JCI115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, Chambon P. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Prog Neurobiol. 2005;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. Infundibular tanycytes as modulators of neuroendocrine function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed. 2007;78 (Suppl 1):84–98. [PubMed] [Google Scholar]
- Lehrmann E, Colantuoni C, Deep-Soboslay A, Becker KG, Lowe R, Huestis MA, Hyde TM, Kleinman JE, Freed WJ. Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS ONE. 2006;1:e114. doi: 10.1371/journal.pone.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lull ME, Freeman WM, Vrana KE, Mash DC. Correlating human and animal studies of cocaine abuse and gene expression. Ann N Y Acad Sci. 2008;1141:58–75. doi: 10.1196/annals.1441.013. [DOI] [PubMed] [Google Scholar]
- Martinez de Arrieta C, Morte B, Coloma A, Bernal J. The human RC3 gene homolog, NRGN contains a thyroid hormone-responsive element located in the first intron. Endocrinology. 1999;140:335–343. doi: 10.1210/endo.140.1.6461. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Drager UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci USA. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla A, Kalantarhormozi MR, Khazraee S. Clinical characteristics of patients with major depressive disorder with and without hypothyroidism: a comparative study. J Psychiatr Pract. 2011;17:67–71. doi: 10.1097/01.pra.0000393848.35132.ff. [DOI] [PubMed] [Google Scholar]
- van Neerven S, Kampmann E, Mey J. RAR/RXR and PPAR/RXR signaling in neurological and psychiatric diseases. Prog Neurobiol. 2008;85:433–451. doi: 10.1016/j.pneurobio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. National Institutes of Health; Bethesda: 1997–2009. [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Royland JE, Parker JS, Gilbert ME. A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol. 2008;20:1319–1338. doi: 10.1111/j.1365-2826.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- Samad TA, Krezel W, Chambon P, Borrelli E. Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor-retinoid X receptor family. Proc Natl Acad Sci USA. 1997;94:14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60:593–599. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Greenspan SL, Ridgway EC. Transcriptional regulation of thyrotropin subunit genes by thyrotropin-releasing hormone and dopamine in pituitary cell culture. J Biol Chem. 1986;261:12675–12679. [PubMed] [Google Scholar]
- Sugawara A, Yen PM, Qi Y, Lechan RM, Chin WW. Isoform-specific retinoid-X receptor (RXR) antibodies detect differential expression of RXR proteins in the pituitary gland. Endocrinology. 1995;136:1766–1774. doi: 10.1210/endo.136.4.7895689. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Woods BT, Mello NK, Hallgring E, Anfinsen P, Douglas A, Mercer G. Pituitary volume in men with concurrent heroin and cocaine dependence. J Clin Endocrinol Metabol. 1993;76:1529–1532. doi: 10.1210/jcem.76.6.8501161. [DOI] [PubMed] [Google Scholar]
- Tong H, Chen GH, Liu RY, Zhou JN. Age-related learning and memory impairments in adult-onset hypothyroidism in Kunming mice. Physiol Behav. 2007;91:290–298. doi: 10.1016/j.physbeh.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Vallortigara J, Alfos S, Micheau J, Higueret P, Enderlin V. T3 administration in adult hypothyroid mice modulates expression of proteins involved in striatal synaptic plasticity and improves motor behavior. Neurobiol Dis. 2008;31:378–385. doi: 10.1016/j.nbd.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Pezzarossa A. Thyrotropin-releasing hormone-induced GH release after cocaine withdrawal in cocaine addicts. Neuropeptides. 1999;33:522–525. doi: 10.1054/npep.1999.0773. [DOI] [PubMed] [Google Scholar]
- Wang JF, Yang Y, Sullivan MF, Min J, Cai J, Zeldin DC, Xiao YF, Morgan JP. Induction of cardiac cytochrome p450 in cocaine-treated mice. Exp Biol Med (Maywood) 2002;227:182–188. doi: 10.1177/153537020222700305. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Lindqvist E, Mata de Urquiza A, Tomac A, Eriksson U, Perlmann T, Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37:11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]