Abstract
γ-Secretase cleaves the carboxyl-terminal fragment (βCTF) of APP not only in the middle of the transmembrane domain (γ-cleavage), but also at sites close to the membrane/cytoplasm boundary (ε-cleavage), to produce the amyloid β protein (Aβ) and the APP intracellular domain (AICD), respectively. The AICD49–99 and AICD50–99 species were identified as counterparts of the long Aβ species Aβ48 and Aβ49, respectively. We found that Aβ40 and AICD50–99 were the predominant species in cells expressing wild-type APP and presenilin, whereas the production of Aβ42 and AICD49–99 was enhanced in cells expressing familial Alzheimer's disease mutants of APP and presenilin. These long Aβ species were identified in cell lysates and mouse brain extracts, which suggests that ε-cleavage is the first cleavage of βCTF to produce Aβ by γ-secretase. Here, we review the progress of research on the mechanism underlying the proteolysis of the APP transmembrane domain based on tri- and tetrapeptide release.
1. Introduction
The amyloid precursor protein (APP) is a type I membrane protein. After ectodomain shedding by β-secretase, the carboxyl-terminal fragment (βCTF) of APP becomes a direct substrate of γ-secretase and is processed into the amyloid β protein (Aβ) and the APP intracellular domain (AICD) [1–5]. γ-secretase is an enigmatic protease composed of presenilin 1/2, nicastrin, Aph-1, and Pen-2 that catalyzes proteolysis in the hydrophobic environment of the lipid bilayer [6–15]. Currently, over 50 molecules are reported as γ-secretase substrates, which reflects the physiological importance of this enzyme [16]. For instance, the Notch receptor on the plasma membrane is cleaved by γ-secretase upon ligand binding and the liberated Notch intracellular domain (NICD) translocates into the nucleus and activates the expression of transcription factors to suppress neuronal differentiation [17, 18]. This indicates that inhibition of γ-secretase for suppression of Aβ production causes harmful side effects. To avoid this risk in anti-Alzheimer's disease (AD) therapeutics, it is very important to elucidate the molecular mechanism underlying γ-secretase-dependent proteolysis. Recently, it was revealed that γ-secretase forms a hydrophilic pore and three water-accessible cavities [19–23]. Here, we review the progress of research on the mechanism underlying the proteolysis of the transmembrane domain of βCTF.
2. Discovery of ε-Cleavage during APP Processing
After the β-secretase-dependent cleavage of APP, the ectodomain of APP is released into the extracellular space and βCTF (as a stub in the lipid bilayer) is the direct substrate of γ-secretase [2, 3, 24]. βCTF is composed of 99 amino acids and is eventually processed into the 38–43-residue-long Aβ, suggesting that the counterparts of those Aβ species should contain 56–61 residues [4, 25–29]. However, 50-51-residue-long AICDs were identified that correspond to residues 49–99 and 50–99 of βCTF (AICD49–99 and AICD50–99), instead of 56–61-residue-long species (Figure 1) [30–32]. These AICD species were suppressed by L-685,458, a transition state analogue γ-secretase inhibitor, and by expression of a dominant-negative mutant of presenilin (PS), suggesting that γ-secretase cleaves βCTF not only in the middle of the transmembrane domain (γ-cleavage), but also at sites close to the membrane/cytoplasm boundary (ε-cleavage), releasing AICD49–99 and AICD50–99. ε-Cleavage sites are analogues of the Notch S3 cleavage site, which is located at the membrane, near the cytoplasm (Figure 1). Cleavages similar to the APP ε-cleavage were identified in other proteins, such as amyloid precursor-like protein 1 (APLP-1), APLP-2, CD44, Delta 1, E-cadherin, ErbB4, and LRP1 [30, 33–37]. It is reasonable to consider that the water molecules required for proteolysis have access to the catalytic center of γ-secretase from the cytoplasm, rather than from the extracellular space, and that ε-cleavage precedes γ-cleavage during APP processing.
Figure 1.
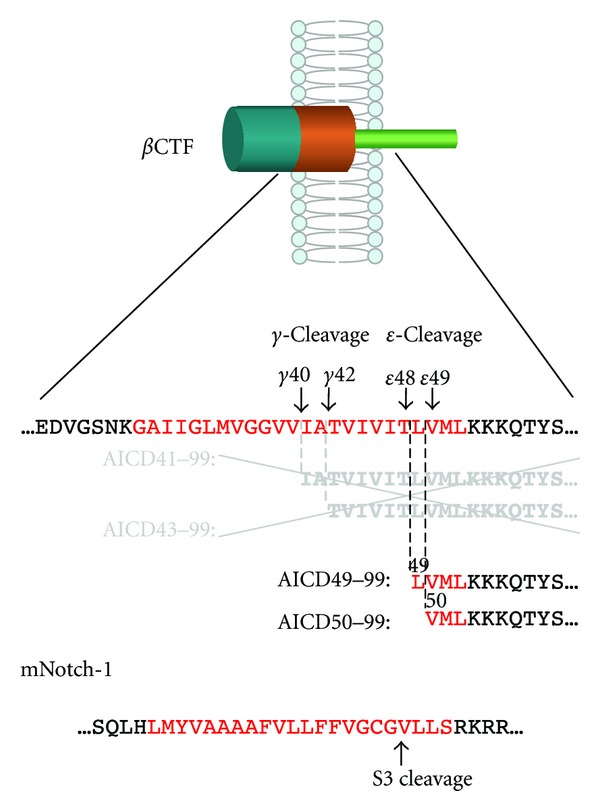
βCTF is cleaved at the membrane-cytoplasm boundary and not in the middle of the transmembrane domain (ε-cleavage), to release the AICD49–99 and AICD50–99 species. The production of AICD species was inhibited in the presence of a γ-secretase inhibitor. ε-Cleavage is analogous to the S3 cleavage of mNotch-1. Red indicates the transmembrane domain.
3. Relationship between γ- and ε-Cleavage
CHO cells expressing familial AD (FAD) mutants of PS or APP increase production ratio of Aβ42 (Aβ43) to Aβ40 compared to cells expressing wild-type PS or APP these longer Aβ species are more hydrophobic and more prone to form neurotoxic aggregates. CHO cells expressing wild-type PS preferentially release AICD50–99, whereas those expressing a subset of familial AD (FAD) mutants of PS or APP exhibit an increased proportion of AICD49–99 (Figure 2(a)) [42]. As those FAD mutations cause an increase in the Aβ42/Aβ40 ratio, a potential link between γ- and ε-cleavage was assumed. To test this, we expressed Aβ49 and Aβ48, which are potential counterparts of AICD50–99 and AICD49–99, respectively, in CHO cells. The cells expressing Aβ49 predominantly secreted Aβ40, whereas those expressing Aβ48 exhibited a significantly increased proportion of Aβ42/Aβ40 (Figure 2(b)) [43]. These data indicate that ε-cleavage sites determine the preference for γ- and ε-cleavage sites to produce Aβ40 and Aβ42. Long Aβ species, Aβ49 and Aβ48, have been identified in cell lysates and mouse brain extracts, which suggests that ε-cleavage is the first cleavage of βCTF to produce Aβ by γ-secretase [44]. On the other hand, ε-cleavage can be considered as endopeptidase activity of γ-secretase. FAD mutations did not consistently impair the endopeptidase activity on APP, Notch, ErbB4, and N-Cadherin, but altered γ-cleavage of APP, especially fourth cleavage to produce Aβ40 and Aβ38 from Aβ43 and Aβ42, respectively [45]. Such dissociation between ε-cleavage and γ-cleavage was also proposed by Quintero-Monzon et al. [46].
Figure 2.
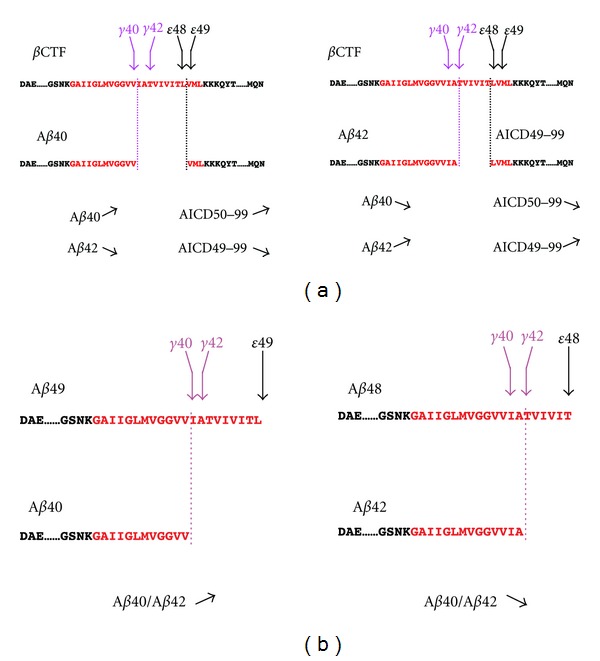
Relationship between γ- and ε-cleavage. (a) Cells expressing wild-type PS or APP predominantly produce Aβ40 and AICD50–99, while cells expressing a FAD mutant of PS or APP exhibited increased proportion of Aβ42 and AICD49–99. (b) Expression of Aβ49 results in an increase in Aβ40/Aβ42 ratio, whereas expression of Aβ48 leads to opposite results. ↗ increase, ↘ decrease.
4. Tripeptide Hypothesis
Treatment with N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor, suppressed extracellular Aβ in cells expressing APP [47]. The levels of the intracellular Aβ40 and Aβ42 species also decreased after DAPT treatment; however, intracellular Aβ43 and Aβ46 increased in a dose-dependent manner [44, 48, 49]. Tryptophan substitutions of γ-cleavage site (41–43) of APP attenuated Aβ secretion, but accumulated Aβ45 species in cell lysate. Tryptophan substitutions of ε-cleavage site (48–52) of APP decreased Aβ production and allowed longer AICD46–99 production. Tryptophan substitutions of ξ-cleavage site (45–47) also suppressed Aβ production. These substitution studies also implied successive cleavage of APP for Aβ production after ε-cleavage [50].
γ-Secretase containing mature nicastrin accumulates in lipid rafts, which indicates that active γ-secretase mainly localizes to the lipid raft of cells [51]. Lipid rafts are an ideal material to investigate Aβ production in the membrane environment. Aβ46 was the dominant species in a lipid raft isolated from DAPT-treated cells. Interestingly, incubating this lipid raft in the absence of DAPT resulted in production of Aβ40 and Aβ43, but not of Aβ42 [52]. These data suggest that Aβ46 is mainly converted into Aβ40 by releasing VIV and IAT tripeptides (successive tripeptide release, tripeptide hypothesis; Aβ40 product line) (Figure 3(a)). On the other hand, CHO cells expressing an FAD mutant of presenilin 2 exhibited a decrease in intracellular Aβ42 and a concomitant increase in intracellular Aβ45 levels in the presence of DAPT, suggesting that Aβ45 is a precursor of Aβ42 by releasing TVI (Aβ42 product line) (Figure 3(a)) [53]. It is reasonable to consider that two major product lines lead to Aβ40 and Aβ42 production (Figure 3(a)).
Figure 3.
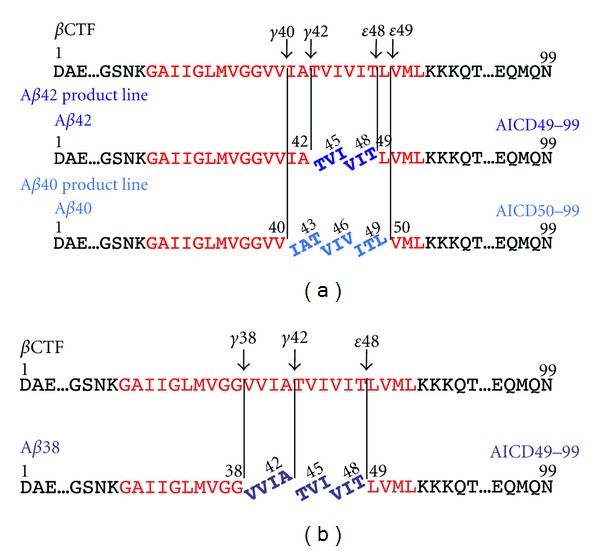
Tri- and tetrapeptide release from βCTF. (a) Upon ε-cleavage at ε48, γ-secretase releases the VIT and TVI tripeptides successively to produce Aβ42. (b) In the Aβ40 product line, after ε-cleavage at ε49, βCTF is converted into Aβ40 by releasing ITL, VIV, and IAT. Aβ42 is a direct substrate during Aβ38 production, which acts by releasing the VVIA tetrapeptide.
5. Identification of Tri- and Tetrapeptides Released from βCTF
The most effective approach to confirm tripeptide release from βCTF is the identification of those tripeptides directly in the reaction mixture of Aβ production. CHAPSO soluble γ-secretase was isolated and incubated with the βCTF substrate. LC-MS/MS analysis identified five major tripeptides, and γ-secretase inhibitors abolished the production of these molecules. ITL, VIV, and IAT were predicted tripeptides in the Aβ40 product line (Figure 3(a)). The amounts of Aβ40 and Aβ43 in the reaction mixture, as assessed using Western blotting, corresponded roughly to the predicted Aβ40 and Aβ43 levels, respectively [38]. VIT and TVI were also detected in the Aβ42 product line, as predicted (Figure 3(a)). Interestingly, the VVIA tetrapeptide was detected in the reaction mixture only in the absence of γ-secretase inhibitors (Figure 3(b)). We postulated that VVIA was released from Aβ42 to produce Aβ38. No significant difference was detected between the level of Aβ42 by Western blot quantification and that by LC-MS/MS quantitative estimation. These results indicate that γ-secretase releases tri- and tetrapeptides successively upon ε-cleavage of βCTF, to produce Aβ species. These tri- and tetrapeptides released from βCTF were detected even in the lipid raft fraction (Takami, unpublished observation).
6. Is Tripeptide Release a General Property of Substrate Cleavage by γ-Secretase?
Successive tripeptide release was observed in βCTF processing by γ-secretase. We also found that γ-secretase released tri- and tetrapeptides successively from αCTF substrate (Takami, unpublished observation). Recently, tripeptide spacing of endoproteolysis on presenilin has been reported [54]. These suggest that successive tri- and tetrapeptide release is a general property of γ-secretase-mediated intramembrane proteolysis.
Yanagida et al. reported that APLP-1 was also cleaved into three Aβ-like peptides [39]. As three ε-like cleavages are known, it is likely that APLP-1 is processed in three product lines by successive tripeptide release [30] (Figure 4). The transmembrane domain of mNotch-1 is cleaved by γ-secretase after ectodomain shedding to liberate NICD (S3 cleavage). NICD containing V1744 was found as the prominent species produced by S3 cleavage [55]. To date, it seems reasonable to suppose that there is a single cleavage site in S3. γ-Secretase also cleaves mNotch-1 at the lumen-membrane boundary (S4 cleavage) to release Notch β peptides (Nβ) (Figure 4) [40, 56, 57]. Fenofibrate treatment increased the proportion of Nβ25, but not that of Nβ21, which implies that Nβ25 and Nβ21 correspond to Aβ42 and Aβ40, respectively [57]. However, it is unlikely that several Nβ product lines exist in Notch processing because of the single S3 site. The production of Nβ species may not fit the tripeptide-processing model (Figure 4). CD44 is cleaved not only at the membrane-cytoplasm boundary, but also at the middle of the transmembrane domain, which results in the release of Aβ-like peptides [33, 41]. Similar to Notch, the processing of the CD44 transmembrane domain may not fit the tripeptide-processing model (Figure 4).
Figure 4.
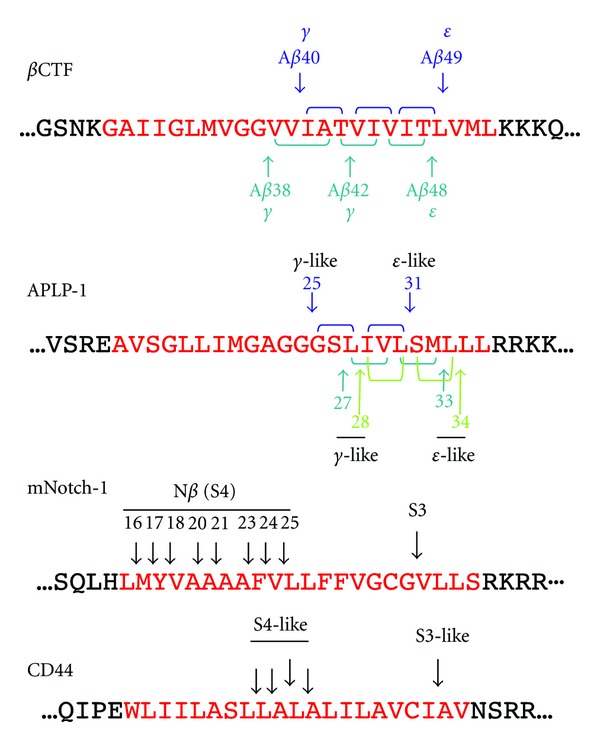
Multiple cleavage sites on the transmembrane domain of γ-secretase substrates. APP [38], APLP-1 [30, 39], mNotch-1 [40], and CD44 [41].
7. Conclusion and Perspectives
The tripeptide hypothesis was confirmed in the processing of the APP transmembrane domain, which accounts for the production of Aβ species. Although the physiological significance of the multiple cleavage of the transmembrane domain is unknown, it is important to illustrate the cleavage mechanisms of other γ-secretase substrates, because the limitation of this stepwise mechanism would help to elucidate the substrate-specific inhibition of Aβ production. As shown in Figure 4, APLP-1 may be cleaved by tripeptide release; however, Notch and CD44 do not fit this processing model [40, 41]. γ-Secretase is widely believed to be a promiscuous protease; however, the cleavage mechanisms of APP and Notch, at least, seem to be different (Figure 4), which indicates that γ-secretase distinguishes substrates during proteolysis. Perhaps absence of helix breaker glycine residues in mid-portion of transmembrane domain allows multiple S4 cleavages even after single S3 cleavage in Notch. From this point of view, uncovering the mechanisms underlying γ-secretase-dependent cleavage offers a basis for new therapeutic approaches that are aimed at substrate-specific Aβ inhibition.
Acknowledgments
The authors thank Nobuto Kakuda for critical comments on this paper. Work in the authors' laboratories is supported in part by the Core Research for Evolutional Science and Technology of JST and by MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2012–2016.
Abbreviations
- Aβ:
Amyloid β protein
- AICD:
APP intracellular domain
- APP:
Amyloid precursor protein
- βCTF:
Carboxyl terminal fragment of APP
- DAPT:
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester
- FAD:
Familial Alzheimer's disease
- LC-MS/MS:
Liquid chromatography-tandem mass spectrometry
- Nβ:
Notch β peptide
- NICD:
Notch intracellular domain
- PS:
Presenilin.
References
- 1.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 2.Citron M, Teplow DB, Selkoe DJ. Generation of amyloid β protein from its precursor is sequence specific. Neuron. 1995;14(3):661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- 3.Vassar R, Bennett BD, Babu-Khan S, et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 4.Pinnix I, Musunuru U, Tun H, et al. A novel γ-secretase assay based on detection of the putative C-terminal fragment-γ of amyloid β protein precursor. Journal of Biological Chemistry. 2001;276(1):481–487. doi: 10.1074/jbc.M005968200. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiological Reviews. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 6.De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 7.De Strooper B, König G. Alzheimer's disease. A firm base for drug development. Nature. 1999;402(6761):471–472. doi: 10.1038/44973. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398(6727):513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 9.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of notch in drosophila. Nature. 1999;398(6727):522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 10.Yu G, Nishimura M, Arawaka S, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 2000;407(6800):48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 11.Goutte C. Genetics leads the way to the accomplices of presenilins. Developmental Cell. 2002;3(1):6–7. doi: 10.1016/s1534-5807(02)00213-7. [DOI] [PubMed] [Google Scholar]
- 12.Francis R, McGrath G, Zhang J, et al. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Developmental Cell. 2002;3(1):85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 13.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ-secretase activity. Nature Cell Biology. 2003;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 14.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takasugi N, Tomita T, Hayashi I, et al. The role of presenilin cofactors in the γ-secratase complex. Nature. 2003;422(6930):438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 16.Beel AJ, Sanders CR. Substrate specificity of γ-secretase and other intramembrane proteases. Cellular and Molecular Life Sciences. 2008;65(9):1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100(4):391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe MS, Kopan R. Intramembrane proteolysis: theme and variations. Science. 2004;305(5687):1119–1123. doi: 10.1126/science.1096187. [DOI] [PubMed] [Google Scholar]
- 19.Tolia A, Horré K, De Strooper B. Transmembrane domain 9 of presenilin determines the dynamic conformation of the catalytic site of γ-secretase. Journal of Biological Chemistry. 2008;283(28):19793–19803. doi: 10.1074/jbc.M802461200. [DOI] [PubMed] [Google Scholar]
- 20.Takagi S, Tominaga A, Sato C, Tomita T, Iwatsubo T. Participation of transmembrane domain 1 of presenilin 1 in the catalytic pore structure of the γ-secretase. Journal of Neuroscience. 2010;30(47):15943–15950. doi: 10.1523/JNEUROSCI.3318-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato C, Takagi S, Tomita T, Iwatsubo T. The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the γ-secretase. Journal of Neuroscience. 2008;28(24):6264–6271. doi: 10.1523/JNEUROSCI.1163-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeo K, Watanabe N, Tomita T, Iwatsubo T. Contribution of the γ-secretase subunits to the formation of catalytic pore of presenilin 1 protein. Journal of Biological Chemistry. 2012;287(31):25834–25843. doi: 10.1074/jbc.M111.336347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osenkowski P, Li H, Ye W, et al. Cryoelectron microscopy structure of purified γ-secretase at 12Å resolution. Journal of Molecular Biology. 2009;385(2):642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Citron M, Oltersdorf T, Haass C, et al. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature. 1992;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 25.Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Sweeney D, Gandy SE, Sisodia SS. The profile of soluble amyloid β protein in cultured cell media. Detection and quantification of amyloid β protein and variants by immunoprecipitation-mass spectrometry. Journal of Biological Chemistry. 1996;271(50):31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 27.Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Medicine. 1997;3(1):67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 28.Clarke NJ, Tomlinson AJ, Ohyagi Y, Younkin S, Naylor S. Detection and quantitation of cellularly derived amyloid β peptides by immunoprecipitation-HPLC-MS. FEBS Letters. 1998;430(3):419–423. doi: 10.1016/s0014-5793(98)00706-6. [DOI] [PubMed] [Google Scholar]
- 29.Beher D, Wrigley JDJ, Owens AP, Shearman MS. Generation of C-terminally truncated amyloid-β peptides is dependent on γ-secretase activity. Journal of Neurochemistry. 2002;82(3):563–575. doi: 10.1046/j.1471-4159.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling gamma-secretase-like cleavage of Notch. Journal of Biological Chemistry. 2001;276(38):35235–35238. doi: 10.1074/jbc.C100357200. [DOI] [PubMed] [Google Scholar]
- 31.Sastre M, Steiner H, Fuchs K, et al. Presenilin-dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Reports. 2001;2(9):835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidemann A, Eggert S, Reinhard FBM, et al. A novel ε-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with notch processing. Biochemistry. 2002;41(8):2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto I, Kawano Y, Murakami D, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. Journal of Cell Biology. 2001;155(5):755–762. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HJ, Jung KM, Huang YZ, et al. Presenilin-dependent γ-secretase-like intramembrane cleavage of ErbB4. Journal of Biological Chemistry. 2002;277(8):6318–6323. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- 35.Marambaud P, Shioi J, Serban G, et al. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO Journal. 2002;21(8):1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May P, Krishna Reddy Y, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. Journal of Biological Chemistry. 2002;277(21):18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 37.Ikeuchi T, Sisodia SS. The Notch ligands, Delta1 and Jagged2, are substrates for presenilin-dependent “γ-secretase” cleavage. Journal of Biological Chemistry. 2003;278(10):7751–7754. doi: 10.1074/jbc.C200711200. [DOI] [PubMed] [Google Scholar]
- 38.Takami M, Nagashima Y, Sano Y, et al. γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. Journal of Neuroscience. 2009;29(41):13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagida K, Okochi M, Tagami S, et al. The 28-amino acid form of an APLPl-derived Aβ-like peptide is a surrogate marker for Aβ42 production in the central nervous system. EMBO Molecular Medicine. 2009;1(4):223–235. doi: 10.1002/emmm.200900026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanngren J, Ottervald J, Parpal S, et al. Second generation γ-secretase modulators exhibit different modulation of notch β and Aβ production. Journal of Biological Chemistry. 2012;287(39):32640–32650. doi: 10.1074/jbc.M112.376541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammich S, Okochi M, Takeda M, et al. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Aβ-like peptide. Journal of Biological Chemistry. 2002;277(47):44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 42.Sato T, Dohmae N, Qi Y, et al. Potential link between amyloid beta-protein 42 and C-terminal fragment gamma 49-99 of beta-amyloid precursor protein. Journal of Biological Chemistry. 2003;278(27):24294–20301. doi: 10.1074/jbc.M211161200. [DOI] [PubMed] [Google Scholar]
- 43.Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of β-amyloid precursor protein are processed to amyloid β-proteins 40 and 42. Biochemistry. 2004;43(42):13532–13540. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- 44.Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, et al. Longer forms of amyloid β protein: implications for the mechanism of intramembrane cleavage by γ-secretase. Journal of Neuroscience. 2005;25(2):436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chévez-Gutiérrez L, Bammens L, Benilova I, et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO Journal. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quintero-Monzon O, Martin MM, Fernandez MA, Cappello CA, Osenkowski P, Wolfe MS. Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer's disease-causing presenilin mutations. Biochemistry. 2011;50(42):9023–9035. doi: 10.1021/bi2007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. Journal of Neurochemistry. 2001;76(1):173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhao G, Mao G, Tan J, et al. Identification of a new presenilin-dependent ζ-cleavage site within the transmembrane domain of amyloid precursor protein. Journal of Biological Chemistry. 2004;279(49):50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]
- 49.Zhao G, Cui MZ, Mao G, et al. γ-cleavage is dependent on ζ-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. Journal of Biological Chemistry. 2005;280(45):37689–37697. doi: 10.1074/jbc.M507993200. [DOI] [PubMed] [Google Scholar]
- 50.Sato T, Tanimura Y, Hirotani N, Saido TC, Morishima-Kawashima M, Ihara Y. Blocking the cleavage at midportion between γ- and ε-sites remarkably suppresses the generation of amyloid β-protein. FEBS Letters. 2005;579(13):2907–2912. doi: 10.1016/j.febslet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 51.Vetrivel KS, Cheng H, Lin W, et al. Association of γ-secretase with lipid rafts in post-golgi and endosome membranes. Journal of Biological Chemistry. 2004;279(43):44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yagishita S, Morishima-Kawashima M, Ishiura S, Ihara Y. Aβ46 is processed to Aβ40 and Aβ43, but not to Aβ42, in the low density membrane domains. Journal of Biological Chemistry. 2008;283(2):733–738. doi: 10.1074/jbc.M707103200. [DOI] [PubMed] [Google Scholar]
- 53.Yagishita S, Morishima-Kawashima M, Tanimura Y, Ishiura S, Ihara Y. DAPT-induced intracellular accumulations of longer amyloid β-proteins: further implications for the mechanism of intramembrane cleavage by γ-secretase. Biochemistry. 2006;45(12):3952–3960. doi: 10.1021/bi0521846. [DOI] [PubMed] [Google Scholar]
- 54.Fukumori A, Fluhrer R, Steiner H, Haass C. Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of γ-secretase-mediated intramembrane proteolysis. Journal of Neuroscience. 2010;30(23):7853–7862. doi: 10.1523/JNEUROSCI.1443-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osawa S, Funamoto S, Nobuhara M, et al. Phosphoinositides suppress γ-secretase in both the detergent-soluble and -insoluble states. Journal of Biological Chemistry. 2008;283(28):19283–19292. doi: 10.1074/jbc.M705954200. [DOI] [PubMed] [Google Scholar]
- 56.Okochi M, Steiner H, Fukumori A, et al. Presenilins mediate a dual intramembranous γ-secretase cleavage of Notch-1. EMBO Journal. 2002;21(20):5408–5416. doi: 10.1093/emboj/cdf541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okochi M, Fukumori A, Jiang J, et al. Secretion of the Notch-1 Aβ-like peptide during Notch signaling. Journal of Biological Chemistry. 2006;281(12):7890–7898. doi: 10.1074/jbc.M513250200. [DOI] [PubMed] [Google Scholar]


