Abstract
Epithelia use a unique process called ‘cell extrusion’ to remove cells from a layer, while preserving their barrier function. Specifically, a cell destined to die triggers formation of an actin- and myosin-ring in the live neighboring epithelial cells surrounding it, which squeeze the dying cell out. During extrusion, the surrounding cells expand toward one another and meet to fill the gap left by the extruded cell. Recent studies have revealed new roles of extrusion in controlling developmental morphogenesis, maintaining homeostatic cell numbers, and how this process is usurped during bacterial pathogenesis. Here, we review recent advances in new processes that require cell extrusion and the signaling pathways controlling it.
Introduction
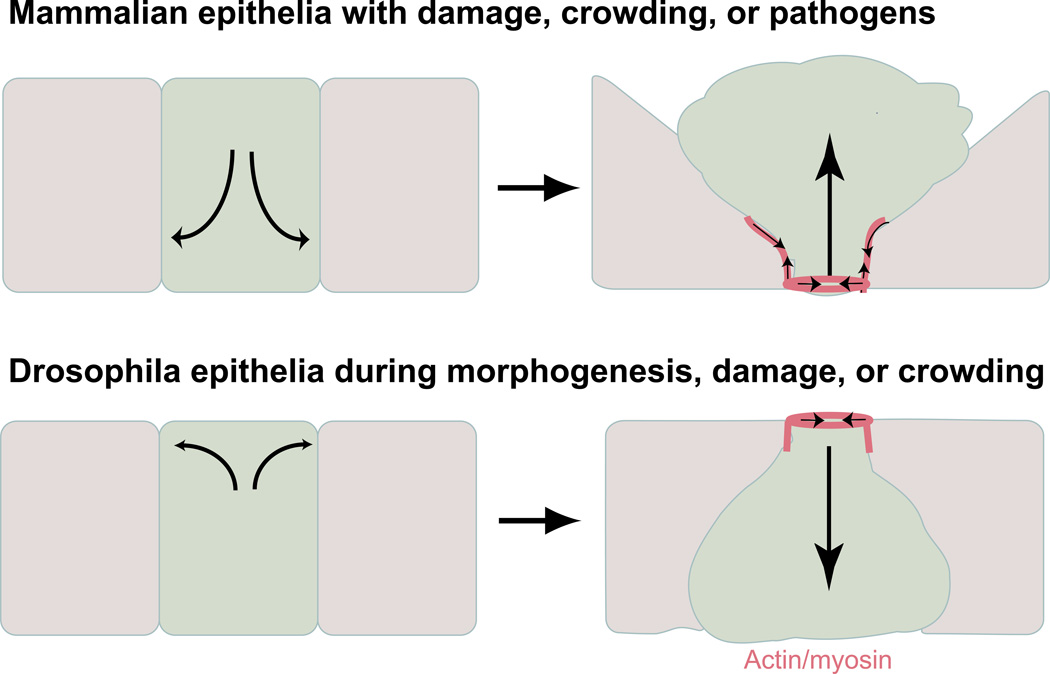
Epithelia made of one or two cell layers encase and protect organs. The cells comprising epithelia are constantly turning over by cell division and death. Cell death, or apoptosis, could compromise the barrier function of the epithelium, but it does not. Instead, epithelia have devised a process called ‘cell extrusion’ to remove cells from a layer, while preserving their barrier function [1]. Specifically, an epithelial cell destined to die triggers formation of an actin- and myosin-ring in the live neighboring cells surrounding it, which then squeeze it out (Figure 1). Extrusion occurs without disrupting epithelial continuity because the surrounding cells constrict and meet to form new multi-cellular junctions below the extruding cell [1–2]. While extrusion clearly functions to maintain epithelial barrier function, its discovery during different developmental morphogenetic events in Drosophila and a variety of vertebrates has led to an understanding that extrusion may be controlling more than just barrier function. While extrusion removes cells apically in most species, cells predominantly extrude basally during Drosophila development, where it is typically referred to as delamination (Figure 1). Here, we discuss new emerging roles for cell extrusion in controlling developmental morphogenesis, maintaining homeostatic cell numbers, and how this process is hijacked during bacterial pathogenesis. Because extrusion plays such an important role in many processes, we also discuss new insights into the signaling pathways that control extrusion.
Figure 1.
Model for apical and basal (delamination) extrusion. Cells can be extruded apically (upper panel) or basally (lower panel) depending on the localization of actin/myosin (dark pink) contraction. Situations where these different types of extrusion occur are also indicated.
Live cell extrusion maintains epithelial homeostasis
Maintenance of epithelial function requires that cell death be exquisitely coupled to cell proliferation. Little was known about how these two processes were linked; yet misregulation of this balance could lead to excess cells or too few cells, which could result in tumor formation or barrier function diseases, respectively. Recently, however, two independent studies demonstrated that overcrowding induces extrusion of live cells to maintain epithelial homeostasis [3••–4••]. Both studies found that during cell turnover in colon epithelia, zebrafish epidermis, and fly notum, very few cells undergo apoptosis within the epithelium, but are instead first pushed out by live cell extrusion. Live cell extrusion occurs at the locations with highest cell densities, such as the fin edges of zebrafish, colon surfaces, and the midline of fly notum. Cell crowding forces result from cell proliferating and migrating from other sites within the epithelium. For instance, in the intestinal epithelium, stem cells divide within the crypts, differentiate, and migrate to the tips of the villi, which become 1.8 times more crowded than crypts [4••]. Individual cells then extrude at the crowded villus tips [5–6] at a rate of approximately 1011 cells per day in the human small intestine [5]. Marinari et al. found that blocking cell growth eliminates the crowding and subsequent delamination, suggesting that crowding forces from proliferation drive delamination [3••].
To test if the overcrowding seen in vivo can directly induce epithelial cell extrusion, Eisenhoffer et al. cultured MDCK cells to confluence on a stretched silicone membrane and then releasing them from stretch [4••]. Using this device, they found that cell crowding triggers extrusion. This also enabled them to dissect the signaling pathway controlling crowd-induced extrusion (referred to below). In doing so, they were able to block homeostatic extrusion in zebrafish larvae, which resulted in formation of epidermal cell masses, suggesting that extrusion is critical for epithelial cell turnover [4••]. Additionally, they found that normally live extruded cells eventually die through a process termed anoikis, or apoptosis due to loss of survival signaling [7–8] but if replated directly were competent to proliferate into a new monolayer [4••]. Similarly, Marinari et al. observed cells dying after their complete removal from the epithelial monolayer in the developing fly. These two studies, demonstrate that cell division and migration within epithelia cause overcrowding, which induce live cells to extrude and die in order to maintain epithelial homeostasis.
Developmental Drosophila morphogenesis requires epithelial cell extrusion
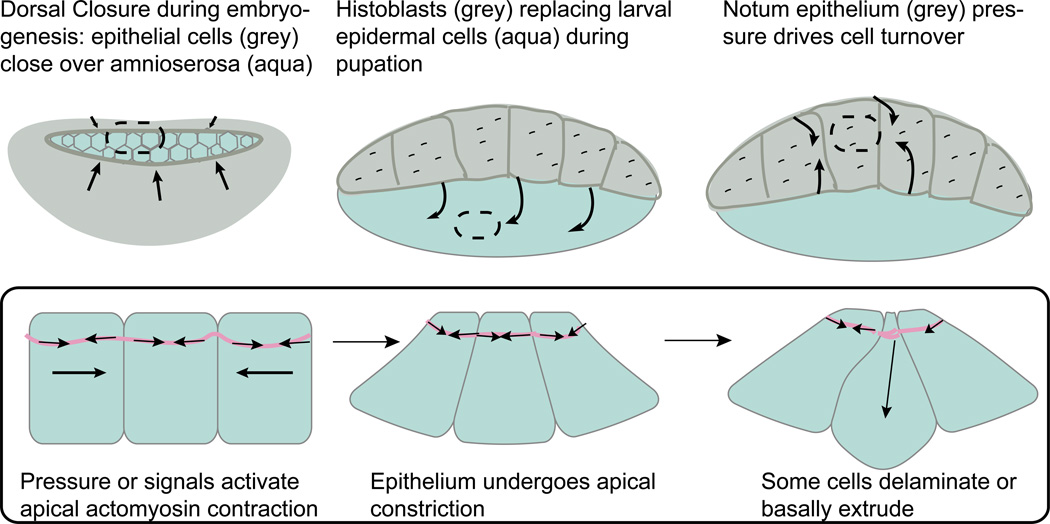
Epithelial cell extrusion plays a key role in several epithelial-based morphogenesis events during Drosophila development. An important process for shaping of the three-dimensional body plan during development is the dynamic replacement of earlier epithelial cell populations with more mature ones [9]. Apoptotic epithelial cell extrusion has been shown to be a driving force for Drosophila epithelial sheet movement and fusion in distinct developmental processes, such as during dorsal closure and development of adult abdominal epidermis [10–11] (Figure 2). Dorsal closure is a process whereby two lateral epithelial cell sheets advance to progressively cover a transient epithelium covering the dorsal side called the amnioserosa (AS). Initially, the lateral epidermis migrates as a sheet to the midline until it forms an eye-shaped opening. As this happens, the whole AS contracts by apically constricting. Simultaneously, cells extrude underneath the epithelium to the interior of the embryo [12]. The combined AS constriction and extrusion helps drive closure of the attached lateral epidermis [11]. AS cell extrusion is estimated to contribute between one third and one half of the net force required to drive dorsal closure [11]. Following dorsal closure, the extruded AS cells die by anoikis and are engulfed by hemocytes, or early macrophages [13].
Figure 2.
Extrusion drives epithelia cell replacement during embryogenesis and pupariation. Insets (below) of cells apically constricting and extruding (delaminating) from oval regions.
Later, during metamorphosis from the larval to pupal stage, another type of epithelial cell replacement occurs. Pupal epidermal cells, histoblasts, replace the larval epidermal cells (LECs) that coat the abdomen. Histoblasts are initially dormant and organized in nests localized around the LECs until pupation when they expand rapidly and fuse to replace LECs, which die and extrude basally [10,14]. Cell sheet replacement requires epithelial cell extrusion of the LECs, as inhibition of apical myosin contraction in these cells results in their retention. Blocking LEC apoptosis slows cell replacement and, interestingly, causes the histoblasts to die and extrude instead [10]. Conversely, blocking histoblast cell division by Dacapo overexpression increases LEC survival, suggesting that cell competition rather than programmed cell death drive extrusion and epithelial cell replacement [10]. Ninov et. al found that LECs secrete Decapentapalegic (Dpp, a TGF-beta/BMP homolog) that recruits histoblasts and keeps the LECs alive until the histoblasts force them out [15•]. Moreover, artificial boundaries of Dpp signaling in Drosophila wing disc epithelia cause live cells to extrude [16–17], as do discrete developmentally induced boundaries of Dpp expression in the fly leg disc [18]. Thus, autocrine and paracrine Dpp signaling may ensure maintenance of a protective barrier as the epidermis undergoes developmental transitions [15•].
When considering these findings on how epithelial cells are replaced, it may be useful to consider that epithelial crowding forces can also control extrusion [3••–4••]. Just as crowding forces control epithelial refinement in the pupal notum by forcing cells to compete for limited space, mechanical forces from movements of new epithelial cell populations during dorsal closure or abdominal cell replacement may also act to extrude the old epithelial cell populations (Figure 2). For instance, when histoblast proliferation is blocked, there is no extrusion of LECs [10], suggesting that normally the new cells similarly force out the old. Additionally, during dorsal closure, higher rates of cell ingression occur closest to the enveloping lateral epidermis, where more force is present [19•] and inhibiting cell death in one tissue can accelerate rates of cell delamination in an adjacent tissue [20]. Both findings support an additional role for force and cell competition controlling epithelial cell replacements during development.
Extrusion facilitates intestinal invasion and dissemination of bacterial pathogens
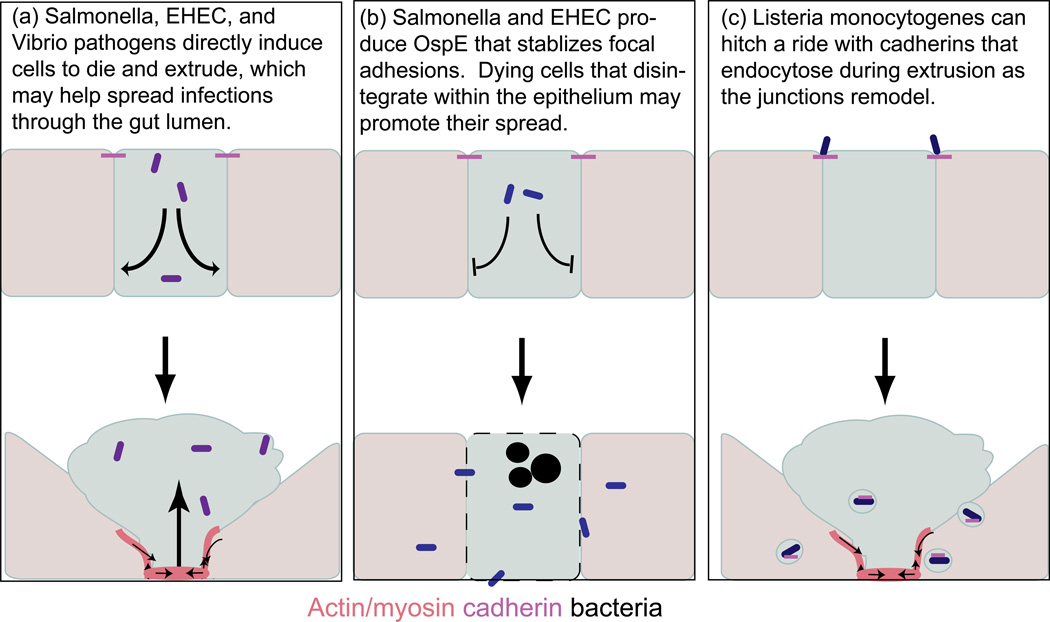
A variety of enteric pathogens have found ways to hijack extrusion to invade host gut epithelium (Figure 3, left panel). Salmonella, Vibrio parahaemolyticus, and enterohemorrhagic E. coli (EHEC) can induce epithelial cell extrusion [21••–23•]. Unlike extrusion induced by apoptotic stimuli or crowding, enteropathogens use a variety of factors to trigger extrusion. Salmonella infection triggers caspase-1activation, which activates both IL-18, a cytokine regulator of inflammation, and extrusion, since inhibiting caspase-1 greatly reduces extrusion rates [21••]. On the other hand, expression of the EHEC protein EspM-2 directly activates RhoA to trigger extrusion in the absence of any apparent cell death [23•]. Alternatively, extrusion of pathogen infected epithelial cells could result indirectly from activating proinflammatory cytokines such Tumor Necrosis Factor, which can also activate apoptotic extrusion [24–26]. Release of Tumor Necrosis Factor or other cytotoxic factors may also cause the extensive extrusion that leads to loss of epithelial barrier function, inflammation, and cavities in the small intestinal epithelium associated with Vibrio parahaemolyticus and Salmonella infections [21••–22•].
Figure 3.
Models showing how bacterial pathogens can highjack extrusion to invade host cells. Different pathogens can either induce (a) or block (b) extrusion, or take advantage of extrusion to enable invasion.
Pathogen-induced cell extrusion could benefit the pathogen or the host. Although inducing extrusion could enable the release of pathogen-infected cells into the lumen to promote their spread [21••], it could also help the host eliminate adherent bacteria. It may be for this reason that both EHEC and Salmonella produce a virulent factor, OspE that inhibits cell shedding by stabilizing focal adhesion by binding integrin-linked kinase [27]. Therefore, these pathogens could essentially trigger apoptosis but prevent extrusion as a way to increase epithelial cell permeability and make it more susceptible to invasion (Figure 3, middle panel). Understanding how these different pathogens promote or inhibit extrusion also gives us hints about how factors like Rho, caspase-1, and integrin-linked kinase may normally regulate extrusion.
For Listeria monocytogenes, extrusion provides an opportunity for cell entry (Figure 3, right panel) [28]. Pentecost et al. showed that L. monocytogenes bind junctions during cell extrusion at the villus tips [28]. L. monocytogenes invasion requires binding of its surface protein Internalin A with host E-cadherin. Because E-cadherins are engaged in cell-cell interactions and, therefore, not exposed in a gut epithelium, L. monocytogenes bind E-cadherins that are transiently exposed as adherens junctions are remodeled during cell extrusion [28]. Entry of this pathogen then takes advantage of E-cadherins endocytosing to invade the cell and proliferate [29••]. These beautiful studies showing how L. monocytogenes can hijack extrusion to gain access to the cell cytoplasm also reveal new information on the mechanism of extrusion. By rapidly recycling adherens junctions through endocytosis, the epithelium can maintain a tight barrier throughout the extrusion process.
Signaling pathways controlling epithelial cell extrusion
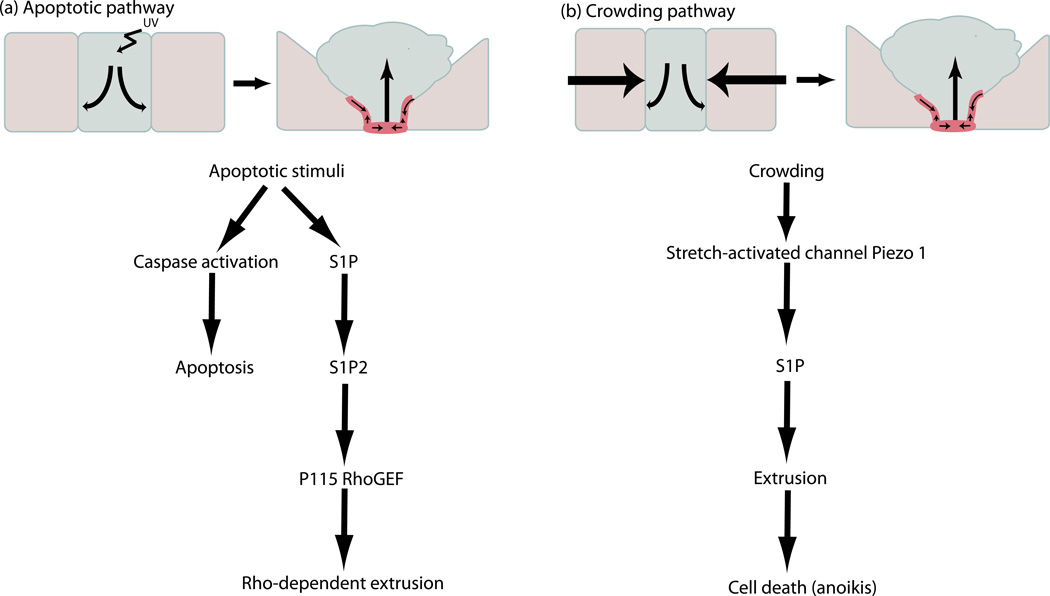
Since extrusion is involved in so many important physiological processes, it is essential to understand signaling pathways that drive cell extrusion. Recent studies in vertebrate systems have revealed that the Sphingosine 1-Phosphate (S1P) pathway controls both apoptotic and live epithelial cell extrusion [4,30••]. S1P is a bioactive sphingolipid that regulates diverse cellular processes including survival, proliferation, and cytoskeleton remodeling in a paracrine/autocrine fashion [31]. Most S1P driven processes are mediated through five specific G-protein coupled receptors (designated S1P1–5) located on the cell surface. Cells destined to extrude produce and release S1P, which binds to S1P2 on the surface of surrounding cells to trigger formation and contraction of an actin/myosin ring [30••]. p115 RhoGEF, a guanine nucleotide exchange factor (GEF) for Rho GTPase, and Rho, both of which are S1P2 effectors, are also required for epithelial cell extrusion [1,32]. While the S1P-S1P2 pathway is critical for both live during homeostasis and dying cell extrusion in response to apoptotic stimuli, different signaling activates these pathways in either case (Figure 4). Generation of S1P during apoptotic cell extrusion requires apoptotic stimuli [26,30••]. In contrast, crowding-induced live cell extrusion requires the stretch activated channel Piezo 1 [4••], an ion channel activated by mechanical deformation of the plasma membrane [33]. Because S1P is a key regulator of macrophage and T-cell migration and function [34], it will be interesting to determine if pathogen-induced extrusion also uses S1P, which could also activate an immune cell response. Additionally, future studies will also need to determine if the S1P signaling pathway controls basal cell delaminations in Drosophila epithelia.
Figure 4.
The signaling pathways that control apoptosis- and crowding-induced extrusion. This schematic overview shows the most recent findings of important factors required for extrusion.
Signaling controlling the direction a cell extrudes
Because tumors typically upregulate survival signaling, the direction a tumor cell extrudes could be important for its fate. Apical extrusion appears to play a pivotal role in controlling cell numbers by removing excess cells and could eliminate tumor cells, even if their survival signaling was upregulated. On the other hand, basal extrusion could enable tumor cells to invade the tissue and potentially initiate metastasis. Interestingly, loss of the tumor suppressor adenomatous polyposis coli (APC) drives cells to extrude predominantly basally, whereas wild type cells typically extrude apically [35]. Mutations or loss of APC severely misregulate microtubule dynamics, which are critical for controlling the direction a cell extrudes. Dynamic microtubules target the microtubule-associated p115 RhoGEF to activate contraction at the base of the cell and extrude it apically [35]. The lack of the microtubule-binding sites in Drosophila APC [36] could account for the basal extrusion in Drosophila epithelia. Future work will need to identify other mechanisms controlling the direction a cell extrudes and if basal extrusion could promote invasion for tumors where APC is mutated.
Concluding remarks
Since its discovery over ten years ago [1], several labs have found new roles for extrusion in driving epithelial cell turnover, morphogenetic changes during development, and bacterial pathogenesis. Future studies should bring new insights into the mechanisms that govern it and how they are misregulated to drive tumor initiation and progression, epithelial barrier function diseases, and enable pathogenic infection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yapeng Gu, Email: Yapeng.gu@hci.utah.edu.
Jody Rosenblatt, Email: Jody.rosenblatt@hci.utah.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 2.Corfe BM, Dive C, Garrod DR. Changes in intercellular junctions during apoptosis precede nuclear condensation or phosphatidylserine exposure on the cell surface. Cell Death Differ. 2000;7:234–235. doi: 10.1038/sj.cdd.4400634. [DOI] [PubMed] [Google Scholar]
- 3. Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–545. doi: 10.1038/nature10984. This study shows that live cells are extruded from Drosophila notum to maintain constant cell numbers following crowding from growth of other cells within the same epithelium.
- 4. Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. The authors demonstrate that overcrowding induce extrusion of live cells to maintain epithelial homeostasis. They also find that crowding activates extrusion through the Piezo-1 stretch-activated channel.
- 5.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 6.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 7.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 9.Martin P, Wood W. Epithelial fusions in the embryo. Curr Opin Cell Biol. 2002;14:569–574. doi: 10.1016/s0955-0674(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 10.Ninov N, Chiarelli DA, Martin-Blanco E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development. 2007;134:367–379. doi: 10.1242/dev.02728. [DOI] [PubMed] [Google Scholar]
- 11.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed BH, Wilk R, Schock F, Lipshitz HD. Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr Biol. 2004;14:372–380. doi: 10.1016/j.cub.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Madhavan MM, Madhavan K. Morphogenesis of the epidermis of adult abdomen of Drosophila. J Embryol Exp Morphol. 1980;60:1–31. [PubMed] [Google Scholar]
- 15. Ninov N, Menezes-Cabral S, Prat-Rojo C, Manjon C, Weiss A, Pyrowolakis G, Affolter M, Martin-Blanco E. Dpp signaling directs cell motility and invasiveness during epithelial morphogenesis. Curr Biol. 2010;20:513–520. doi: 10.1016/j.cub.2010.01.063. The authors demonstrate that Dpp secreted from LECs stimulates dormant histoblasts to expand and migrant to replace LECs while simultaneously keeping the LECs alive until the histoblasts force them out.
- 16.Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- 17.Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- 18.Manjon C, Sanchez-Herrero E, Suzanne M. Sharp boundaries of Dpp signalling trigger local cell death required for Drosophila leg morphogenesis. Nat Cell Biol. 2007;9:57–63. doi: 10.1038/ncb1518. [DOI] [PubMed] [Google Scholar]
- 19. Sokolow A, Toyama Y, Kiehart DP, Edwards GS. Cell ingression and apical shape oscillations during dorsal closure in Drosophila. Biophys J. 2012;102:969–979. doi: 10.1016/j.bpj.2012.01.027. The authors present a mathematical model for forces that drive cell ingression during dorsal closure.
- 20.Muliyil S, Krishnakumar P, Narasimha M. Spatial, temporal and molecular hierarchies in the link between death, delamination and dorsal closure. Development. 2011;138:3043–3054. doi: 10.1242/dev.060731. [DOI] [PubMed] [Google Scholar]
- 21. Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci U S A. 2010;107:17733–17738. doi: 10.1073/pnas.1006098107. This study shows that Salmonella induces extrusion of infected epithelial cells into the lumen, which may allow Salmonella to infect secondary cells rapidly and contributes to host-host transmission
- 22. Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, Davis BM, Bronson RT, Waldor MK. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002593. e1002593. This paper shows that Vibrio parahaemolyticus induce re-distribution of cytoskeletal and tight junction proteins and extrusion of infected epithelial cells, which contributes to loss of epithelial barrier function, inflammation, and cavities in the small intestinal epithelium.
- 23. Simovitch M, Sason H, Cohen S, Zahavi EE, Melamed-Book N, Weiss A, Aroeti B, Rosenshine I. EspM inhibits pedestal formation by enterohaemorrhagic Escherichia coli and enteropathogenic E. coli and disrupts the architecture of a polarized epithelial monolayer. Cell Microbiol. 2010;12:489–505. doi: 10.1111/j.1462-5822.2009.01410.x. This paper shows that EHEC pathogens can trigger extrusion directly with a bacterial protein EspM that activates RhoA.
- 24.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 25.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218. e1201–e1202. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade D, Rosenblatt J. Apoptotic regulation of epithelial cellular extrusion. Apoptosis. 2011;16:491–501. doi: 10.1007/s10495-011-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, Koyama T, Nagai S, Lange A, Fassler R, Sasakawa C. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 2009;459:578–582. doi: 10.1038/nature07952. [DOI] [PubMed] [Google Scholar]
- 28.Pentecost M, Otto G, Theriot JA, Amieva MR. Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog. 2006;2:e3. doi: 10.1371/journal.ppat.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pentecost M, Kumaran J, Ghosh P, Amieva MR. Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000900. e1000900. This paper follows previous findings by the Amieva group that Listeria invade at sites of extrusion. Here, they show that binding endocytosing E-cadherin enables Listeria invasion.
- 30. Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol. 2011;193:667–676. doi: 10.1083/jcb.201010075. This paper shows that apoptotic epithelial cells produce and release S1P, which binds to the S1P receptor 2 in surrounding cells. Activation of this receptor triggers formation and contraction of an actin-myosin ring that extrude the S1P-producing cell.
- 31.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slattum G, McGee KM, Rosenblatt J. P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J Cell Biol. 2009;186:693–702. doi: 10.1083/jcb.200903079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell. 2011;22:3962–3970. doi: 10.1091/mbc.E11-05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]