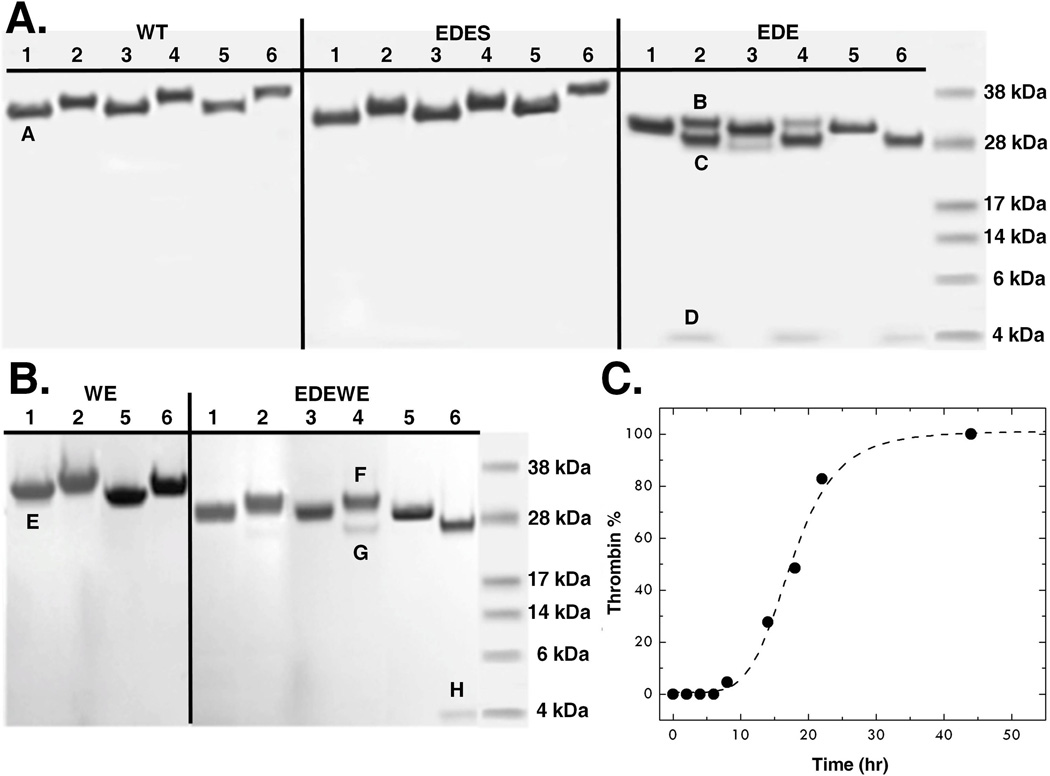
Figure 4. Auto-activation of prethrombin-2.
(A) The prethrombin-2 mutant E14eA/D14lA/E18A (EDE) shows evidence of auto-activation, which is not seen in the wild-type (WT) and is selectively abrogated by the additional mutation S195A (EDES). After heparin-sepharose purification, the concentration of each protein was adjusted to 0.27 mg/ml and auto-activation was followed at room temperature for 0 (lanes 1, 2), 4 (lanes 3, 4) and 90 (lanes 5, 6) hr. (B) Auto-activation is also observed when the E14eA/D14lA/E18A mutation is introduced in the prethrombin-2 mutant W215A/E217A (WE) to yield the construct E14eA/D14lA/E18A/W215A/E217A (EDEWE). In this case, the concentration was adjusted to 3 mg/ml and the reaction was followed at room temperature for 0 (lanes 1, 2), 3 (lanes 3, 4) and 7 (lanes 5, 6) days. No evidence of auto-activation is detected for WE over the same time scale. Samples were analyzed under non-reducing (lanes 1, 3, 5) and reducing (lanes 2, 4, 6) conditions. In the case of EDE and EDEWE, the two bands pertaining to the A and B chains of the mature enzyme are easily detected under reducing conditions and conversion to thrombin is complete after 90 h or 7 days, respectively. The chemical identity of the A and B chains was confirmed by N-terminal sequencing. Bands in the gel are labeled as follows: A and E mapped to N-terminal sequence GRGSE and refer to prethrombin-2 constructs with the T7tag from the expression vector partially cleaved and then processed during E. coli expression as reported (57, 58); B and F mapped to N-terminal sequence TFGSG and refer to prethrombin-2 with a single N-terminus starting at T1h; C and G mapped to N-terminal sequence IVAGS and refer to the B chain of thrombin with the N-terminus I16 and the mutation E18A introduced in the EDE and EDEWE constructs; D and H mapped to N-terminal sequence TFGSG and refer to the A chain of thrombin with the N-terminus T1h.