Abstract
Evidence has accumulated that emotion recognition performance varies with menstrual cycle phase. However, according to some empathy models, facial affect recognition constitutes only one component of empathic behavior, besides emotional perspective taking and affective responsiveness. It remains unclear whether menstrual cycle phase and thus estradiol and progesterone levels are also associated with the two other empathy constructs.
Therefore, we investigated 40 healthy right-handed females, 20 during their follicular phase and 20 during their midluteal phase and compared their performance in three tasks tapping the empathic components as well as self-report data. Salivary hormone levels were obtained and correlated with performance parameters. Subjects were matched for age and education and did not differ in neuropsychological function. Analysis of empathy performance revealed a significant effect of phase in emotion recognition, showing higher accuracy in the follicular group. Regarding affective responsiveness, we observed a significant difference in reaction times, with faster responses for sad and angry stimuli in the midluteal group. No significant group difference emerged for emotional perspective taking. Furthermore, significant correlations between progesterone levels and emotion recognition accuracy and affective responsiveness emerged only in the luteal group. However, groups did not differ in self-reported empathy.
Our results indicate that menstrual cycle phase and thus ovarian hormone concentration are differentially related to empathic behavior, particularly emotion recognition and responsiveness to negative situations, with progesterone covarying with both in the luteal phase.
Keywords: Empathy, Emotion recognition, Perspective taking, Affective responsiveness, Menstrual cycle, Progesterone, Estrogen, Social interaction
Highlights
► First study to investigate the relationship between menstrual cycle and a broader array of empathic abilities. ► Females during the follicular phase showed better emotion recognition performance. ► Females in the luteal phase showed faster responses when asked to imagine a sad or angry situation. ► Emotional perspective taking performance did not differ between the two phase groups. ► Progesterone correlated with emotion recognition and affective responsiveness in the luteal phase.
Introduction
Behavioral evidence documents that sex hormone concentration affects cognition, emotion and nonverbal behavior, thus a broad spectrum of human behavior, across the whole lifespan starting within the first trimester of pregnancy (for reviews see Boulware et al., 2011; Hines, 2010; Vincent and Tracey, 2010). Concerning emotional abilities in females, previous studies mostly focused on the impact of menstrual cycle phase on facial emotion processing: Pearson and Lewis (2005) reported a significant positive association of estrogen levels with fear recognition, with highest accuracy during the preovulatory phase when estrogen levels are high. Conway et al. (2007) demonstrated that progesterone levels in healthy female subjects are related to intensity ratings of disgusted and fearful but not happy faces. Thus, the authors assume that elevated progesterone levels are associated with increased sensitivity to facial cues carrying sources of threat or contagion. Our preceding findings (Derntl et al., 2008a,b) showed a significant difference in emotion recognition performance across the menstrual cycle, with higher accuracy during the follicular phase which was further supported by a significant negative correlation of progesterone levels with recognition accuracy. Notably, analysis of error tendencies strongly corroborated results by Conway et al. (2007) as we observed statistically significant higher recognition errors for anger and disgust during the luteal phase, thus further supporting the assumption that raised progesterone levels bias behavioral tendencies towards threatening stimuli with the possible aim of protection from any source of threat or danger (e.g., illness). Moreover, Guapo et al. (2009) reported a significant difference in the identification of anger and sadness between females at three different stages of the menstrual cycle, again with better performance during the follicular phase. Authors also observed a significantly negative correlation between estrogen levels and recognition performance for angry male faces, while no significant association between progesterone and performance was mentioned. Thus, previous research showed that performance in facial affect recognition is modulated by menstrual cycle phase. However, studies investigating a broader spectrum of emotional abilities such as empathy are missing.
Empathy, the ability to infer and share another's internal emotional states, is a multidimensional phenomenon. Due to the complexity of the construct, empathy has various definitions (e.g., Preston and de Waal, 2002; Singer and Lamm, 2009; Walter, 2012), but most models differentiate between cognitive and affective empathy. Cognitive empathy refers to the ability to understand the feelings of others. It is very closely related to theory of mind (ToM), i.e. the ability to represent and understand the mental states of others in general. Mentalizing about affective states of others is also called affective theory of mind—which is more or less synonymous with cognitive empathy. Affective empathy refers to an affective response that is elicited by the perceived, imagined, or inferred affective state of another person, which has also been called affect sharing or affective responsiveness. In their comprehensive model, Decety and Jackson (2004) postulate that at least three core components of empathy can be derived: (1) recognition of emotions in oneself and others via facial expressions, speech or behavior, (2) an affective component. i.e. the ability to experience similar emotions as others while being conscious that this is the simulation of the emotional feeling and it is not one's own emotion (affective responsiveness), and (3) a cognitive component, i.e. to take the perspective of another person, though the distinction between self and other remains intact (emotional perspective taking).
As shown above several studies investigated emotion recognition across the menstrual cycle and observed significant group differences, but no such study exists for emotional perspective taking or affective responsiveness.
Hence, we examined whether menstrual cycle phase and sex hormone levels (estradiol and progesterone) are associated with the three core components of empathy by comparing females during the early follicular phase with females during the midluteal phase of their menstrual cycle. Our aim was to analyze whether menstrual cycle phase is significantly related to empathy performance, thus higher order emotional abilities. According to previous results (Derntl et al., 2008a,b; Guapo et al., 2009; Pearson and Lewis, 2005) we hypothesized that females during their follicular phase show better emotion recognition performance due to a probably higher social sensitivity even in the early follicular phase (Guapo et al., 2009; Macrae et al., 2002). Moreover, we expected a significant menstrual phase group effect on the other two components, again with better performance of females during the follicular phase.
Materials and methods
Sample
Forty right-handed healthy females aged 19–34 years (mean age 25.3 years, SD = 3.4) participated in the study. When contacted, female participants were asked about their menstrual cycle phase and cycle duration and were then assigned a testing date. Only females who reported regular cycle duration (range: 25–35 days, M = 29.0, SD = 1.8) were included. Twenty females were in their early follicular phase (days 2–5 of menstrual cycle; low estradiol and progesterone levels; FO), and the other 20 were in their midluteal phase (days 18–25 of menstrual cycle; high estradiol and progesterone levels, LU).
Participants were recruited by advertisements at the University of Vienna and the Medical University of Vienna, Austria. The female participants were screened for history of any psychiatric or mental disorder by using the German version of the structured interview of DSM IV (SCID; Wittchen et al., 1997). Written informed consent was obtained from all subjects prior to the examination.
Saliva samples
To obtain actual estradiol and progesterone levels saliva samples were collected on the day of testing. Saliva samples have been shown to have great potential for studying ovarian hormone levels as a reliable, feasible and non-invasive method (e.g., Gandara et al., 2007). Due to the circadian secretion pattern of steroid hormones all samples were collected between 10 am and 12 am. Furthermore, to exclude external hormone influences only females without oral contraceptives or any hormone treatment were included. Before we started obtaining saliva samples we asked participants to wash out their mouth with water. In order to obtain more representative measures, we collected saliva samples for each hormone every half hour, thus we collected three samples per hormone in total (multiple sampling). For data analysis, the values were then averaged across the three samples for each participant and these mean values were used for further analyses. Participants were instructed to fill a small plastic vial with at least 1.5 ml saliva (max. 3 ml) using a straw to stimulate saliva flow. Participants' collection vials were sealed after each collection and frozen immediately in accordance with previous research on sample storage (see Gröschl, 2008).
Saliva samples were analyzed by the European Institute for Salivary Analysis (Swiss Health Med, Aying, Germany) using an enzyme-linked immunoassay method from DRG (DRG Marburg, Germany; Salivary Estradiol ELISA SLV-4188 and DRG Salivary Progesterone ELISA SLV-2931). Analytical sensitivities (confidence interval 95%) were 0.4 pg/ml (Estradiol) and 3.9 pg/ml (Progesterone). For estradiol, intra- and interassay coefficients were 3.8% and 2.6% respectively. For Progesterone, intra- and interassay coefficients were 7.7% and 5.3%, respectively.
For details on hormone concentration and sociodemographic data see Table 1.
Table 1.
Demographic information showing mean values and standard deviation in parentheses. Groups differed significantly in their progesterone and estradiol levels (p-values in bold) as expected but had similar age and years of education.
| Early follicular n = 20 |
Midluteal n = 17 |
p-Value | |
|---|---|---|---|
| Age (years) | 25.1 (3.3) | 26.0 (3.3) | .39 |
| Education (years) | 18.3 (2.2) | 18.4 (1.7) | .81 |
| Estradiol (pg/ml) | 2.6 (1.3) | 3.7 (1.2) | .02 |
| Progesterone (pg/ml) | 61.1 (17.9) | 221.5 (109.9) | < .001 |
Material
Emotion recognition
We used the Vienna Emotion Recognition Task—Shortversion (VERT-K) that consisted of 36 facial expressions of five basic emotions (anger, disgust, fear, happiness, and sadness) as well as neutral expressions taken from a validated stimulus set (Gur et al., 2002). The instruction was to recognize the emotion depicted and a forced-choice answering format with all emotions and neutral was listed on the right side of the screen. The stimuli were balanced with respect to gender, age, intensity, valence, and brightness. All actors were Caucasians and appeared only once. Facial expressions were presented maximally for 5 s and manual response triggered the next stimulus. Scores were calculated as the percent of items judged correctly and reaction times were assessed. The emotion recognition task is described in more detail elsewhere (Derntl et al., 2008a) and has been used in several studies of our group (e.g., Derntl et al., 2008a, 2009, 2011; Seidel et al., 2010a,b).
Emotional perspective taking
Participants viewed 60 pictures each presented for 4 s depicting scenes showing two Caucasians involved in social interaction thereby portraying five basic emotions and neutral scenes (10 stimuli per condition). The face of one person was masked and participants were asked to infer the corresponding emotional expression of the masked face that would fit the emotional situation. Responses were made by selecting between two different emotional facial expressions or a neutral expression presented after each scene. Facial alternatives were taken from the same stimulus set described above. One option was correct and the other was selected at random from all other choices. Again, scores were calculated as percent of items judged correctly and reaction times were assessed.
Affective responsiveness
We presented 150 short written sentences describing real-life situations, which induced basic emotions (the same emotions as described above), and situations that were emotionally neutral (25 stimuli per condition). Participants were asked to imagine how they would feel if they were experiencing those situations. Stimuli were presented for 4 s and response format was the same as for emotional perspective taking. Response format was kept maximally similar across tasks allowing comparisons between tasks, i.e. differences could be traced back to different task requirements not to different response formats. Similar to both other tasks, percent correct and reaction times were calculated and used for data analysis.
Prior to the study, the stimuli of the emotional perspective taking and the affective responsiveness tasks were rated by 30 healthy adults and only those stimuli correctly identified by over 70% of the sample were included in the main study. Additionally, the emotional perspective taking and affective responsiveness tasks have been used in clinical studies (e.g., Derntl et al., 2009; Seidel et al., 2010a,b) and neuroimaging studies (Derntl et al., 2010, 2012).
To control for order effects, the tasks were presented in counterbalanced order. Regarding behavioral performance, no gender differences for the three tasks were observed in our previous studies.
Empathy questionnaires and neurocognitive tests
Three questionnaires measuring cognitive and affective empathy were administered: the Questionnaire Measure of Emotional Empathy (QMEE, Mehrabian and Epstein, 1972), the German Questionnaire for Assessment of Empathy, Prosociality and Aggression (FEPAA, Lukesch, 2006) and the German version of the Interpersonal Reactivity Index (IRI, Davis, 1983; German version: Saarbrückener Persönlichkeitsfragebogen, SPF, Paulus, 2009). In a previous study on gender differences in empathic abilities, we observed a significant effect for the SPF only, with females describing themselves as more empathic than males (Derntl et al., 2010), thereby supporting existing literature (e.g., Rueckert and Naybar, 2008). However, for both other self-report measures (QMEE, FEPAA), no significant gender difference emerged.
Moreover, to assess neuropsychological functioning, all participants completed tests tapping crystallized verbal intelligence (Mehrfachwahl-Wortschatz-Test, MWT-B, Lehrl, 1996) and executive functions (Trail-Making-Test, TMT-A/-B, Reitan, 1956).
Data analysis
Statistical analyses were performed using SPSS 17.0 and level of significance was set at p = .05. Mean percent correct and reaction times were analyzed for each empathy task using repeated-measures ANOVAs with emotion as within-subject factor and group (FO vs. LU) as between-subject factors. For significant effects partial-eta squares are listed as estimates of effect size. In cases of violations of sphericity, statistical tests involving the emotion factor employed Greenhouse–Geisser correction. All post hoc results were Bonferroni corrected.
Group differences in the empathy questionnaires and the neurocognitive tests were assessed using two sample t-tests. For significant differences Cohen's d are listed as estimates of effect size.
Pearson correlations (two-tailed) between accuracy measures of the empathy paradigms and self-report measures (SPF empathy score, FEPAA empathy score, and QMEE) were computed.
Since progesterone levels were not normally distributed as tested with the Kolmogorov–Smirnov test (FO group: p = .04, LU group: p = .32), we transformed the values taking the square root, which is an adequate tool to apply to right skewed data (Bortz, 1999). The transformed progesterone values then were normally distributed (FO group: p = .24, LU group: p = .50) and thus were entered into further analyses. Pearson correlations (two-tailed) were calculated to investigate the association of female sex hormone levels with empathic performance and self-report measures for each group separately. To adjust for significant inter-hormonal correlations additional partial correlations were calculated, controlling for estradiol/progesterone influence on the correlations between performance and hormone levels, respectively. Moreover, estradiol/progesterone ratio was calculated and entered in the correlation analyses.
Results
Three females during their luteal phase were excluded since their progesterone and estradiol levels were out of range for luteal phase. Hence, final analysis was performed for 20 follicular and 17 luteal females.
Females of the two groups (FO vs. LU) did not differ in their age (t(35) = − 0.87, p = .39), and education (t(35) = − 0.25, p = .808), nor in the duration of their menses (t(35) = 1.04, p = .31). However, FO and LU differed in their estradiol (t(35) = − 2.36, p = .02) and progesterone levels (t(35) = − 6.72, p < .001), with higher values in the LU group, thus indicating that their hormonal profiles differed significantly.
Empathy tasks
Emotion recognition
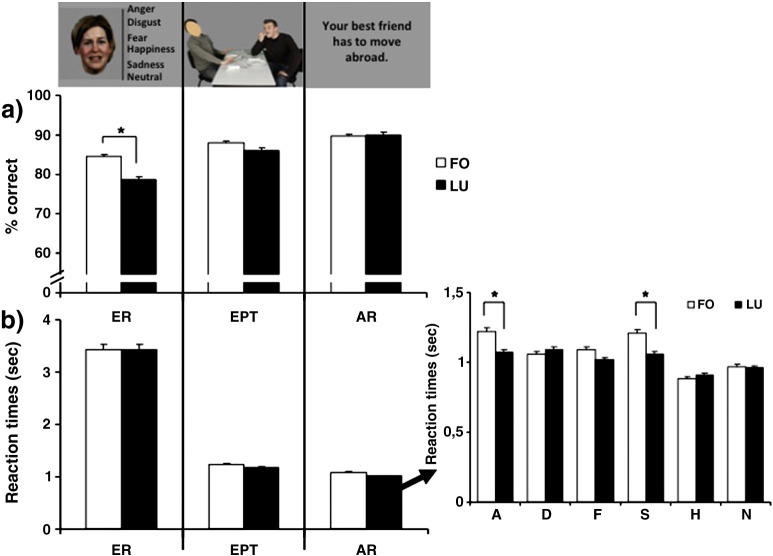
For accuracy, the repeated measures ANOVA revealed a significant emotion effect (F(3.80,133.11) = 12.73, p < .001, part eta sq = .27) and a significant group effect (F(1,35) = 4.33, p = .04, part-eta sq = .11), with higher accuracy in the FO group. However, no significant emotion-by-group interaction (F(3.80,133.11) = 1.68, p = .16) occurred. Post hoc analysis of the significant emotion effect indicated that happy expressions were recognized significantly better than sad, disgust, and fear (all p < .001), and neutral expressions (p = .02) but no difference emerged to angry faces (p = .08).
For reaction times, analysis revealed a significant emotion effect (F(2.11,69.72) = 10.09, p < .001, partial eta sq. = .23), but no significant group effect (F(1,35) = 1.02, p = .32) and no significant emotion-by-group interaction (F(2.11,69.72) = 0.53, p = .60). Post hoc analysis of the significant emotion effect demonstrated fastest reaction times for happy faces (happy vs. sad: p = .01, happy vs. anger: p = .002, happy vs. fear/disgust: p < .001; happy vs. neutral: p = .04) followed by neutral, anger, disgust, sadness, and fear.
Emotional perspective taking
Analysis of accuracy revealed a significant emotion effect (F(3.74, 131.09) = 23.24, p < .001, part-eta sq = .40), but no significant group effect (F(1,35) = 2.11, p = .16), nor a significant emotion-by-group interaction (F(3.74, 131.09) = 0.47, p = .75). Post hoc analysis of the significant emotion effect showed highest accuracy for happy conditions (happy vs. disgust/sad/anger/fear: p < .001; happy vs. neutral: p = .01) followed by neutral, sadness, anger, disgust and fear conditions.
Analysis of reaction times revealed a significant emotion effect (F(5,175) = 20.05, p < .001, partial eta sq. = .36), no group effect (F(1,35) = 2.07, p = .16) and no significant emotion-by-group interaction (F(5,175) = 0.98, p = .43). Post hoc analysis of the significant emotion effect yielded fastest times for happy conditions (happy vs. anger/disgust/fear/neutral/sad: p < .001) followed by neutral, anger, sadness, disgust, and fear conditions.
Affective responsiveness
For accuracy, repeated measures analysis demonstrated a significant emotion effect (F(3.55, 124.14) = 35.23, p < .001, part-eta sq = .50), but no significant group effect (F(1,35) = 0.28, p = .60) and no significant emotion-by-group interaction (F(3.55, 124.14) = 0.76, p = .54). Post hoc analysis of the significant emotion effect demonstrated best performance again for happy conditions (happy vs. anger/disgust/fear/sad: p < .001; happy vs. neutral: p = .01), followed by neutral, fear, disgust, sadness, and anger conditions.
Analysis of reaction times showed a significant emotion effect (F(3.66,128.08) = 30.30, p < .001, partial eta sq. = .46), no significant group effect (F(1,35) = 2.58, p = .12), but a significant emotion-by-group interaction (F(3.66,128.08) = 7.94, p < .001, partial eta sq. = .19). Post hoc analysis of the significant emotion effect yielded fastest times for happy conditions (happy vs. anger/disgust/fear/sad: p < .001; happy vs. neutral: p = .02) followed by neutral, sadness, disgust, fear, and anger. Disentangling the significant emotion-by-group interaction, post hoc analyses revealed significant group differences for reaction times to sad (p = .01) and angry stimuli (p = .01), always showing faster responses in females during the luteal phase, while no group differences emerged for the other comparisons (fear: p = .06, disgust: p = .53, happy: p = .68; neutral: p = .67).
Fig. 1 illustrates performance parameters (accuracy and reaction times) for the three empathy tasks for both groups and Table 2 lists means and p-values of the various ANOVAs (for the main effect of phase).
Fig. 1.
Recognition accuracy (a) and reaction times (b) with standard error of mean for the separate empathy tasks for both groups (early follicular: n = 20 females, FO; midluteal: n = 17 females, LU). Data analysis yielded a significant group effect for emotion recognition accuracy (p = 0.01), revealing better performance of the females during their early follicular phase marked with an asterisk. Moreover, analysis of reaction times for affective responsiveness revealed significantly faster responses of LU group for sad (p = .02) and angry stimuli (p = .02).
Table 2.
Mean values for accuracy (% correct) and reaction times (in seconds) of the three empathy paradigms for each group and p-values as indicated by the repeated measures ANOVA (main effect group). Groups only differed in emotion recognition accuracy (marked with bold p-value).
| Early follicular n = 20 |
Midluteal n = 17 |
p-value | |
|---|---|---|---|
| Emotion recognition (%) | 84.9% (6.5) | 79.2% (3.9) | .04 |
| Emotion recognition (rt) | 3.4 s (0.8) | 3.5 s (0.7) | .32 |
| Emotional perspective taking (%) | 88.2% (4.5) | 85.4% (7.1) | .16 |
| Emotional perspective taking (rt) | 1.3 s (0.2) | 1.2 s (0.2) | .16 |
| Affective responsiveness (%) | 90.7% (3.9) | 91.2% (2.6) | .60 |
| Affective responsiveness (rt) | 1.0 s (0.2) | 1.0 s (0.1) | .12 |
Empathy questionnaires and neurocognitive tests
Regarding the self-report measures of empathy, analysis of group effects revealed no differences for any of the self-report measures (QMEE: t(35) = 1.16, p = .26; SPF (fantasy: t(35) = 0.66, p = .51; perspective taking: t(35) = 0.30, p = .77; empathic concern: t(35) = − 1.24, p = .22; personal distress: t(35) = − 0.11, p = .91; empathy: t(35) = − 0.74, p = .46); FEPAA: t(35) = − 0.23, p = .82).
Moreover, regarding neurocognitive performance, groups did not differ in verbal crystallized intelligence (t(35) = 0.32, p = .75), nor in executive functions (TMT-A, t(35) = 0.70, p = .49; TMT-B, t(35) = − 0.96, p = .34).
Mean values of the questionnaire data and neurocognitive tests are given in Table 3.
Table 3.
Neurocognitive and self-report data showing mean values and standard deviation in parentheses. Groups showed similar neurocognitive performance and similar self-reports in empathy questionnaires.
| Early follicular n = 20 |
Midluteal n = 17 |
p-Value | |
|---|---|---|---|
| MWT-B (raw score) | 28.2 (3.6) | 27.7 (3.1) | .75 |
| TMT-A (s) | 19.3 (5.6) | 18.2 (5.9) | .49 |
| TMT-B (s) | 34.8 (7.8) | 38.3 (14.3) | .34 |
| SPF empathy | 46.0 (6.3) | 47.5 (5.7) | .46 |
| SPF fantasy | 15.6 (3.4) | 15.2 (2.8) | .91 |
| SPF distress | 10.8 (3.1) | 9.9 (2.3) | .32 |
| SPF perspective taking | 15.4 (2.6) | 15.8 (2.9) | .68 |
| SPF empathic concern | 15.3 (2.4) | 16.4 (2.2) | .12 |
| QMEE | 16.6 (4.2) | 15.2 (2.7) | .26 |
| FEPAA empathy | 21.7 (3.4) | 21.9 (2.6) | .82 |
Note: MWT-B = Mehrfachwahl-Wortschatz-Test-B measures verbal intelligence, TMT-A = Trail-Making-Test A measures information processing speed, and TMT-B = Trail-Making-Test B measures cognitive flexibility; SPF = Saarbrückener Persönlichkeitsfragebogen (German version of the Interpersonal Reactivity Index), QMEE = Questionnaire Measure for Emotional Empathy; FEPAA = German Questionnaire for Assessment of Empathy, Prosociality and Aggression.
Correlation analyses
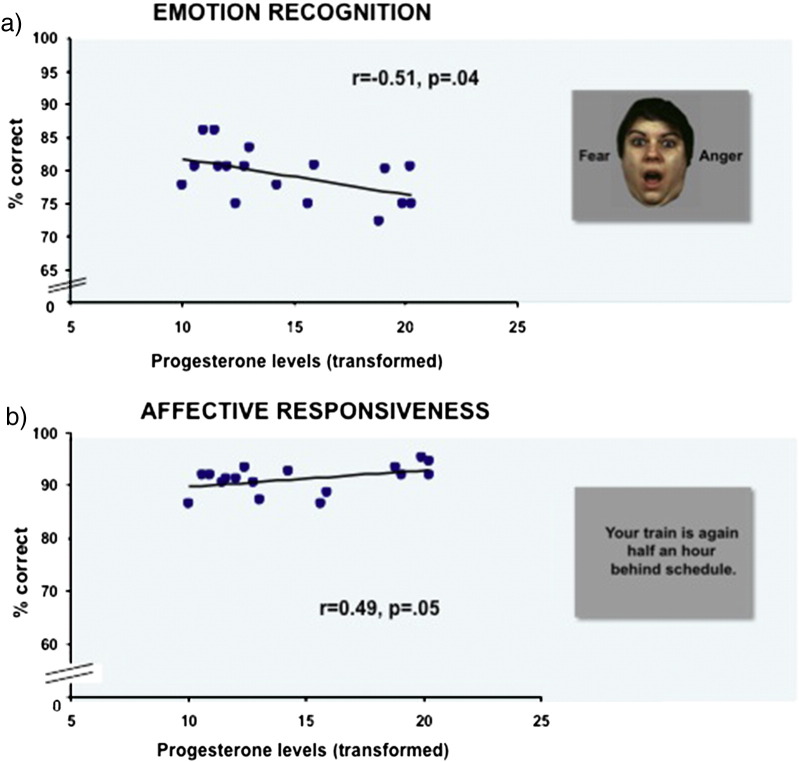
Results of the correlation analyses between hormone levels and performance in empathy tasks (total percent correct and mean reaction times) are listed in Table 4. Correlation analyses for FO revealed no significant association between hormone levels (estradiol or progesterone) and behavioral performance (all p > .13). For LU, a significant negative correlation between progesterone levels and emotion recognition accuracy (r = − 0.51, p = .04) and a significant positive correlation between progesterone levels and affective responsiveness accuracy (r = 0.49, p = .05) emerged.
Table 4.
Results of the correlational analyses (two-tailed) between hormone concentration (estradiol, transformed progesterone, estradiol:progesterone ratio) and performance parameters (% correct and reaction time, rt) for the three empathy tasks separately for each group (FO = follicular group; LU = luteal group). Significant correlations are marked in bold.
| ER % | EPT % | AR % | ER rt | EPT rt | AR rt | ||
|---|---|---|---|---|---|---|---|
| Estradiol | FO | r = 0.17, p = .48 | r = 0.24, p = .31 | r = − 0.13, p = .59 | r = 0.35, p = .13 | r = − 0.28, p = .23 | r = − 0.01, p = .68 |
| LU | r = − 0.30, p = .24 | r = − 0.36, p = .15 | r = − 0.20, p = .44 | r = 0.02, p = .93 | r = 0.16, p = .53 | r = − 0.25, p = .34 | |
| Progesterone | FO | r = − 0.33, p = .16 | r = 0.19, p = .36 | r = 0.09, p = .72 | r = − 0.24, p = .30 | r = − 0.09, p = .71 | r = 0.14, p = .55 |
| LU | r = − 0.51, p = .04 | r = − 0.29, p = .27 | r = 0.49, p = .05 | r = 0.44, p = .08 | r = 0.02, p = .95 | r = − 0.16, p = .54 | |
| E:P ratio | FO | r = 0.26, p = .27 | r = 0.01, p = .96 | r = − 0.19, p = .43 | r = 0.37, p = .13 | r = − 0.01, p = .96 | r = − 0.27, p = .25 |
| LU | r = − 0.08, p = .76 | r = − 0.32, p = .21 | r = − 0.33, p = .18 | r = − 0.20, p = .44 | r = 0.07, p = .79 | r = 0.12, p = .65 |
Note: ER = emotion recognition, EPT = emotional perspective taking, AR = affective responsiveness.
As estradiol and progesterone concentrations are often correlated, we conducted additional partial correlations to control for estradiol influence on the significant progesterone correlations in the LU group. For emotion recognition, partial correlation with progesterone and emotion recognition performance controlling for estradiol revealed a marginally significant association (r = − 0.49, p = .05), while the correlation for affective responsiveness and progesterone (controlled for estradiol) remained similar (r = 0.50, p = .05).
However, additional corollary analyses with the estradiol/progesterone ratio and performance parameters (% correct and reaction times for the three tasks) revealed no significant association between performance and hormone data neither for FO (all p > .13) nor for LU (p = .18).
The significant correlations are depicted in Fig. 2.
Fig. 2.
Correlation analyses revealed significant results for the LU group only: (a) for emotion recognition, a significant negative association between transformed progesterone levels (pg/mL) and accuracy (r = − 0.51, p = .04) occurred. (b) A marginally significant positive correlation between transformed progesterone levels and accuracy in affective responsiveness (r = 0.49, p = .05) emerged.
Correlation analyses between self-report measures and task performance showed a significant positive association between emotion recognition accuracy and QMEE scores (r = 0.38, p = .02), while no other correlation reached significance (all p > .11).
Discussion
In this study we demonstrated a significant association of menstrual cycle phase and ovarian hormone levels with specific components of empathic behavior. More precisely, we observed significantly higher emotion recognition accuracy in females during their early follicular phase (FO group) compared to females during the midluteal phase (LU group), thereby supporting previous results (Derntl et al., 2008a,b) and partly in accordance with existing literature (Guapo et al., 2009; Pearson and Lewis, 2005). Regarding reaction times, data analysis revealed that females in the LU group showed faster responses than the other group in affective responsiveness to negative stimuli, specifically sad and angry situations. Notably, emotion recognition performance showed a significant negative correlation with progesterone levels, indicating better performance during phases of lower progesterone levels, however only in the LU group. Also in the LU group, progesterone levels were positively associated with accuracy in the affective responsiveness task, pointing to better performance with higher progesterone levels. Against our expectations, we did not observe any group difference for emotional perspective taking or self-report empathy.
Taken together, these results suggest a variation in emotion recognition accuracy across the menstrual cycle but only a weaker or even no association with the two other empathy components.
Besides the reported results for empathic abilities, females only differed in their estradiol and progesterone levels, but showed similar neuropsychological performance and demographic variables.
Pearson and Lewis (2005) demonstrated that the ability to correctly recognize fearful faces varies across the menstrual cycle: females during the preovulatory phase (highest estrogen level) showed the highest accuracy for fear. In contrast, Guapo et al. (2009) observed significantly higher recognition accuracy for sad and angry faces in early follicular females compared to midluteal females; females during the ovulatory phase demonstrated better fear recognition than males. Moreover, a significant association of estrogen levels with anger recognition, particularly male anger recognition, emerged. Hence, our results only partly support previous findings and differences might be due to several methodological issues: 1) Pearson and Lewis (2005) only indirectly inferred hormone levels via cycle phase without obtaining hormone samples, thus only a rough estimation of a hormone association was possible, 2) while we and Guapo et al. (2009) compared females during the early follicular phase and midluteal phase, Pearson and Lewis measured females during various stages of the menstrual cycle (menstruation, pre-ovulation, ovulation, and luteal), thus hormonal profiles of the follicular groups are different, 3) similar to our study, Pearson and Lewis as well as Guapo et al. (2009) relied on an explicit emotion recognition paradigm, however, both studies included surprised faces instead of neutral expressions as presented in our study, and 4) Guapo and colleagues presented faces only for 0.5 s and their emotional faces varied in intensities (from 10% to 100%), however, in the end only a total correct score was used for analysis, thus it remains unclear how intensity levels influenced results.
It seems noteworthy that we were able to replicate previous findings from our group, where we also showed better emotion recognition performance in females during the follicular phase compared to the luteal phase for the third time (Derntl et al., 2008a,b), in a new and independent female sample. However, we were not able to replicate the significant correlation of progesterone levels with recognition accuracy in both menstrual cycle groups. Nevertheless, taken our results and those from previous studies, variations in recognition accuracy across the menstrual cycle seem to be a reliable effect, though the underlying causality is less clear.
Results from Macrae et al. (2002) point out that females are more interested in social signals and interactions during the follicular phase when they are fertile and in better mood than during the luteal phase. Accordingly, they might also tend to exert more attention on emotional expressions as a basis for more successful interaction and generally higher social competence which might already be observable in the first days of the follicular phase as suggested by our results and results from Guapo et al. (2009). Moreover, in a prior fMRI study (Derntl et al., 2008b), we showed that amygdala activation varies according to the menstrual cycle phase, with stronger activation during emotion recognition in the follicular phase. Hence, besides differences in social interaction and mood states, differences in the neural correlates might also underlie this finding.
Regarding affective responsiveness, we did not observe any group differences for accuracy. However, we noted a significant difference for reaction times, with faster responses to angry and sad stimuli in the LU group compared to the FO group. Hence, despite similar accuracy, females during the second half of the menstrual cycle demonstrate faster responsiveness and reactivity to certain emotionally negative stimuli. In our affective responsiveness task we asked females to put themselves in a certain situation and then tell us how they would feel. Hence, this is the only self-centered emotional task in our study. Thus, our findings may support results showing that females during the luteal phase are more reactive to social stress (Kirschbaum et al., 1999) and experience more intrusive recollections about negative events than females during the follicular phase (Ferree and Cahill, 2009). Moreover, studies of daily moods reported higher negative moods and depression scores during the luteal phase compared to the follicular phase (Allen et al., 2009; Reed et al., 2008) which might trigger a mood-congruent bias as seen in females suffering from premenstrual dysphoric disorder (PMDD, Rubinow et al., 2007) or more frequently reported in depressed patients (for review see Bourke et al., 2010) and in subjects at high-risk for depression (Watters and Williams, 2011). However, we did not observe any difference in accuracy nor a general facilitation effect or mood-congruent bias in emotion processing irrespective of the task requirements, i.e. better recognition of or faster reaction times to angry/sad faces in the other tasks. Hence, we speculate that this faster responsiveness in the LU group might be emotion-dependent and specific to self-centered tasks where females are asked about their mood or emotional states. Alternatively, and despite unaffected reaction times in both other empathy tasks and neuropsychological tests (e.g. TMT), females of the FO group might struggle with a finer distinction between internal emotional states, reflected in the slower responses to sad and angry stimuli in the affective responsiveness task. Thus, while females during the first half of their menstrual cycle are very sensitive to external emotional cues they might show some difficulties in differentiating specific internal emotions.
To further evaluate these hypotheses, data from larger samples at various stages of the menstrual cycle are needed. Moreover, answering formats presenting all emotions simultaneously would be favorable, enabling a thorough error analysis and thereby may help to shed light on the underlying processes.
For emotional perspective taking, we did not observe any difference across the menstrual cycle phase nor any correlation with hormone concentration and performance parameters (% correct and reaction times). While previous studies addressed the impact of testosterone or oxytocin administration on cognitive empathy, more specifically the ability to infer emotional states from the eye region (testosterone: Van Honk et al., 2011; oxytocin: Domes et al., 2007) or measured (neural) responses of synchronous vs. intrusive mothers (Atzil et al., 2011), this is the first study exploring the association of estradiol and progesterone concentration with emotional perspective taking. Although we expected better performance of females during the first half of the menstrual cycle due to their suggested higher sensitivity for social signals, emotional perspective taking does not seem to be associated with menstrual cycle phase or hormone concentration to the same extent as e.g., emotion recognition. This lack of a significant hormonal association with perspective taking might be due to several reasons 1) we used a simple task design, particularly the response format with only two choices given might not be sensitive enough to detect differences, 2) we did not investigate females during their periovulatory phase where they are most fertile and most interested in social signals (cf. Macrae et al., 2002), thus preventing us to thoroughly investigate the impact of menstrual cycle phase on and the association of estradiol (unopposed by progesterone) with this competency.
Notably, comparable to previous findings from our lab (Derntl et al., 2008a,b) we did not see a significant association of estrogen levels with performance in empathy related tasks but instead our results again show a significant correlation of progesterone with empathic competencies, i.e. facial affect recognition and affective responsiveness, however only in the LU group. Accordingly, Conway et al. (2007) showed that females during their luteal phase with raised progesterone levels judged fearful and disgusted faces with averted gaze as more intense than expressions with direct gaze, indicating that progesterone levels may modulate emotional behavior. It has frequently been reported that mood is more negative during the luteal phase and we speculate that high progesterone levels might be linked to a reduced sensitivity to certain social signals coming from others, such as emotional expressions, whereas mood-congruent or distress oriented stimuli concerning the self elicit increased responsivity (Kirschbaum et al., 1999).
Regarding the influence of progesterone on neural activation during emotion processing, van Wingen et al. (2008) showed that a single dose of progesterone during the early follicular phase led to an increase in amygdala reactivity to threatening faces. Furthermore, in the same study authors report that progesterone increased functional coupling of the amygdala with the medial prefrontal cortex (mPFC), indicating that progesterone influences the communication between the amygdala and mPFC, hence, two key regions regarding emotion processing and empathy. However for emotional memory, decreased amygdala activity during the memorization of happy and neutral faces after a single dose of progesterone was observed (van Wingen et al., 2007). Moreover, Derntl et al. (2008b) report decreased amygdala activation during emotion recognition in females during the luteal phase compared to the follicular phase. Taken together these data suggest that the influence of progesterone on amygdala reactivity is task- and probably dose-dependent (for review see van Wingen et al., 2011). Multiple studies indicate that progesterone and the progesterone derivative allopregnanolone have significant modulatory effects upon neurotransmitter systems involved in the regulation of affect and behavior such as serotonin and noradrenalin (e.g. Bethea et al., 1998; Epperson et al., 1999). Interestingly regarding negative mood, allopregnanolone seems to exert a paradoxical effect mediated via the GABA-A receptor probably causing higher excitability of some brain regions (e.g., Bäckström et al., 2011). Since we did not assess neural activation or experimentally administer progesterone, we can only speculate about the underlying associations. Future neuroimaging studies that explore how progesterone affects the neural correlates of a broader spectrum of social–emotional abilities are mandatory to further highlight the impact of menstrual cycle phase and sex hormone concentration on the neural basis of emotional behavior.
While this study provides new insight into the relation of menstrual cycle phase and thus ovarian hormones with emotional competencies, i.e. empathic behavior, several methodological constraints have to be considered: besides the small sample we did not examine empathic abilities in a longitudinal approach investigating females at various stages during the menstrual cycle to analyze intra-individual differences. However, groups are well matched for various neuropsychological parameters and were carefully screened as well as selected, hence we are convinced that the results presented in this manuscript are representative. Future studies exploring behavioral and neural correlates of empathic abilities and emotional competencies should include females during the periovulatory phase where conception risk is high to better characterize any association of cycle phase and ovarian hormones with emotional behavior.
We did not examine women using hormone contraceptives, since this external hormone intake might alter emotion processing. However, investigating the effect and alterations of oral contraceptives on neural activation and emotional performance would be of high interest, as the number of females taking oral contraceptives is steadily rising and short- as well as long-term effects on neural activation and performance are still unclear (Kurshan and Neill Epperson, 2006). Recently, Pletzer et al. (2010) reported a significant correlation of the volume of the parahippocampal gyrus with duration of oral contraceptive intake. Hence, investigating the effect and alterations of oral contraceptives on cognitive and social–emotional competencies as well as the underlying neural correlates is of high interest.
Due to the fact that we only investigated females, interaction of gender and hormone levels and their association with empathic behavior were not examined. However, investigation of hormonal influences has important implications for the understanding of gender-specific emotional functioning, thus probably helps to further characterize previously reported gender differences in the neural correlates of empathic abilities (e.g., Derntl et al., 2010; Li et al., 2008; Schulte-Rüther et al., 2008).
Conclusions
We conclude that menstrual cycle phase and progesterone concentration are only associated with specific components of empathy and not a broader spectrum of emotional competencies. Despite significantly reduced emotion recognition accuracy, we observed no group difference for perspective taking but faster affective responsiveness to negative stimuli, i.e. angry and sad situations, during the luteal phase. Moreover, emotion recognition accuracy correlated with progesterone levels in the LU group, indicating better performance in females with lower progesterone levels. And, progesterone levels were also positively associated with affective responsiveness, suggesting better performance with higher progesterone levels in the LU group. Thus, our findings suggest the possibility that elevated progesterone levels facilitate responsivity to distressing situations concerning the self i.e. internal emotions, while inhibiting facial affect recognition, thus emotional expressions from others i.e. external emotions. Summarizing, our data underline that emotion recognition performance is better during the first phase of the menstrual cycle and females during the luteal phase may be faster in responsiveness to negative emotions, but no group difference was observed for emotional perspective taking.
Acknowledgments
BD and UH were supported by the German Research Foundation (DFG, IRTG 1328, KFO 112, Ha3202/7-2). BD, IKE and UH were also supported by the Austrian Science Fund (FWF P23533). UH was further supported by the Interdisciplinary Centre for Clinical Research (IZKF) within the Faculty of Medicine at the RWTH Aachen University (TV N70, VV N68-j), the Federal Ministry of Education and Research (BMBF: FKZ 01GW0751) as well as the Helmholtz Alliance (016W0751). None of these funding institutions had a further role in study design; the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
References
- Allen S.S., Allen A.M., Pomerleau C.S. Influence of phase-related variability in premenstrual symptomatology, mood, smoking withdrawal, and smoking behavior during ad libitum smoking, on smoking cessation outcome. Addict. Behav. 2009;34:107–111. doi: 10.1016/j.addbeh.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström T., Haage D., Löfgren M., Johansson I.M., Strämberg J., Nyberg S., Andréen L., Ossewaarde L., van Wingen G.A., Turkmen S., Bengtsson S.K. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 2011;191:46–54. doi: 10.1016/j.neuroscience.2011.03.061. [DOI] [PubMed] [Google Scholar]
- Bethea C.L., Pecins-Thompson M., Schutzer W.E., Gundlah C., Lu Z.N. Ovarian steroids and serotonin neural function. Mol. Neurobiol. 1998;18:87–123. doi: 10.1007/BF02914268. [DOI] [PubMed] [Google Scholar]
- Bortz J. Springer; Berlin: 1999. Statistik für Sozialwissenschafter. [Google Scholar]
- Boulware M.I., Kent B.A., Frick K.M. The impact of age-related ovarian hormone loss on cognitive and neural function. Curr. Top. Behav. Neurosci. 2011 doi: 10.1007/7854_2011_122. [DOI] [PubMed] [Google Scholar]
- Bourke C., Douglas K., Porter R. Processing of facial emotion expression in major depression: a review. Aust. N. Z. J. Psychiatry. 2010;44:681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Conway C.A., Jones B.C., DeBruine L.M., Welling L.L.M., Law Smith M.J., Perrett D.I., Sharp M.A., Al-Dujaili E.A.S. Salience of emotional displays of danger and contagion in faces is enhanced when progesterone levels are raised. Horm. Behav. 2007;51:202–206. doi: 10.1016/j.yhbeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Davis M.H. The effects of dispositional empathy on emotional reactions and helping: a multidimensional approach. J. Pers. 1983;51:167–184. [Google Scholar]
- Decety J., Jackson P.L. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Derntl B., Kryspin-Exner I., Fernbach E., Moser E., Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm. Behav. 2008;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Derntl B., Windischberger C., Robinson S., Lamplmayr E., Kryspin-Exner I., Gur R.C., Moser E., Habel U. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33:1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Toygar T.K., Hülsmann A., Schneider F., Falkenberg D.I., Habel U. Generalized deficit in all core components of empathy in schizophrenia. Schizophr. Res. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Eickhoff S., Kellermann T., Falkenberg D.I., Schneider F., Habel U. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Derntl B., Seidel E.-M., Eickhoff S.B., Kellermann T., Gur R.C., Schneider F., Habel U. Neural correlates of social approach and withdrawal in patients with major depression. Soc. Neurosci. 2011 doi: 10.1080/17470919.2011.579800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Voss B., Eickhoff S.B., Kellermann T., Schneider F., Habel U. Neural correlates of the core facets of empathy in schizophrenia. Schizophr. Res. 2012;136:70–81. doi: 10.1016/j.schres.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves "mind-reading" in humans. Biol. Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Epperson C.N., Wisner K.L., Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom. Med. 1999;61:676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Ferree N.K., Cahill L. Post-event spontaneous intrusive recollections and strength of memory for emotional events in men and women. Conscious. Cogn. 2009;18:126–134. doi: 10.1016/j.concog.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara B.K., Leresche L., Mancl L. Patterns of salivary estradiol and progesterone across the menstrual cycle. Ann. N. Y. Acad. Sci. 2007;1098:446–450. doi: 10.1196/annals.1384.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschl M. Current status of salivary hormones analysis. Clin. Chem. 2008;54:1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- Guapo V.G., Graeff F.G., Zani A.C.T., Labate C.M., dos Reis R.M., Del-Ben C.M. Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology. 2009;34:1087–1094. doi: 10.1016/j.psyneuen.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Gur R.C., Radim S., Hagendoorn M., Marom O., Hughett P., Macy L., Turner T., Bajcsy R., Posner A., Gur R.E. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends Cogn. Sci. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kurshan N., Neill Epperson C. Oral contraceptives and mood in women with and without premenstrual dysphoria: a theoretical model. Arch. Womens Ment. Health. 2006;9:1–14. doi: 10.1007/s00737-005-0102-z. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Der MWT- ein Intelligenztest für die ärztliche Praxis. Prax. Neurol. Psychiatr. 1996;7:488–491. [Google Scholar]
- Li H., Yuan J., Lin C. The neural mechanism underlying the female advantage in identifying negative emotions: an event-related potential study. NeuroImage. 2008;40:1921–1929. doi: 10.1016/j.neuroimage.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Lukesch H. Beltz; Göttingen: 2006. FEPAA. Fragebogen zur Erfassung von Empathie, Prosozialität, Aggressionsbereitschaft und aggressivem Verhalten. [Google Scholar]
- Macrae C.N., Alnwick K.A., Milne A.B., Schloerscheidt A.M. Person perception across the menstrual cycle: hormonal influences on social-cognitive functioning. Psychol. Sci. 2002;13:532–536. doi: 10.1111/1467-9280.00493. [DOI] [PubMed] [Google Scholar]
- Mehrabian A., Epstein N. A measure of emotional empathy. J Pers. 1972;40:525–543. doi: 10.1111/j.1467-6494.1972.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Paulus C. Der Saarbrückener Persönlichkeitsfragebogen SPF (IRI) zur Messung von Empathie: Psychometrische Evaluation der deutschen Version des Interpersonal Reactivity Index. 2009. http://psydok.sulb.uni-saarland.de/volltexte/2009/2363/
- Pearson R., Lewis M.B. Fear recognition across the menstrual cycle. Horm. Behav. 2005;47:267–271. doi: 10.1016/j.yhbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Pletzer B., Kronbichler M., Aichhorn M., Bergmann J., Ladurner G., Kerschbaum H.H. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Preston S.D., de Waal F.B. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–71. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Reed S.C., Levin F.R., Evans S.M. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder) Horm. Behav. 2008;54:185–193. doi: 10.1016/j.yhbeh.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M. Indianapolis; 1956. Trail Making Test: Manual for Administration, Scoring and Interpretation. [Google Scholar]
- Rubinow D.R., Smith M.J., Schenkel L.A., Schmidt P.J., Dancer K. Facial emotion discrimination across the menstrual cycle in women with premenstrual dysphoric disorder (PMDD) and controls. J. Affect. Disord. 2007;104:37–44. doi: 10.1016/j.jad.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L., Naybar N. Gender differences in empathy: the role of the right hemisphere. Brain Cogn. 2008;67:162–167. doi: 10.1016/j.bandc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M., Markowitsch H.J., Shah N.J., Fink G.R., Piefke M. Gender differences in brain networks supporting empathy. NeuroImage. 2008;42:393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Seidel E.-M., Habel U., Finkelmeyer A., Schneider F., Gur R.C., Derntl B. Implicit and explicit behavioral tendencies in male and female depression. Psychiatry Res. 2010;177:124–130. doi: 10.1016/j.psychres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Seidel E.-M., Habel U., Kirschner M., Gur R.C., Derntl B. The impact of facial emotional expressions on behavioral tendencies in women and men. J. Exp. Psychol. Hum. Percept. Perform. 2010;36:500–507. doi: 10.1037/a0018169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Lamm C. The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- van Honk J., Schutter D.J., Bos P.A., Kruijt A.-W., Lentjes E.G., Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen G., van Broekhoven F., Verkes R.J., Peteresson K.M., Bäckström T., Buitellaar J., Fernandez G. How progesterone impairs memory for biologically salient stimuli in healthy young women. J. Neurosci. 2007;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wingen G., van Broekhoven F., Verkes R.J., Petersson K.M., Bäckström T., Buitelaar J.K., Fernandez G. Progesterone selectively increases amygdala reactivity in women. Mol. Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Van Wingen G.A., Ossewaarede L., Bäckström T., Hermans E.J., Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Vincent K., Tracey I. Sex hormones and pain: the evidence from functional imaging. Curr. Pain Headache Rep. 2010;14:396–403. doi: 10.1007/s11916-010-0139-1. [DOI] [PubMed] [Google Scholar]
- Walter H. Social cognitive neuroscience of empathy: concepts, circuits, and genes. Emot. Rev. 2012;4:9–17. [Google Scholar]
- Watters A.J., Williams L.M. Negative biases and risk for depression; integrating self-report and emotion task markers. Depress. Anxiety. 2011;28:703–718. doi: 10.1002/da.20854. [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Zaudig M., Fydrich T. Hogrefe; Göttingen: 1997. Strukturiertes Klinisches Interview für DSM-IV. [Google Scholar]