Summary
The mechanisms responsible for the transcriptional silencing of pluripotency genes in differentiated cells are poorly understood. We have observed that cells lacking the tumor suppressor p27 can be reprogrammed into induced pluripotent stem cells (iPSCs) in the absence of ectopic Sox2. Interestingly, cells and tissues from p27 null mice, including brain, lung, and retina, present an elevated basal expression of Sox2, suggesting that p27 contributes to the repression of Sox2. Furthermore, p27 null iPSCs fail to fully repress Sox2 upon differentiation. Mechanistically, we have found that upon differentiation p27 associates to the SRR2 enhancer of the Sox2 gene together with a p130-E2F4-SIN3A repressive complex. Finally, Sox2 haploinsufficiency genetically rescues some of the phenotypes characteristic of p27 null mice, including gigantism, pituitary hyperplasia, pituitary tumors, and retinal defects. Collectively, these results demonstrate an unprecedented connection between p27 and Sox2 relevant for reprogramming and cancer and for understanding human pathologies associated with p27 germline mutations.
Graphical Abstract
Highlights
► Loss of the tumor suppressor p27 upregulates the pluripotency gene Sox2 ► Absence of p27 allows reprogramming without ectopic Sox2 ► p27 associates with a Sox2 enhancer together with a repressive complex ► SOX2 mediates some of the main phenotypic defects of p27 null mice
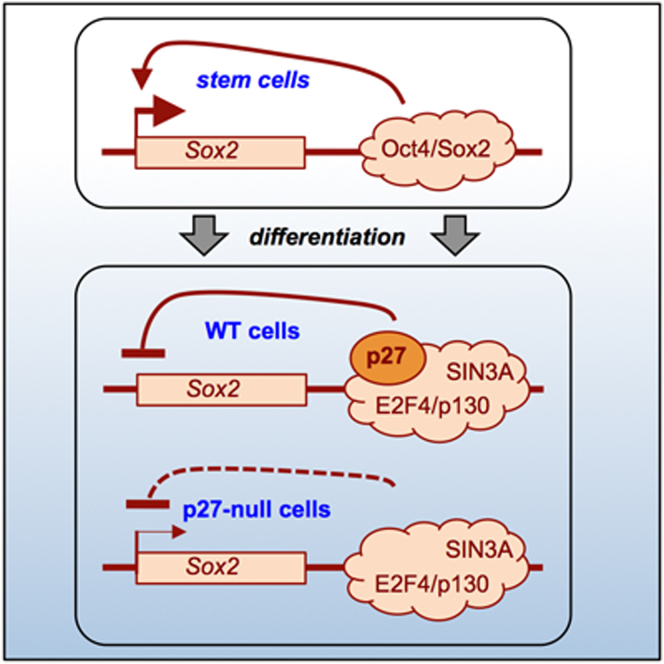
The tumor suppressor p27Kip1 has an unexpected role in direct transcriptional regulation of Sox2 during the differentiation of pluripotent cells.
Introduction
Differentiated cells can be converted into induced pluripotent stem cells (iPSCs) through the combined action of transcription factors, most notably OCT4, KLF4, and SOX2 (Takahashi and Yamanaka, 2006). Importantly, the mechanisms involved in this process might provide clues about the molecular mechanisms governing stem cell biology and cancer. Recently, we and others have shown that tumor suppressors, such as those encoded by the p53 gene and the Ink4a/Arf locus, oppose reprogramming and limit the efficiency of the process (Banito et al., 2009; Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marión et al., 2009; Utikal et al., 2009; Zhao et al., 2008).
The tumor suppressor p27Kip1 binds and inhibits multiple cyclin-dependent kinases (Besson et al., 2008). Importantly, low protein levels of p27 constitute a poor prognosis marker for several types of cancer (Chu et al., 2008) and germline mutations of the p27 gene (also known as CDKN1B) are responsible for a subset of human multiple endocrine neoplasia (MEN) syndromes, notably characterized by pituitary tumors (Marinoni and Pellegata, 2011; Vandeva et al., 2010). The cyclin-dependent kinase 2 (CDK2) is one of the main CDKs inhibited by p27 (Besson et al., 2008). Paradoxically, however, the main phenotypes of p27 null mice, namely, increased body size, organ hyperplasia, pituitary tumors, and retinal dysplasia (Fero et al., 1996; Kiyokawa et al., 1996; Nakayama et al., 1996), are not rescued by concomitant deletion of Cdk2, thus suggesting that these p27 null phenotypes are not primarily caused by uncontrolled CDK2 activity (Aleem et al., 2005; Martín et al., 2005).
In the context of investigating the role of tumor suppressors during reprogramming, we studied p27 null cells and we noticed that these cells can be reprogrammed into iPSCs without ectopic expression of Sox2. This observation led us to explore the potential link between these two previously unrelated proteins, p27 and SOX2.
Results
Cells Lacking p27 Express Higher Levels of Sox2 and Can Be Reprogrammed without Ectopic Sox2
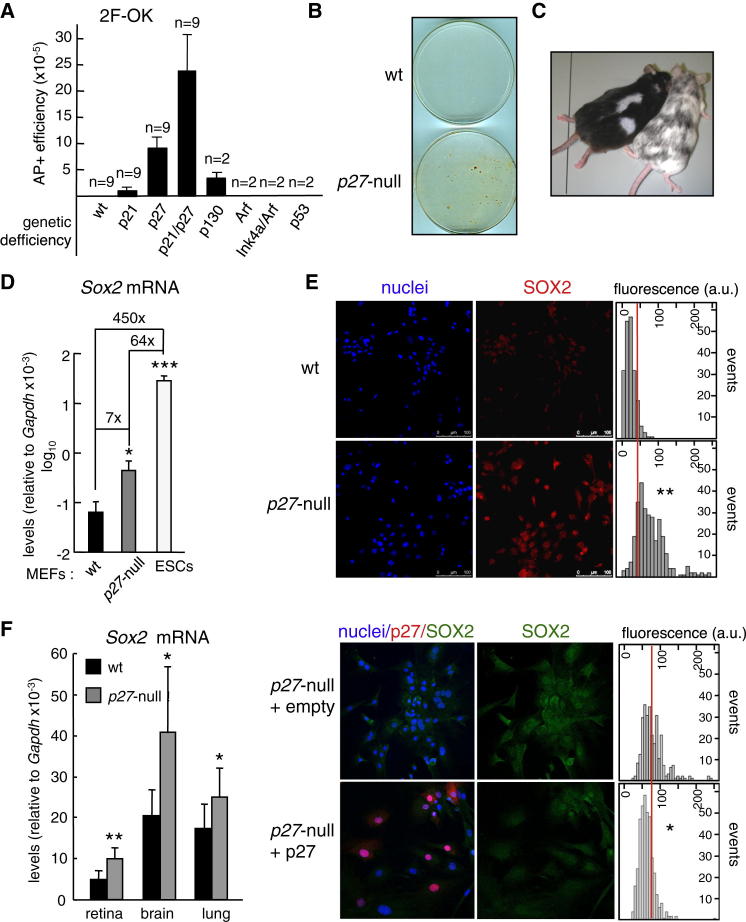
While investigating the effect of tumor suppressor genes on the process of reprogramming to induced pluripotent stem cells (iPSCs) by the three Yamanaka factors (Oct4, Klf4, and Sox2) (Takahashi and Yamanaka, 2006), we tested the three possible combinations of two factors (abbreviated as 2F-OK, 2F-OS, and 2F-KS in reference to Oct4, Klf4, and Sox2) in a series of primary mouse embryo fibroblasts (MEFs) lacking cell cycle regulators and tumor suppressors. After repeated attempts, we were unable to obtain alkaline-phosphatase-positive (AP+) colonies in any of the tested MEFs using 2F-OS or 2F-KS. Interestingly, however, p27 null MEFs and, to a lesser extent, p130 null MEFs gave rise to AP+ colonies with 2F-OK (Figures 1A and 1B). Absence of the p27-related protein p21 also produced AP+ colonies and further increased the number of AP+ colonies when combined with p27 deficiency, thus suggesting some degree of functional redundancy between p27 and p21. In all these MEFs, the emergence of visible AP+ colonies was delayed compared to the standard three-factor cocktail (3F-OKS) (4 weeks versus 2 weeks) and the average efficiency was about 100-fold lower (9 × 10−5 in p27 null/2F-OK versus 8 × 10−3 in WT/3F-OKS). In contrast to this, WT MEFs or MEFs deficient in p53, Arf, or Ink4a/Arf could not be reprogrammed by 2F-OK despite the fact that these cells are reprogrammed with very high efficiency by 3F-OKS (Banito et al., 2009; Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marión et al., 2009; Utikal et al., 2009; Zhao et al., 2008). Also, absence of p27 had a modest stimulatory effect on 3F-OKS reprogramming (Figure S1A available online). Together, these observations suggest that the absence of p27 selectively renders cells susceptible to reprogramming in the absence of ectopic Sox2. The p27 null/2F-OK AP+ colonies were confirmed to be bona fide iPSCs based on their expression of endogenous pluripotency genes (Nanog, Sox2, and Oct4; Figure S1B), production of teratomas (Figure S1C), and efficient contribution to chimeric mice (Figure 1C). To further validate the use of alkaline phosphatase as a marker of reprogramming under our experimental conditions (most notably characterized by the absence of ectopic c-Myc and by the use of serum-free medium), we obtained WT and p27 null MEFs carrying a transgenic GFP reporter under the Sox2 promoter (D'Amour and Gage, 2003), and we observed that >90% of the AP+ colonies were GFP+. All together, these results indicate that the absence of p27 eliminates the absolute requirement for ectopic Sox2 in reprogramming.
Figure 1.
Absence of p27 Allows Two-Factor (Oct4 and Klf4) Reprogramming
(A) Two-factor (Oct4 and Klf4, 2F-OK) reprogramming of primary MEFs of the indicated genotype. Efficiency is measured as the number of alkaline-phosphatase-positive (AP+) colonies relative to the total number of infected cells. n values correspond to independent MEF isolates.
(B) Representative picture of AP stained plates.
(C) Picture of chimeric mice generated from p27 null/2F-OK iPSCs (black-C57BL6 genetic background) after microinjection into albino-C57BL6 blastocysts.
(D) Sox2 mRNA levels in WT (n = 3) and p27 null (n = 5) MEFs and ESCs. mRNA levels were determined by qRT-PCR.
(E) Upper panel: representative picture of SOX2 immunofluorescence in WT and p27 null MEFs and quantification of the immunofluorescence corresponding to one experiment. A total of two experiments were performed, each with different MEF isolates, with similar results obtained in both of them. Lower panel: representative picture of p27 and SOX2 immunofluorescence in p27 null MEFs infected with empty vector or with pBabe-p27. A total of three independent experiments were performed, each with different MEF isolates, and similar results were obtained in the three of them. The average ± SD of each distribution was compared with its corresponding control using the Student's t test.
(F) Sox2 mRNA levels in WT (n = 6) and p27 null (n = 10) mice (∼1 year old).
All data correspond to the average ± SD. Statistical significance was assessed by two-tailed Student's t test: ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05. See also Figure S1.
Mouse fibroblasts express low, but detectable, levels of Sox2 (Eminli et al., 2008), and therefore, we wondered whether p27 deficiency affected Sox2 expression. Indeed, p27 null MEFs had a significant increase in Sox2 mRNA levels compared to WT controls (7-fold), although these levels were still about 64-fold lower than in embryonic stem cells (ESCs) (Figure 1D). We also detected SOX2 protein by immunofluorescence. Consistent with the mRNA data, quantitative image analyses indicated that SOX2 protein levels were globally increased in p27 null MEFs (Figure 1E). Of note, the distribution of SOX2 fluorescence intensity in p27 null MEFs is broad, and therefore, it is conceivable that only those p27 null cells with the highest SOX2 levels are the ones susceptible of 2F-OK reprogramming. It is also worth mentioning that WT and p27 null MEFs have the same proliferative rate (Coats et al., 1999), thus implying that their different SOX2 levels are not secondary to a different proliferative activity. We tested whether the increased levels of SOX2 observed in p27 null MEFs could be reverted by ectopic overexpression of p27. Interestingly, quantitative immunofluorescence of p27-overexpressing p27 null MEFs indicated a downregulation of SOX2 levels (Figure 1E). Finally, we also observed higher Sox2 mRNA levels in the retina, brain, and lung of adult p27 null mice (Figure 1F). Together, these data indicate that p27 contributes to the silencing of Sox2 in differentiated cells and tissues.
Sox2 Expression Is Repressed by p27
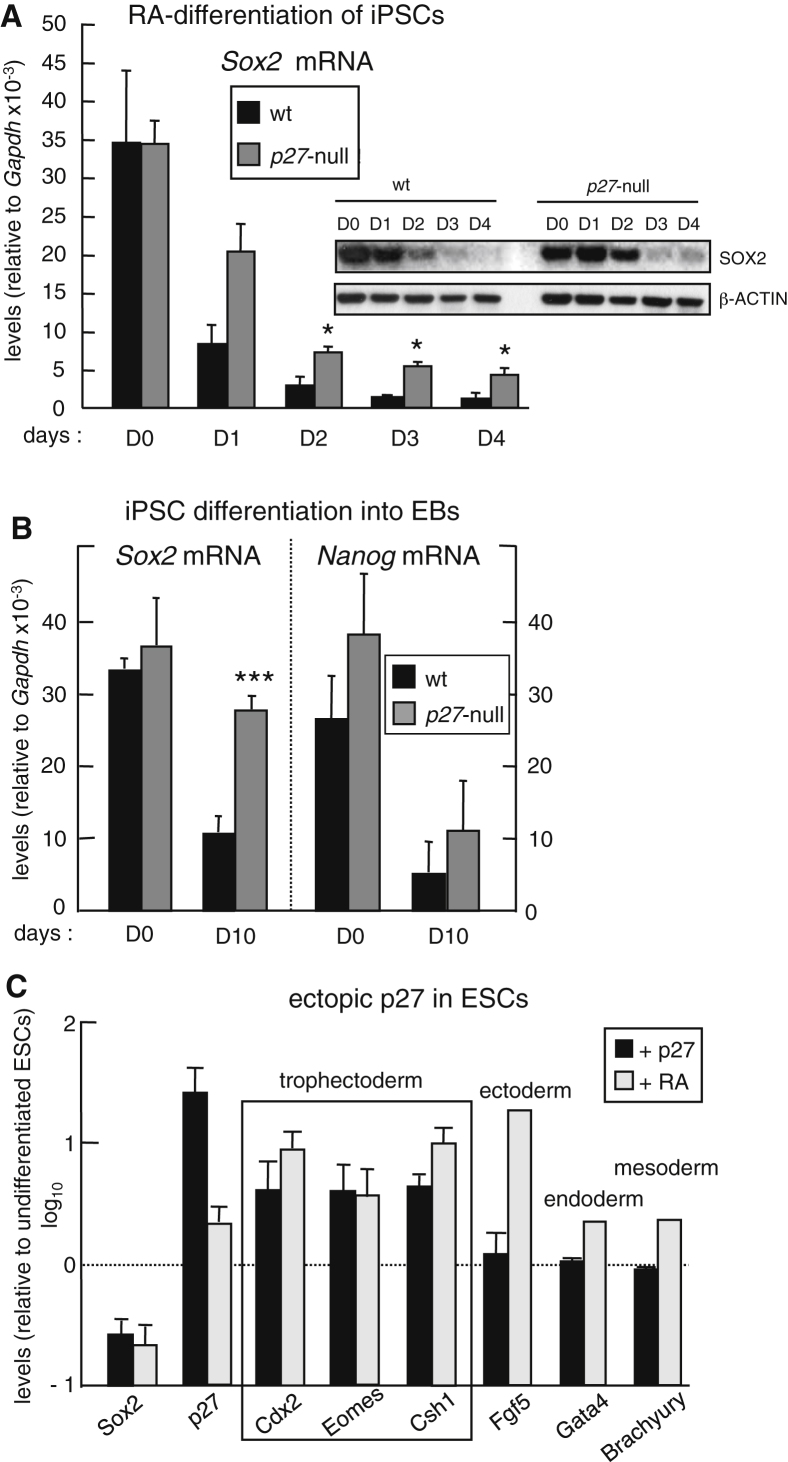
To further define the repressive role of p27 on Sox2, we analyzed the differentiation of pluripotent stem cells upon treatment with retinoic acid (RA). This differentiation protocol efficiently reduces SOX2 protein and mRNA concomitantly with a dramatic upregulation of p27 (Figure S2A). The upregulation of p27 during RA-induced differentiation is in agreement with a previous report on differentiating human embryonic carcinoma cells (Bahrami et al., 2005). We wondered whether the absence of p27 would affect the kinetics of Sox2 repression. For this, we generated iPSCs derived from WT or p27 null MEFs after reprogramming them with Oct4, Klf4, and Sox2. The levels of Sox2 mRNA were similar in undifferentiated WT or p27 null iPSCs (Figure 2A). However, upon RA-induced differentiation, Sox2 levels in p27 null iPSCs were not reduced as efficiently as in WT iPSCs (Figure 2A). Similarly, we tested Sox2 expression during iPSC differentiation into embryoid bodies (EBs). Again, Sox2 mRNA levels were abnormally high in p27-deficient EBs compared to WT ones (Figure 2B). These observations are in agreement with a previous study reporting numerous abnormalities in EBs from p27 null ESCs (Bryja et al., 2005). We considered the possibility that the increased Sox2 levels in p27 null EBs could reflect a skewed neural differentiation, but the levels of Nestin mRNA were similar in WT and p27 null EBs (Figure S2B). To directly test the repressive activity of p27, we retrovirally transduced ESCs with a pMSCV vector expressing p27, and interestingly, p27 overexpression was able to reduce the levels of Sox2 to an extent comparable to RA (Figure 2C). Previous studies have demonstrated that Sox2 null ESCs spontaneously differentiate into trophectoderm-like cells (Masui et al., 2007). We asked whether p27 overexpression in ESCs promotes the trophectoderm-like differentiation characteristic of Sox2 null ESCs. Indeed, this was the case and p27 overexpression selectively induced trophectoderm markers to a similar extent as RA differentiation, whereas ectoderm, endoderm, and mesoderm markers were not induced by p27 but were induced by RA (Figure 2C). Moreover, p27-overexpressing ESCs produced cells with giant trophoblast-like morphology, while this type of cell was absent in RA-differentiated ESCs (Figure S2C). To extend the generality of these findings to other pluripotent cells, we overexpressed p27 in murine P19 embryonal carcinoma (P19EC) cells at levels that did not affect proliferation and found a significant decrease of Sox2 mRNA levels (Figure S2D). Together, these results indicate that p27 exerts a repressive effect on Sox2 that is relevant during differentiation.
Figure 2.
p27 Represses Sox2 Expression
(A) Sox2 mRNA and protein levels in iPSCs undergoing in vitro differentiation by the addition of retinoic acid (RA) in the absence of LIF for the indicated number of days. mRNA values correspond to the average ± SD. (n = 6 independent clones per genotype.)
(B) Sox2 and Nanog mRNA levels in iPSCs before and after 10 days of induction of embryoid bodies (EBs). mRNA values correspond to the average ± SD. (n = 6 independent clones per genotype.)
(C) Sox2 and p27 mRNA levels in ESCs after infection (3 days) with an empty vector or with a plasmid overexpressing p27 (pMSCV-p27). ESCs at day 4 of the RA-differentiation protocol were used as a differentiation control. Trophectoderm markers and markers for other lineages were tested. In the case of ESCs infected with pMSCV-p27, values are relative to ESCs infected with empty vector. In the case of RA-differentiated ESCs, values are relative to nondifferentiated ESCs. Three independent assays were performed for overexpression of p27 (n = 3).
mRNA levels were determined by qRT-PCR. All data correspond to the average ± SD. Statistical significance was assessed by the two-tailed Student's t test: ∗∗∗p < 0.01; ∗p < 0.05. See also Figure S2.
p27 Associates to the Sox2-SRR2 Enhancer Together with Repressive Complex p130-E2F4-SIN3A
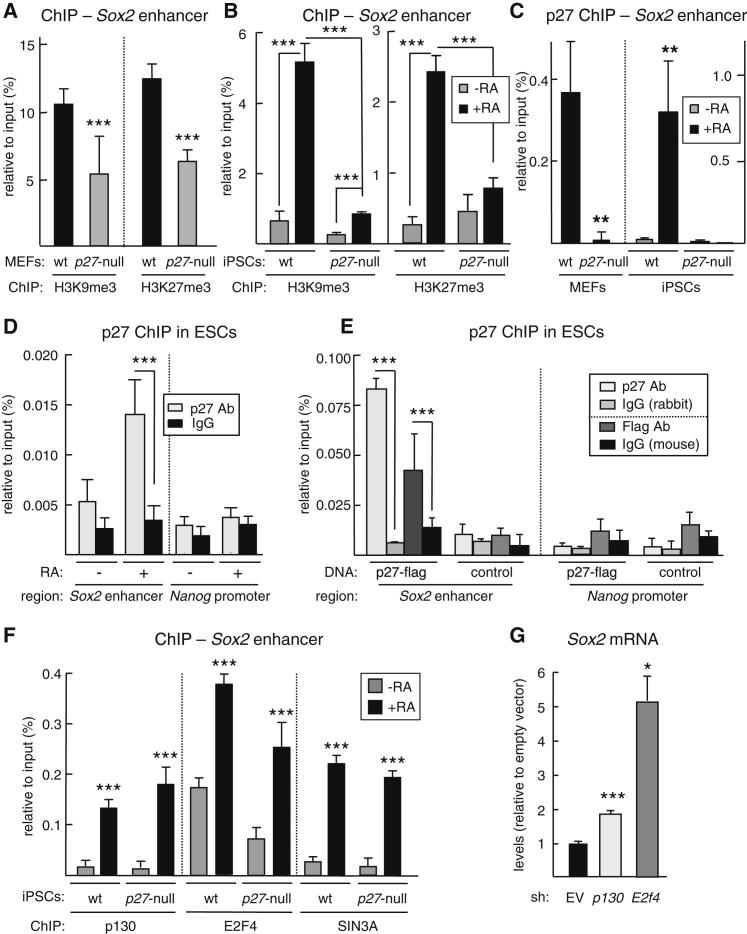
The main regulatory element responsible for the expression of Sox2 in pluripotent stem cells is located ∼4 kb downstream of the single Sox2 coding exon and it is named SRR2 (Sikorska et al., 2008; Tomioka et al., 2002). Based on our above observations in MEFs and in differentiating pluripotent cells, we asked whether the presence or absence of p27 had an effect on the repressive epigenetic marks on the Sox2-SRR2 enhancer. Interestingly, we observed that p27 null MEFs present lower levels of the H3K9me3 and H3K27me3 repressive marks at the Sox2-SRR2 enhancer as evidenced by chromatin immunoprecipitation (ChIP) analysis (Figure 3A). Similarly, RA-induced differentiation of WT iPSCs dramatically increased the levels of H3K9me3 and H3K27me3 at the Sox2-SRR2 enhancer, while these epigenetic marks were modestly increased in p27 null iPSCs (Figure 3B). These results indicate that the absence of p27 leads to a defective epigenetic remodeling of the Sox2-SRR2 enhancer both in differentiated cells (MEFs) and during the differentiation of pluripotent cells.
Figure 3.
p27 Directly Binds to the Sox2-SRR2 Enhancer
(A) Chromatin immunoprecipitation (ChIP) of H3K9me3 and H3K27me3 in the Sox2-SRR2 enhancer of WT and p27 null MEFs. Data correspond to one representative assay from a total of three independent assays, each of them with different MEF isolates.
(B) ChIP of the indicated proteins in the Sox2-SRR2 enhancer of WT and p27 null iPSCs before and after RA differentiation. Data correspond to one representative assay from a total of two independent assays, each of them with different iPSC clones.
(C) ChIP of p27 in the Sox2-SRR2 enhancer of WT and p27 null MEFs, and in WT and p27 null iPSCs before and after RA differentiation. Data correspond to one representative assay from a total of three independent assays, each of them with different MEF isolates and iPSCs clones.
(D) ChIP of p27 on the Sox2-SRR2 enhancer of ESCs before and after RA differentiation. Data correspond to one representative assay from a total of two independent assays.
(E) ChIP of p27 in ESCs 2 days after transfection with empty vector (control) or a plasmid expressing flag-tagged p27 (p27-flag).
(F) ChIP of the indicated proteins in the Sox2-SRR2 enhancer before and after RA differentiation of WT and p27 null iPSCs.
(G) Sox2 mRNA levels in WT MEFs 48 hr after retroviral transduction with shp130, shE2f4, or empty vector (EV). Data correspond to two independent assays (n = 2).
All data correspond to the average ± SD. Statistical significance was assessed by the two-tailed Student's t test: ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05. See also Figure S3.
Based on the above data and the recent report that p27 can associate to gene promoters in association with the repressive complex p130-E2F4-SIN3A (Pippa et al., 2012), we hypothesized that p27 might be recruited in this manner to the Sox2-SRR2 enhancer. To directly test this, we performed ChIP with anti-p27 antibodies in MEFs and we detected p27 associated to the Sox2-SRR2 enhancer (Figure 3C). As before, we wondered whether this was also the case in differentiating pluripotent cells. Interestingly, RA-induced differentiation of iPSCs was accompanied by a strong recruitment of p27 to the Sox2-SRR2 enhancer (Figure 3C). To further extend these observations, we used ESCs and, as in the case of iPSCs, p27 was immunoprecipated at the Sox2-SRR2 enhancer upon RA-induced differentiation, but not at the Nanog promoter used here as a control (Figure 3D). We sought additional proof by performing ChIP from ESCs transfected with flag-tagged p27 and we also found p27 bound to the Sox2-SRR2 enhancer after immunoprecipitation with antibodies against p27 or against the flag tag (Figure 3E). Similar results were obtained in P19EC cells, both upon RA-induced differentiation (Figure S3A) and upon transfection of flag-tagged p27 (Figure S3B).
To examine the presence of a p130-E2F4-SIN3A repressive complex at the Sox2-SRR2 enhancer, we performed ChIP assays using antibodies against p130, E2F4, and SIN3A in WT or p27 null iPSCs undergoing RA-induced differentiation. Interestingly, the three proteins were detected in the Sox2-SRR2 enhancer of RA-differentiated cells regardless of the presence or absence of p27 (Figure 3F). We also detected binding of p130, E2F4, and SIN3A to the Sox2-SRR2 enhancer in WT and p27 null MEFs (Figure S3C) and RA-differentiated P19EC cells (Figure S3D). These results indicate that a repressive p130-E2F4-SIN3A complex is assembled at the Sox2-SRR2 enhancer upon differentiation and independently of p27.
Finally, we asked whether the direct inhibition of the p130-E2F4-SIN3A complex would also result in derepression of Sox2. In agreement with previous reports (Dannenberg et al., 2005), depletion of SIN3A had a dramatic effect on cell viability that precluded us from further examining the effect on Sox2 expression. Interestingly, however, knockdown of p130 or E2f4 with shRNAs resulted in a severe reduction of their expression (Figure S3E) and, importantly, this was accompanied in both cases by a significant upregulation of Sox2 expression (Figure 3G). In summary, we conclude that p27 associates to the Sox2-SRR2 enhancer together with the repressive complex p130-E2F4-SIN3A, and together contribute to the repression of Sox2 upon cell differentiation.
Sox2 Heterozygosity Rescues p27 Deficiency in Mice
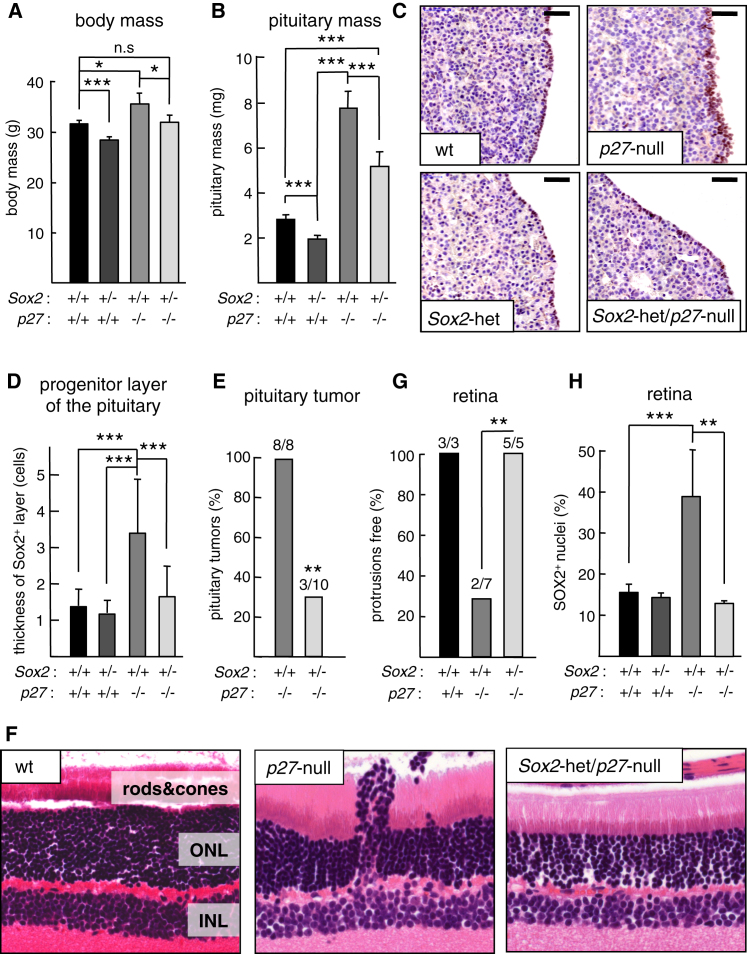
Based on our above data, we wondered whether the incomplete repression of Sox2 observed in p27-deficient cells and tissues could mediate some of the phenotypes characteristic of p27 null mice. To evaluate this in a genetic manner, we generated compound Sox2 heterozygous (Sox2-het) and p27 null mice. Sox2 null mice are not viable, but Sox2-het are viable and display a moderate reduction in body size and hypopituitarism (Kelberman et al., 2006). Interestingly, the characteristic gigantism of p27 null mice was normalized by deletion of one Sox2 allele (Figure 4A; Figure S4A), as was also the case for the pituitary mass in young 3- to 6-month-old mice (at this age, p27 null mice present pituitary hyperplasia, but do not have pituitary tumors yet) (Figure 4B). However, given the fact that Sox2-het mice have a modest, but detectable, defect in growth, the above results leave open the possibility that p27 and Sox2 simply have opposite effects on growth that balance each other. For this reason, we decided to focus in the progenitor cell layer that surrounds the pituitary cleft and which is formed by SOX2-positive (SOX2+) cells (Fauquier et al., 2008; Garcia-Lavandeira et al., 2009; Gleiberman et al., 2008). The progenitor layer in Sox2-het pituitaries had the same thickness as in WT pituitaries (Figures 4C and 4D; Figure S4B). Interestingly, the thickness of the progenitor layer was significantly increased in p27 null mice compared to WT or to Sox2-het mice, and this defect was absent in Sox2-het/p27-null mice (Figures 4C and 4D). The progenitor cells of the pituitary have been proposed to constitute the origin of pituitary adenomas (Gleiberman et al., 2008). In this regard and in line with our above observations, the incidence of pituitary tumors was significantly reduced in Sox2-het/p27-null mice compared to p27 null littermates (Figure 4E).
Figure 4.
p27 Null Phenotypes Are Rescued by Sox2 Haploinsufficiency
(A) Body mass of 2-month-old males of the indicated genotypes (n = 3 for WT; n = 6 for Sox2-het; n = 5 for p27-null; and n = 3 for Sox2-het/p27-null).
(B) Pituitary mass (n = 9 for WT; n = 12 for Sox2-het; n = 7 for p27-null; and n = 10 for Sox2-het/p27-null, males and females pooled, 3–6 months old).
(C) Representative pictures of the progenitor layer of the pituitary cleft stained with SOX2. Bars correspond to 50 μm.
(D) Thickness of the progenitor layer of the pituitary cleft expressed as number of cells (n = 3 for each genotype, males and females pooled, 3–6 months old).
(E) Incidence of pituitary adenomas in 3- to 6-month-old mice.
(F) Representative pictures of the retina (H&E staining). A focal protrusion is apparent in the p27 null retina. ONL = outer nuclear layer; INL = inner nuclear layer.
(G) Incidence of retinas free of protrusions (n = 3 for WT; n = 7 for p27-null; and n = 5 for Sox2-het/p27-null, males and females pooled, 1 year old).
(H) Relative number of SOX2+ nuclei in the retina (n = 3 for each genotype, males and females pooled, 1 year old).
Data in (A), (B), (D), and (H) correspond to the average ± SD and statistical significance was assessed by the two-tailed Student's t test. Data in (E) and (G) correspond to ratios and statistical significance was assessed by the Fisher's test. ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05; n.s., not significant. See also Figure S4.
Having established that Sox2 heterozygosity rescues the gigantism and the pituitary phenotypes of p27 null mice, we wondered whether the same was true for the retinal defects of p27 null mice. In agreement with previous reports, the retinas of Sox2-het mice were normal (Taranova et al., 2006) (Figure S4C), while p27 null retinas presented focal protrusions of the outer nuclear layer (ONL) (Nakayama et al., 1996) (Figures 4F and 4G). Interestingly, these protrusions were absent in Sox2-het/p27-null retinas (Figures 4F and 4G). Also, we observed that p27 null retinas present an increased abundance of SOX2+ nuclei at the inner nuclear layer (INL) and mislocalized SOX2+ nuclei in the outer plexiform layer (OPL) (Figure S4C). We quantified the number of SOX2+ nuclei in complete retinal sections and confirmed that p27 null retinas present an excess of SOX2+ nuclei and, importantly, we found that this defect was absent in Sox2-het/p27-null retinas (Figure 4H). We wanted to corroborate the increased abundance of SOX2+ nuclei in the absence of p27, and for this we used transgenic mice with GFP under the control of a Sox2 promoter region that marks neural multipotent progenitors associated to Sox2 expression (Sox2-GFP mice) (D'Amour and Gage, 2003). Reinforcing our above observations, p27-het/Sox2-GFP retinas presented a significant increase in GFP+ nuclei when compared with WT/Sox2-GFP retinas (Figure S4D). Together, we conclude that a decrease in the gene dosage of Sox2 rescues the main phenotypes associated with p27 deficiency, namely, gigantism, pituitary hyperplasia, pituitary adenomas, and retinal abnormalities. In addition to these phenotypes, p27 null mice also have adrenal gland hyperplasias and tumors (pheochromocytomas) and female sterility (Fero et al., 1996; Kiyokawa et al., 1996; Nakayama et al., 1996). We examined WT adrenal glands and p27 null pheochromocytomas by immunostaining for SOX2, but they were negative and for this reason we did not further pursue this phenotype. Regarding the sterility of p27 null females, Sox2-het/p27-null females remained sterile, suggesting that this phenotype is independent of Sox2. Collectively, these results provide genetic support to the concept that p27 is a negative regulator of Sox2 in the pituitary and in the retina.
Discussion
The mechanisms responsible for the transcriptional silencing of pluripotency genes in differentiated cells are poorly understood. The results reported here demonstrate that the tumor suppressor p27 contributes to the transcriptional repression of Sox2. We have observed that the absence of p27 leads to a defective repression of Sox2 in fibroblasts, lung, retina, and brain, and to a delayed and incomplete silencing of Sox2 during differentiation of pluripotent cells, including iPSCs, ESCs, and P19EC cells. These observations led us to identify p27 as a transcriptional regulator of Sox2 together with a repressive complex formed by p130, E2F4, and SIN3A at a critical enhancer responsible for Sox2 expression. These findings are in line with a recent report describing the capacity of p27 to interact with the p130/E2F4/SIN3A complex and contribute to its transcriptional repressive activity (Pippa et al., 2012). We have found that p27 deficiency leads to an expansion of SOX2+ cells in the progenitor layer of the pituitary and in the retina, which results in pituitary hyperplasia and tumors and morphological defects of the retina. Importantly, these defects are rescued when p27 deficiency is combined with Sox2 heterozygosity. In humans, germline mutations in p27 and SOX2 also affect the pituitary and the retina. On one hand, loss-of-function mutations in p27 produce MEN syndrome, notably characterized by pituitary tumors (Marinoni and Pellegata, 2011; Vandeva et al., 2010). On the other hand, loss-of-function mutations in SOX2 produce syndromes characterized by anophthalmia and hypopituitarism (Engelen et al., 2011; Fantes et al., 2003; Kelberman et al., 2006; Williamson et al., 2006). Our current findings unveil a mechanistic connection between p27 and SOX2, and thereby contribute to our understanding of the molecular basis of the human pathologies associated with the deregulation of these two proteins.
Experimental Procedures
Mice
Mice p27 null (Fero et al., 1996), Sox2-het (Avilion et al., 2003), and Sox2-promoter/GFP transgenic (D'Amour and Gage, 2003) have been previously described. All comparisons were made among mice derived from the same sets of crosses, and they therefore shared the same genetic background. Animal experimentation at the CNIO, Madrid was performed according to protocols approved by the CNIO-ISCIII Ethics Committee for Research and Animal Welfare (CEIyBA) and animal experimentation at the MRC-NIMR, Mill Hill, London was carried out in accordance with the UK Animals (Scientific Procedures) Act 1986.
ChIP, RNA Quantification, and Protein Analyses
ChIP and quantitative PCR was performed following standard methods (detailed in the Supplemental Experimental Procedures). PCR primer sequences, shRNA encoding plasmids, and other DNA constructs, as well as antibodies and other standard molecular biology methods, are all detailed in Supplemental Experimental Procedures.
Generation of iPSCs
Reprogramming of primary (passage 2–4) MEFs was performed as previously described by us (Li et al., 2009) using plasmids pMXs-Klf4, pMXs-Sox2, or pMXs-Oct4 (obtained from Addgene and previously described; Takahashi and Yamanaka, 2006). For additional details, see Supplemental Experimental Procedures.
Differentiation with RA
Differentiation with RA was performed essentially as described (Savatier et al., 1996). ESCs or iPSCs were adapted to grow on gelatin-coated plates (and in the absence of feeder cells). Cells were grown to near confluency in their corresponding complete medium (day 0) and then were trypsinized and seeded at lower density in the absence of LIF for 1 day (day 1). During the following 2 days (days 2 and 3), RA was added at a concentration of 10−6 M, and day 4 cells were without LIF and without RA. In the case of P19EC cells, differentiation was induced by addition of RA (10−6 M) for 4 days.
Immunohistochemistry and Immunofluorescence
For immunohistochemical stainings, quantifications were performed on representative fields at the same magnification, on a minimum of three different areas per sample and a minimum of three different samples per genotype. For immunofluorescence, cells were inspected under a Leica TCS-SP5 confocal microscope (AOBS) and analyzed using Definiens Developer XD 1.5 software, under the same exposure conditions. For additional details, see Supplemental Experimental Procedures.
Statistical Analysis
Unless otherwise specified (Figures 4E and 4G), quantitative data are presented as mean ± SD and significance was assessed by the two-tailed Student's t test.
Acknowledgments
We are indebted to Diego Megías from the CNIO for technical assistance, and to the Biological Services staff at NIMR. H.L. has been funded by the Spanish Association Against Cancer (AECC). H.L. and M. Collado have a “Ramon y Cajal” contract from the Spanish Ministry of Economy (MINECO). Work in the laboratory of M.S. is funded by the CNIO and by grants from the MINECO (SAF and CONSOLIDER), the Regional Government of Madrid, the European Research Council (ERC), the Botin Foundation, the AXA Foundation, and the Ramon Areces Foundation. A.M. is supported by a long-term fellowship of the Human Frontiers Science Program; K.R., R.L.-B., and work in the R.L.-B. laboratory are funded by the UK Medical Research Council (U117512772). Work in the A. Vidal laboratory is funded by grants from the MINECO (SAF) and from the Xunta de Galicia. H.L. and M. Collado performed most of the experiments and contributed to experimental design, data analysis, discussion, and writing the paper; A. Villasante, C.J.L., and C.C. performed the chromatin immunoprecipitations; M. Cañamero performed the histological analyses; A.M., K.R., and R.L.-B. provided the Sox2 mouse models, performed mouse manipulations, and contributed to the analysis of the mouse phenotypes; C.C., G.M., and A. Vidal provided MEFs and contributed to the analysis of the pituitary phenotype; M. Collado and M.S. designed and supervised the study, secured funding, analyzed the data, and wrote the manuscript. All authors discussed the results and commented on the manuscript. The authors declare no competing financial interests with this paper.
Published: December 7, 2012
Footnotes
Supplemental Information for this article includes four figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2012.09.014.
Contributor Information
Manuel Collado, Email: manuel.collado.rodriguez@sergas.es.
Manuel Serrano, Email: mserrano@cnio.es.
Supplemental Information
References
- Aleem E., Kiyokawa H., Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 2005;7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- Avilion A.A., Nicolis S.K., Pevny L.H., Perez L., Vivian N., Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A.R., Matin M.M., Andrews P.W. The CDK inhibitor p27 enhances neural differentiation in pluripotent NTERA2 human EC cells, but does not permit differentiation of 2102Ep nullipotent human EC cells. Mech. Dev. 2005;122:1034–1042. doi: 10.1016/j.mod.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Banito A., Rashid S.T., Acosta J.C., Li S., Pereira C.F., Geti I., Pinho S., Silva J.C., Azuara V., Walsh M. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Dowdy S.F., Roberts J.M. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Bryja V., Cajánek L., Pacherník J., Hall A.C., Horváth V., Dvorák P., Hampl A. Abnormal development of mouse embryoid bodies lacking p27Kip1 cell cycle regulator. Stem Cells. 2005;23:965–974. doi: 10.1634/stemcells.2004-0174. [DOI] [PubMed] [Google Scholar]
- Chu I.M., Hengst L., Slingerland J.M. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Coats S., Whyte P., Fero M.L., Lacy S., Chung G., Randel E., Firpo E., Roberts J.M. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr. Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- D'Amour K.A., Gage F.H. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl 1):11866–11872. doi: 10.1073/pnas.1834200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg J.H., David G., Zhong S., van der Torre J., Wong W.H., Depinho R.A. mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev. 2005;19:1581–1595. doi: 10.1101/gad.1286905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminli S., Utikal J., Arnold K., Jaenisch R., Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- Engelen E., Akinci U., Bryne J.C., Hou J., Gontan C., Moen M., Szumska D., Kockx C., van Ijcken W., Dekkers D.H. Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat. Genet. 2011;43:607–611. doi: 10.1038/ng.825. [DOI] [PubMed] [Google Scholar]
- Fantes J., Ragge N.K., Lynch S.A., McGill N.I., Collin J.R., Howard-Peebles P.N., Hayward C., Vivian A.J., Williamson K., van Heyningen V., FitzPatrick D.R. Mutations in SOX2 cause anophthalmia. Nat. Genet. 2003;33:461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- Fauquier T., Rizzoti K., Dattani M., Lovell-Badge R., Robinson I.C. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. USA. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fero M.L., Rivkin M., Tasch M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Lavandeira M., Quereda V., Flores I., Saez C., Diaz-Rodriguez E., Japon M.A., Ryan A.K., Blasco M.A., Dieguez C., Malumbres M., Alvarez C.V. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS ONE. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman A.S., Michurina T., Encinas J.M., Roig J.L., Krasnov P., Balordi F., Fishell G., Rosenfeld M.G., Enikolopov G. Genetic approaches identify adult pituitary stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H., Takahashi K., Ichisaka T., Aoi T., Kanagawa O., Nakagawa M., Okita K., Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Suzuki J., Wang Y.V., Menendez S., Morera L.B., Raya A., Wahl G.M., Izpisúa Belmonte J.C. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D., Rizzoti K., Avilion A., Bitner-Glindzicz M., Cianfarani S., Collins J., Chong W.K., Kirk J.M., Achermann J.C., Ross R. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J. Clin. Invest. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R.D., Manova-Todorova K.O., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Li H., Collado M., Villasante A., Strati K., Ortega S., Cañamero M., Blasco M.A., Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinoni I., Pellegata N.S. p27kip1: a new multiple endocrine neoplasia gene? Neuroendocrinology. 2011;93:19–28. doi: 10.1159/000320366. [DOI] [PubMed] [Google Scholar]
- Marión R.M., Strati K., Li H., Murga M., Blanco R., Ortega S., Fernandez-Capetillo O., Serrano M., Blasco M.A. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín A., Odajima J., Hunt S.L., Dubus P., Ortega S., Malumbres M., Barbacid M. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1) Cancer Cell. 2005;7:591–598. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Masui S., Nakatake Y., Toyooka Y., Shimosato D., Yagi R., Takahashi K., Okochi H., Okuda A., Matoba R., Sharov A.A. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Pippa R., Espinosa L., Gundem G., García-Escudero R., Dominguez A., Orlando S., Gallastegui E., Saiz C., Besson A., Pujol M.J. p27(Kip1) represses transcription by direct interaction with p130/E2F4 at the promoters of target genes. Oncogene. 2012;31:4207–4220. doi: 10.1038/onc.2011.582. [DOI] [PubMed] [Google Scholar]
- Savatier P., Lapillonne H., van Grunsven L.A., Rudkin B.B., Samarut J. Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene. 1996;12:309–322. [PubMed] [Google Scholar]
- Sikorska M., Sandhu J.K., Deb-Rinker P., Jezierski A., Leblanc J., Charlebois C., Ribecco-Lutkiewicz M., Bani-Yaghoub M., Walker P.R. Epigenetic modifications of SOX2 enhancers, SRR1 and SRR2, correlate with in vitro neural differentiation. J. Neurosci. Res. 2008;86:1680–1693. doi: 10.1002/jnr.21635. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taranova O.V., Magness S.T., Fagan B.M., Wu Y., Surzenko N., Hutton S.R., Pevny L.H. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M., Nishimoto M., Miyagi S., Katayanagi T., Fukui N., Niwa H., Muramatsu M., Okuda A. Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic Acids Res. 2002;30:3202–3213. doi: 10.1093/nar/gkf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utikal J., Polo J.M., Stadtfeld M., Maherali N., Kulalert W., Walsh R.M., Khalil A., Rheinwald J.G., Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeva S., Vasilev V., Vroonen L., Naves L., Jaffrain-Rea M.L., Daly A.F., Zacharieva S., Beckers A. Familial pituitary adenomas. Ann. Endocrinol. (Paris) 2010;71:479–485. doi: 10.1016/j.ando.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Williamson K.A., Hever A.M., Rainger J., Rogers R.C., Magee A., Fiedler Z., Keng W.T., Sharkey F.H., McGill N., Hill C.J. Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum. Mol. Genet. 2006;15:1413–1422. doi: 10.1093/hmg/ddl064. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yin X., Qin H., Zhu F., Liu H., Yang W., Zhang Q., Xiang C., Hou P., Song Z. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.