Abstract
Objective
The histologic types of borderline ovarian tumors (BOTs) exhibit striking differences in clinical behavior and prognosis. Yet, there is no information available on the histologic distribution of BOTs according to geographic region. The purpose of this study was to systematically review this issue worldwide.
Methods
A comprehensive search of the literature was conducted using electronic databases. Studies were eligible if BOTs were investigated and the histologic distribution of the data was shown. The studies were grouped by geographic region and totaled by country.
Results
Of 487 potentially relevant studies, 51 met our inclusion criteria, as follows: 8 studies from North America (2 countries); 26 studies from Europe (14 countries); 7 studies from the Middle East (3 countries); and 10 studies from East Asia (5 countries). The histologic distribution of BOTs was considerably different in different parts of the world, but follows specific patterns. In general, serous-type BOTs were the predominantly identified histology in North America, the Middle East, and Europe, while mucinous-type BOTs predominated in East Asia.
Conclusion
Significant geographic variation is evident among BOT histology in different parts of the world. More research is needed to understand this phenomenon.
Keywords: Borderline ovarian tumor, Histology, Low malignant potential, Systematic review
INTRODUCTION
Borderline ovarian tumors (BOTs) or ovarian tumors of low malignant potential were first described by Taylor in 1929 [1]. BOTs account for 10-15 of all ovarian cancers; they typically affect younger women, as compared with invasive epithelial ovarian tumors, and are mostly diagnosed at earlier stages, which results in a more favorable prognosis. However, a small fraction of BOTs such as advanced-stage diseases with invasive implants are associated with poor prognosis and high recurrence rates of 20-50 [2]. BOTs can be classified histologically according to their epithelial characteristics as serous, mucinous, endometrioid, clear cell or Brenner tumors. As the various histologic types exhibit striking differences in clinical presentation and behavior [3-5], determination of the cell type is critical in the assessment of BOTs, and the different types should be evaluated separately.
Interestingly, there appears to be a difference in the histologic distribution of BOTs according to geographic region. Studies in the USA [6], France [7], and Italy [8] have reported serous-type BOTs as the most common (60-74), while studies in Korea [9] and Japan [10] have reported mucinous-type BOTs as the most common (68-76). However, these studies are too limited in number to reach firm conclusions about regional differences. Therefore, we performed a systematic review of published data regarding the worldwide histologic distribution of BOTs to determine whether or not a difference exists according to geographic region.
MATERIALS AND METHODS
A comprehensive search of the literature was conducted using electronic databases (Medline, Embase, and Cochrane Library). The title and abstract search terms used were as follows: borderline tumor, borderline tumour, borderline neoplasm, low malignant potential, and ovarian, ovary. These terms were also searched as keywords and medical subheadings (MeSH). Each database was searched from its inception to its most recent update as of 5 April 2011.
1. Study selection: inclusion/exclusion criteria
Two investigators (TS and YYL) independently screened the titles and abstracts in duplicate, using standardized techniques. A study was eligible if BOTs were investigated and if the distribution of tumor histology was included (e.g., serous 50; mucinous 40; and others 10). Even if a full text manuscript was written in a non-English language, the study was eligible if data on the histologic distribution of BOTs was included in the abstract written in English. The exclusion criteria were as follows: study subjects were not representative of an entire population but rather of a specifically defined population (e.g., women with advanced-stage BOTs or women with serous BOTs); the study did not present new data (e.g., case reports, review articles, editorials, and letters); and non-human studies. The abstract was evaluated to determine whether or not a study contained quantitative information on the distribution of tumor histology. If review of the abstract did not permit this determination, the full paper was evaluated. Final inclusion and exclusion were based on the full-text manuscripts. To broaden the search, a secondary strategy was carried out, reviewing reference lists of all available primary eligible studies. In the event of disagreement between the two investigators, the disagreement was solved by consensus.
2. Data extraction
Two independent investigators used a piloted data extraction form to collect all relevant data from the studies, including author, year of publication, country of study, type of cohort (single center, multicenter, and population-based), sample size, and information on the histologic distribution of BOTs. Data were entered into an electronic database so that duplicate entries existed for each study; when the two entries did not match, consensus was reached through discussion. We did not contact the authors of studies for any further information.
3. Data synthesis
The included studies were grouped according to geographic region (e.g., North America, Europe, the Middle East, and East Asia). These regions were then divided into subgroups according to the country studied. If an identical author or group reported several studies using the same or an overlapping cohort, we selected the more recent, larger study and excluded the other. In order to schematically illustrate the worldwide histologic distribution of BOTs, data from each study were totaled according to country, regardless of the design or size of the study, and a world map was created representing the most common histology according to country.
RESULTS
1. Study selection and study characteristics
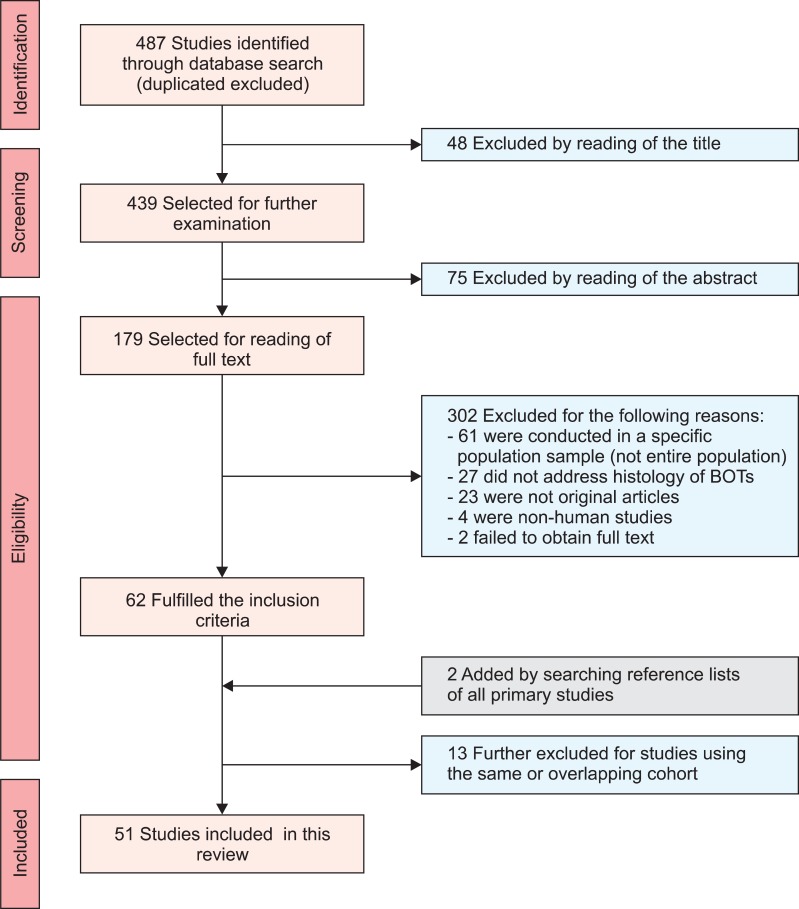
Initially, 487 studies were identified in the 3 online databases, after the removal of duplicates. Of the 487 studies, 123 were excluded because the title and abstract revealed no relevance to the current review, and 302 studies were excluded because they did not meet the inclusion criteria after reading the full text. Finally, 2 studies were added by searching the reference lists of all primary studies, and 13 studies were excluded for using the same or overlapping cohorts, leaving 51 studies eligible for inclusion in the systematic review. The full selection process is summarized in Fig. 1 showing the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review [11].
Fig. 1.
Search process and eligible studies (Preferred Reporting of Systematic Reviews and Meta-Analyses [PRISMA] flow diagram).
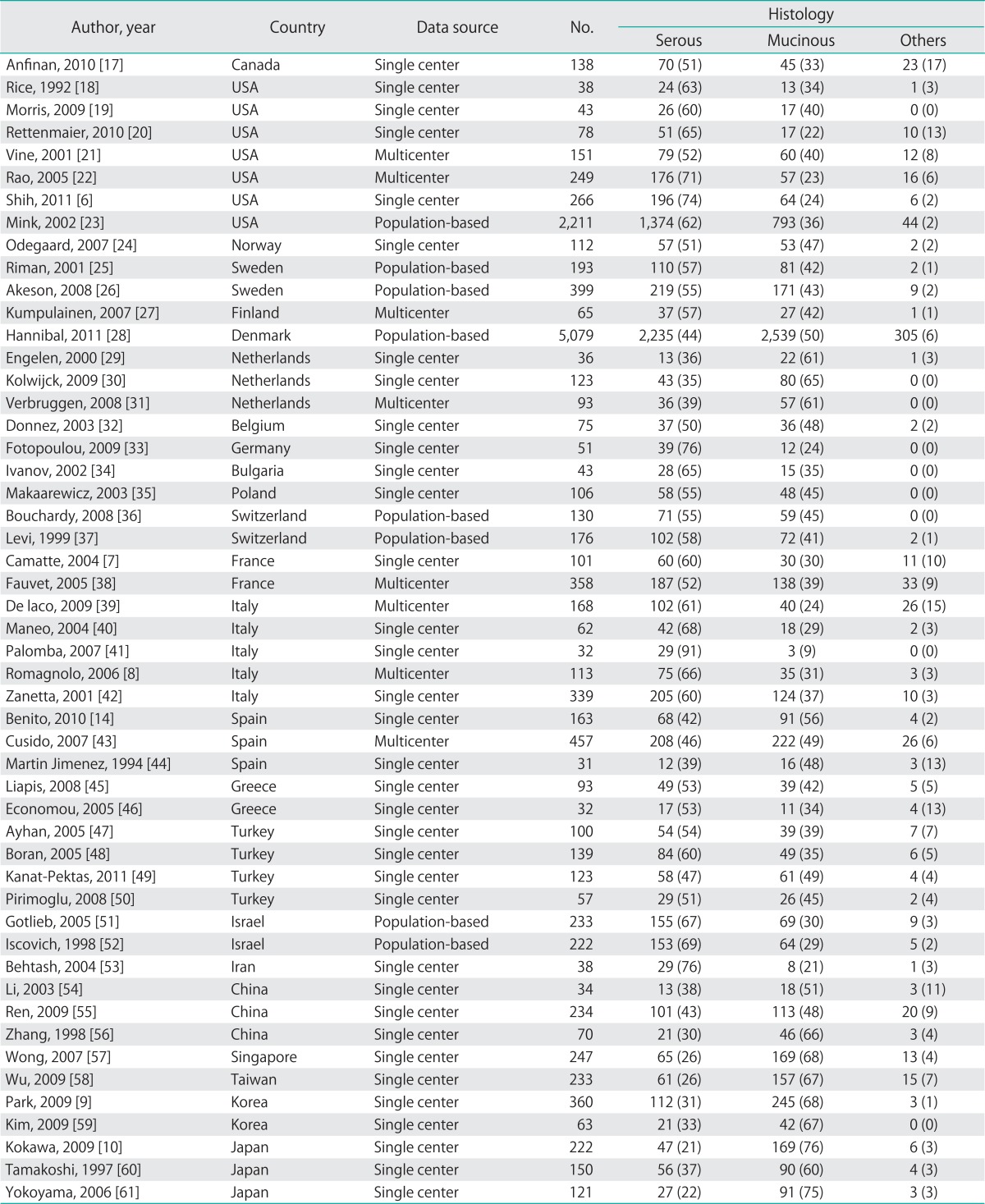
Overall, the 51 eligible studies included 8 studies from North America (2 countries: Canada and USA), 26 studies from Europe (14 countries: Norway, Sweden, Finland, Denmark, Netherlands, Belgium, Germany, Bulgaria, Poland, Switzerland, France, Italy, Spain, and Greece), 7 studies from the Middle East (3 countries: Turkey, Israel, and Iran), and 10 studies from East Asia (5 countries: China, Singapore, Taiwan, Korea, and Japan). Thirty-five studies (68) used a database from a single center, 8 (16) used a database from multi-centers, and 8 (16) used a population-based database (a regional or national cancer registry). The individual studies are described in Table 1.
Table 1.
The 51 studies included in this systematic review
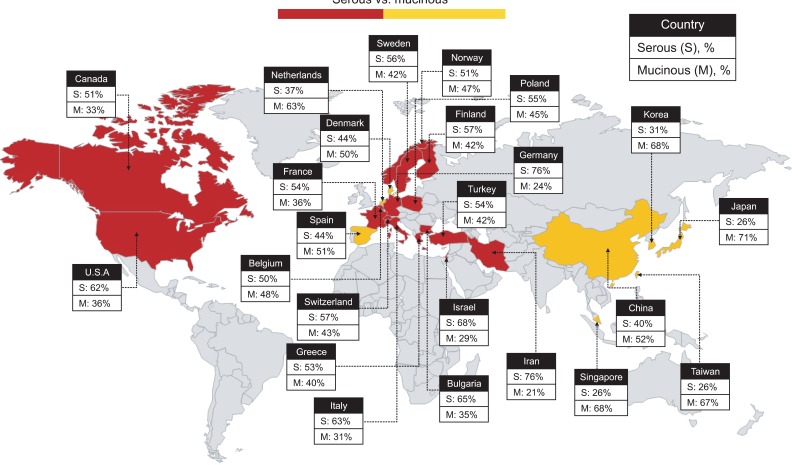
2. Mapping the distribution of tumor histology
Information from the review was used to create a world map illustrating the histologic distribution of BOTs according to country (Fig. 2). Serous histology was the most common type reported in North America, Europe, and the Middle East. In contrast, the mucinous histology was the most common type reported in East Asia. Of 14 European countries, 11 (79) reported serous-type BOTs as the most common, and 3 (21: Spain, Denmark, and Netherlands) reported mucinous-type BOTs as the most common.
Fig. 2.
World map based on tumor histology according to geographic region (country).
DISCUSSION
Our review is the first to systematically review, categorize, and map the worldwide histologic distribution of BOTs. The main finding is that the histologic distribution of BOTs is considerably different in various parts of the world, but follows specific patterns. In general, serous-type BOTs were the predominantly identified histologic type in North America, the Middle East, and most of Europe. In contrast, mucinous-type BOTs predominated in East Asia and parts of Europe. No data were available for other geographic regions of the world.
Although our study found that there is a difference in the histology of BOTs according to geographic region, it is not yet clear which factors are related to this phenomenon. Lifestyle factors appear to be attributable. According to a recent study conducted in Denmark [12], both a history of breastfeeding and use of oral contraceptives reduced the risk of BOTs, the effect being most pronounced for serous tumors. Increasing body mass index was associated with an increased risk of serous tumors (OR, 1.05 per BMI unit; 95 CI, 1.00 to 1.10), whereas current smoking was a strong risk factor for mucinous tumors alone (OR, 2.10; 95 CI, 1.22 to 3.60). Smoking in particular has been found in several studies to be a risk factor associated with benign, borderline, and invasive mucinous ovarian tumors [13-15]. Some studies have reported a strong association between smoking and these three types of tumors, suggesting that smoking is involved early in the neoplastic process. It has been speculated that the relationship between cigarette smoking and the development of mucinous tumors could be due to the similarity of mucinous tumors to gastrointestinal mucosa. Cigarette smoking has consistently been associated with mucinous gastrointestinal tract cancers such as those of the stomach and pancreas [12,13].
The results of the current study have potential implications for the treatment of choice in patients with BOTs. According to recent studies conducted in France [3,16], mucinous BOTs, unlike serous BOTs, do not appear to be a "safe" disease, with a 13 cumulative risk of recurrence in the form of invasive carcinoma at 10 years [16]. The authors of a previous study concluded that the use of salpingo-oophorectomy rather than cystectomy is preferred during conservative surgery for patients with mucinous BOTs because salpingo-oophorectomy decreases the risk of recurrence and does not impair fertility [3]. Therefore, in regions with a high incidence of mucinous BOTs, cystectomy should not be considered as a conservative surgery without the confirmation of tumor histology through intraoperative frozen section analysis. The results of the current study also have potential implications for the diagnosis of BOTs. Being aware of the histological distribution of BOTs over the world would help the pathologists who are to evaluate the ovarian masses with a high index of suspicion for BOTs.
This review has several limitations that merit attention. First, we tried to search many studies as possible through electronic databases, but many of which were not available. Only peer-reviewed articles were included in the systematic review. Second, this review may also be subject to language limitations, although we did not exclude a study if the abstract was written in English without regard to the language of the main text. Third, the histological distribution of BOTs within a certain country/geographical area can be biased by the existence of women belonged to different ethnic groups. That is, a relevant study conducted in Turkey would review the women with BOTs who are living in Turkey but who are not to be Turkish all the time. These women can be Greek, Armenian, Jewish, Kurdish or any immigrant from neighboring countries. Forth, we could not present the distribution of other histologic types such as clear cell and endometrioid cell because they were quite rare in BOTs. Finally, although we mapped the histologic distribution of BOTs, we cannot determine why these geographical differences exist, although they are likely to be a consequence of ethnicity and lifestyle factors. More research is needed to understand this phenomenon.
Despite these limitations, our review is the first to provide evidence on the worldwide histologic distribution of BOTs. While the histologic distribution of BOTs is considerably different in various parts of the world, it appears that specific patterns exist. In general, serous-type BOTs are the predominantly-identified histology in North America, the Middle East, and most of Europe. In contrast, mucinous-type BOTs predominate in East Asia and parts of Europe. The results of this review are important for investigators planning to conduct multi-national clinical trials in patients with BOTs. Without a general understanding of the histologic distribution of BOTs, data reported from one geographic region are difficult to apply to women living in other geographic regions.
ACKNOWLEDGMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920010).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Taylor HC., Jr Malignant and semimalignant tumors of the ovary. Surg Gynecol Obstet. 1929;48:204–230. [Google Scholar]
- 2.Cadron I, Leunen K, Van Gorp T, Amant F, Neven P, Vergote I. Management of borderline ovarian neoplasms. J Clin Oncol. 2007;25:2928–2937. doi: 10.1200/JCO.2007.10.8076. [DOI] [PubMed] [Google Scholar]
- 3.Koskas M, Uzan C, Gouy S, Pautier P, Lhomme C, Haie-Meder C, et al. Fertility determinants after conservative surgery for mucinous borderline tumours of the ovary (excluding peritoneal pseudomyxoma) Hum Reprod. 2011;26:808–814. doi: 10.1093/humrep/deq399. [DOI] [PubMed] [Google Scholar]
- 4.Uzan C, Kane A, Rey A, Gouy S, Duvillard P, Morice P. Outcomes after conservative treatment of advanced-stage serous borderline tumors of the ovary. Ann Oncol. 2010;21:55–60. doi: 10.1093/annonc/mdp267. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg SG, Bell DA, Kurman RJ, Seidman JD, Prat J, Ronnett BM, et al. Borderline ovarian tumors: key points and workshop summary. Hum Pathol. 2004;35:910–917. doi: 10.1016/j.humpath.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Shih KK, Zhou Q, Huh J, Morgan JC, Iasonos A, Aghajanian C, et al. Risk factors for recurrence of ovarian borderline tumors. Gynecol Oncol. 2011;120:480–484. doi: 10.1016/j.ygyno.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camatte S, Morice P, Thoury A, Fourchotte V, Pautier P, Lhomme C, et al. Impact of surgical staging in patients with macroscopic "stage I" ovarian borderline tumours: analysis of a continuous series of 101 cases. Eur J Cancer. 2004;40:1842–1849. doi: 10.1016/j.ejca.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Romagnolo C, Gadducci A, Sartori E, Zola P, Maggino T. Management of borderline ovarian tumors: results of an Italian multicenter study. Gynecol Oncol. 2006;101:255–260. doi: 10.1016/j.ygyno.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Surgical management of borderline ovarian tumors: The role of fertility-sparing surgery. Gynecol Oncol. 2009;113:75–82. doi: 10.1016/j.ygyno.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Kokawa K, Mikami Y, Sakata H, Oki N, Tanakas T, Yamazaki M, et al. Clinical outcome and prognostic factors in borderline tumors of the ovary: results from 17 years' experience in the Kinki District of Japan (1990-2006) Eur J Gynaecol Oncol. 2009;30:155–161. [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Huusom LD, Frederiksen K, Hogdall EV, Glud E, Christensen L, Hogdall CK, et al. Association of reproductive factors, oral contraceptive use and selected lifestyle factors with the risk of ovarian borderline tumors: a Danish case-control study. Cancer Causes Control. 2006;17:821–829. doi: 10.1007/s10552-006-0022-x. [DOI] [PubMed] [Google Scholar]
- 13.Jordan SJ, Whiteman DC, Purdie DM, Green AC, Webb PM. Does smoking increase risk of ovarian cancer? A systematic review. Gynecol Oncol. 2006;103:1122–1129. doi: 10.1016/j.ygyno.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Benito V, Lubrano A, Arencibia O, Medina N, Alvarez Eva E, Andujar M, et al. Serous and mucinous borderline ovarian tumors: are there real differences between these two entities? Eur J Obstet Gynecol Reprod Biol. 2010;153:188–192. doi: 10.1016/j.ejogrb.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Jha P, Ranson MK, Nguyen SN, Yach D. Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health. 2002;92:1002–1006. doi: 10.2105/ajph.92.6.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koskas M, Uzan C, Gouy S, Pautier P, Lhomme C, Haie-Meder C, et al. Prognostic factors of a large retrospective series of mucinous borderline tumors of the ovary (excluding peritoneal pseudomyxoma) Ann Surg Oncol. 2011;18:40–48. doi: 10.1245/s10434-010-1293-8. [DOI] [PubMed] [Google Scholar]
- 17.Anfinan N, Sait K, Ghatage P, Nation J, Chu P. Ten years experience in the management of borderline ovarian tumors at Tom Baker Cancer Centre. Arch Gynecol Obstet. 2011;284:731–735. doi: 10.1007/s00404-010-1713-9. [DOI] [PubMed] [Google Scholar]
- 18.Rice LW, Lage JM, Berkowitz RS, Goodman A, Muto MG, Knapp RC, et al. Preoperative serum CA-125 levels in borderline tumors of the ovary. Gynecol Oncol. 1992;46:226–229. doi: 10.1016/0090-8258(92)90260-p. [DOI] [PubMed] [Google Scholar]
- 19.Morris RT, Gershenson DM, Silva EG, Follen M, Morris M, Wharton JT. Outcome and reproductive function after conservative surgery for borderline ovarian tumors. Obstet Gynecol. 2000;95:541–547. doi: 10.1016/s0029-7844(99)00619-5. [DOI] [PubMed] [Google Scholar]
- 20.Rettenmaier MA, Lopez K, Abaid LN, Brown JV, 3rd, Micha JP, Goldstein BH. Borderline ovarian tumors and extended patient follow-up: an individual institution's experience. J Surg Oncol. 2010;101:18–21. doi: 10.1002/jso.21416. [DOI] [PubMed] [Google Scholar]
- 21.Vine MF, Ness RB, Calingaert B, Schildkraut JM, Berchuck A. Types and duration of symptoms prior to diagnosis of invasive or borderline ovarian tumor. Gynecol Oncol. 2001;83:466–471. doi: 10.1006/gyno.2001.6411. [DOI] [PubMed] [Google Scholar]
- 22.Rao GG, Skinner EN, Gehrig PA, Duska LR, Miller DS, Schorge JO. Fertility-sparing surgery for ovarian low malignant potential tumors. Gynecol Oncol. 2005;98:263–266. doi: 10.1016/j.ygyno.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Mink PJ, Sherman ME, Devesa SS. Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States: results from the SEER Program, 1978-1998. Cancer. 2002;95:2380–2389. doi: 10.1002/cncr.10935. [DOI] [PubMed] [Google Scholar]
- 24.Odegaard E, Staff AC, Langebrekke A, Engh V, Onsrud M. Surgery of borderline tumors of the ovary: retrospective comparison of short-term outcome after laparoscopy or laparotomy. Acta Obstet Gynecol Scand. 2007;86:620–626. doi: 10.1080/00016340701286934. [DOI] [PubMed] [Google Scholar]
- 25.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Risk factors for epithelial borderline ovarian tumors: results of a Swedish case-control study. Gynecol Oncol. 2001;83:575–585. doi: 10.1006/gyno.2001.6451. [DOI] [PubMed] [Google Scholar]
- 26.Akeson M, Zetterqvist BM, Dahllof K, Jakobsen AM, Brannstrom M, Horvath G. Population-based cohort follow-up study of all patients operated for borderline ovarian tumor in western Sweden during an 11-year period. Int J Gynecol Cancer. 2008;18:453–459. doi: 10.1111/j.1525-1438.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumpulainen S, Kuoppala T, Leminen A, Komulainen M, Puistola U, Sankila R, et al. Surgical staging, treatment, and follow-up of borderline tumors in different hospital categories: a prospective nationwide survey in Finland. Acta Obstet Gynecol Scand. 2007;86:610–614. doi: 10.1080/00016340701284707. [DOI] [PubMed] [Google Scholar]
- 28.Hannibal CG, Huusom LD, Kjaerbye-Thygesen A, Tabor A, Kjaer SK. Trends in incidence of borderline ovarian tumors in Denmark 1978-2006. Acta Obstet Gynecol Scand. 2011;90:305–312. doi: 10.1111/j.1600-0412.2010.01060.x. [DOI] [PubMed] [Google Scholar]
- 29.Engelen MJ, de Bruijn HW, Hollema H, ten Hoor KA, Willemse PH, Aalders JG, et al. Serum CA 125, carcinoembryonic antigen, and CA 19-9 as tumor markers in borderline ovarian tumors. Gynecol Oncol. 2000;78:16–20. doi: 10.1006/gyno.2000.5811. [DOI] [PubMed] [Google Scholar]
- 30.Kolwijck E, Thomas CM, Bulten J, Massuger LF. Preoperative CA-125 levels in 123 patients with borderline ovarian tumors: a retrospective analysis and review of the literature. Int J Gynecol Cancer. 2009;19:1335–1338. doi: 10.1111/IGC.0b013e3181a83e04. [DOI] [PubMed] [Google Scholar]
- 31.Verbruggen MB, van Diest PJ, Baak JP, Broeckaert MA, Kenemans P, Verheijen RH. The prognostic and clinical value of morphometry and DNA cytometry in borderline ovarian tumors: a prospective study. Int J Gynecol Pathol. 2009;28:35–40. doi: 10.1097/PGP.0b013e31818131ff. [DOI] [PubMed] [Google Scholar]
- 32.Donnez J, Munschke A, Berliere M, Pirard C, Jadoul P, Smets M, et al. Safety of conservative management and fertility outcome in women with borderline tumors of the ovary. Fertil Steril. 2003;79:1216–1221. doi: 10.1016/s0015-0282(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 33.Fotopoulou C, Schumacher G, Schefold JC, Denkert C, Lichtenegger W, Sehouli J. Systematic evaluation of the intraoperative tumor pattern in patients with borderline tumor of the ovary. Int J Gynecol Cancer. 2009;19:1550–1555. doi: 10.1111/IGC.0b013e3181a84699. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov S. Borderline ovarian tumors. Akush Ginekol (Sofiia) 2002;42:23–25. [PubMed] [Google Scholar]
- 35.Makarewicz H, Emerich J, Olszewski J. Comparison of two groups of patients with serous and mucinous types of borderline ovarian tumors. Ginekol Pol. 2003;74:24–31. [PubMed] [Google Scholar]
- 36.Bouchardy C, Fernandez S, Merglen A, Usel M, Fioretta G, Rapiti E, et al. Increased risk of second cancer among patients with ovarian borderline tumors. Gynecol Oncol. 2008;109:210–214. doi: 10.1016/j.ygyno.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Levi F, La Vecchia C, Randimbison L, Te VC. Borderline ovarian tumours in Vaud, Switzerland: incidence, survival and second neoplasms. Br J Cancer. 1999;79:4–6. doi: 10.1038/sj.bjc.6690002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403–410. doi: 10.1093/annonc/mdi083. [DOI] [PubMed] [Google Scholar]
- 39.De Iaco P, Ferrero A, Rosati F, Melpignano M, Biglia N, Rolla M, et al. Behaviour of ovarian tumors of low malignant potential treated with conservative surgery. Eur J Surg Oncol. 2009;35:643–648. doi: 10.1016/j.ejso.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Maneo A, Vignali M, Chiari S, Colombo A, Mangioni C, Landoni F. Are borderline tumors of the ovary safely treated by laparoscopy? Gynecol Oncol. 2004;94:387–392. doi: 10.1016/j.ygyno.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Palomba S, Zupi E, Russo T, Falbo A, Del Negro S, Manguso F, et al. Comparison of two fertility-sparing approaches for bilateral borderline ovarian tumours: a randomized controlled study. Hum Reprod. 2007;22:578–585. doi: 10.1093/humrep/del381. [DOI] [PubMed] [Google Scholar]
- 42.Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Mangioni C. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study. J Clin Oncol. 2001;19:2658–2664. doi: 10.1200/JCO.2001.19.10.2658. [DOI] [PubMed] [Google Scholar]
- 43.Cusido M, Balaguero L, Hernandez G, Falcon O, Rodriguez-Escudero FJ, Vargas JA, et al. Results of the national survey of borderline ovarian tumors in Spain. Gynecol Oncol. 2007;104:617–622. doi: 10.1016/j.ygyno.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Martin Jimenez A, Miralles Pi RM, Escobedo Sanchez A, Gine Martinez L, Condom Mundo E, Balaguero Llado L. Ovarian tumors of low malignant potential (borderline): a retrospective study of 31 cases. Eur J Gynaecol Oncol. 1994;15:300–304. [PubMed] [Google Scholar]
- 45.Liapis A, Bakalianou K, Iavazzo C, Salakos N, Paltoglou G, Kondi-Pafiti A. A retrospective analysis of borderline ovarian tumors in a Greek university hospital. Eur J Gynaecol Oncol. 2008;29:383–385. [PubMed] [Google Scholar]
- 46.Economou A, Panagopoulos P, Koutras I, Petrakos G, Karadaglis S, Karcanis C. A retrospective study of 32 borderline ovarian tumours: the experience of a non-specialized centre. Eur J Gynaecol Oncol. 2005;26:196–198. [PubMed] [Google Scholar]
- 47.Ayhan A, Guvendag Guven ES, Guven S, Kucukali T. Recurrence and prognostic factors in borderline ovarian tumors. Gynecol Oncol. 2005;98:439–445. doi: 10.1016/j.ygyno.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Boran N, Cil AP, Tulunay G, Ozturkoglu E, Koc S, Bulbul D, et al. Fertility and recurrence results of conservative surgery for borderline ovarian tumors. Gynecol Oncol. 2005;97:845–851. doi: 10.1016/j.ygyno.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Kanat-Pektas M, Ozat M, Gungor T, Sahin I, Yalcin H, Ozdal B. Complete lymph node dissection: is it essential for the treatment of borderline epithelial ovarian tumors? Arch Gynecol Obstet. 2011;283:879–884. doi: 10.1007/s00404-010-1539-5. [DOI] [PubMed] [Google Scholar]
- 50.Pirimoglu ZM, Afsin Y, Guzelmeric K, Yilmaz M, Unal O, Turan MC. Is it necessary to do retroperitoneal evaluation in borderline epithelial ovarian tumors? Arch Gynecol Obstet. 2008;277:411–414. doi: 10.1007/s00404-007-0478-2. [DOI] [PubMed] [Google Scholar]
- 51.Gotlieb WH, Chetrit A, Menczer J, Hirsh-Yechezkel G, Lubin F, Friedman E, et al. Demographic and genetic characteristics of patients with borderline ovarian tumors as compared to early stage invasive ovarian cancer. Gynecol Oncol. 2005;97:780–783. doi: 10.1016/j.ygyno.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Iscovich J, Shushan A, Schenker JG, Paltiel O. The incidence of borderline ovarian tumors in Israel: a population-based study. Cancer. 1998;82:147–151. doi: 10.1002/(sici)1097-0142(19980101)82:1<147::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Behtash N, Modares M, Abolhasani M, Ghaemmaghami F, Mousavi M, Yarandi F, et al. Borderline ovarian tumours: clinical analysis of 38 cases. J Obstet Gynaecol. 2004;24:157–160. doi: 10.1080/01443610310001645460. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Cui H, Shen DH, Zhao Y, Wei LH, Qian HN. Clinical and pathological features of borderline ovarian tumors. Zhonghua Fu Chan Ke Za Zhi. 2003;38:81–84. [PubMed] [Google Scholar]
- 55.Ren J, Lou JY, Liu H, Wang P, Zhang JW, Yang KX, et al. Clinicopathologic features of 234 cases with borderline ovarian tumors. Zhonghua Fu Chan Ke Za Zhi. 2009;44:116–120. [PubMed] [Google Scholar]
- 56.Zhang Z, Hong W, Li L. The clinical features and management of borderline ovarian tumors. Zhonghua Fu Chan Ke Za Zhi. 1998;33:556–559. [PubMed] [Google Scholar]
- 57.Wong HF, Low JJ, Chua Y, Busmanis I, Tay EH, Ho TH. Ovarian tumors of borderline malignancy: a review of 247 patients from 1991 to 2004. Int J Gynecol Cancer. 2007;17:342–349. doi: 10.1111/j.1525-1438.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu TI, Lee CL, Wu MY, Hsueh S, Huang KG, Yeh CJ, et al. Prognostic factors predicting recurrence in borderline ovarian tumors. Gynecol Oncol. 2009;114:237–241. doi: 10.1016/j.ygyno.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Kim JH, Kim TJ, Park YG, Lee SH, Lee CW, Song MJ, et al. Clinical analysis of intra-operative frozen section proven borderline tumors of the ovary. J Gynecol Oncol. 2009;20:176–180. doi: 10.3802/jgo.2009.20.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamakoshi K, Kikkawa F, Nakashima N, Tamakoshi A, Kawai M, Furuhashi Y, et al. Clinical behavior of borderline ovarian tumors: a study of 150 cases. J Surg Oncol. 1997;64:147–152. doi: 10.1002/(sici)1096-9098(199702)64:2<147::aid-jso11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Yokoyama Y, Moriya T, Takano T, Shoji T, Takahashi O, Nakahara K, et al. Clinical outcome and risk factors for recurrence in borderline ovarian tumours. Br J Cancer. 2006;94:1586–1591. doi: 10.1038/sj.bjc.6603139. [DOI] [PMC free article] [PubMed] [Google Scholar]