Abstract
Objective
Deep venous thrombosis and pulmonary embolism are common in patients with epithelial ovarian cancer, resulting in high costs associated with diagnosis and treatment. I aimed to identify subtypes of epithelial ovarian cancer that pose greater and lesser venous thromboembolism (VTE) risk.
Methods
I assessed the outcomes of 641 patients with epithelial ovarian, fallopian tube, and primary peritoneal cancer over a ten-year period. All inpatient, outpatient, and pathology records were reviewed. The rates at which people were evaluated for and diagnosed with venous thromboembolism were assessed.
Results
Of the 641 cases, 30.0% underwent an imaging test to evaluate for deep venous thrombosis (DVT) and 21.7% underwent testing for pulmonary embolism (PE). A 10.8% of all subjects were diagnosed with DVT and 7.2% were diagnosed with PE. Borderline tumors and mucinous showed a strikingly low rate of both DVT and PE. Clear cell and high-grade undifferentiated adenocarcinomas were the most likely to result in VTE. In a multivariate model, pathologic subtype was not only a significant predictor of VTE, but was the single best predictor of VTE.
Conclusion
Clear cell and undifferentiated pathology in epithelial ovarian carcinomas is associated with a higher VTE risk. The underlying reason for this may related to differences in tumor biology. By identifying low and high risk groups, I may both better conserve medical resources and design more effective thromboprophylaxis for my patients.
Keywords: Histology, Ovarian neoplasms, Venous thromboembolism
INTRODUCTION
Ovarian cancer is a tumor with an extremely high thrombotic rate. Despite advances in the prevention of deep venous thrombosis (DVT) and pulmonary embolism (PE) by sequential compression devices and perioperative anticoagulation, venous thromboembolic events remain a serious and common complication for women with ovarian cancer after primary surgical treatment. An estimated 5.0-16.6% of women with ovarian, primary peritoneal and fallopian tube cancers develop a venous thromboembolism (VTE) within thirty days of surgery [1-3], resulting in considerable morbidity [4] and cost [5,6].
The relationship between thromboembolic events and underlying malignancy was first noted by Trousseau [7] in 1865. Further research noted that this relationship is especially strong for tumors of intra-abdominal or pelvic origin [8]. Patients with ovarian cancer have one of the highest incidences of thrombosis among carcinomas. Ovarian cancer patients may be particularly susceptible to VTE due to a need for prolonged abdominal surgeries, immobility during and after surgery, and vascular wall distortion from tumor compression. Perhaps most importantly, ovarian cancer appears to induce a hypercoagulable state. The exact mechanisms of cancer associated hypercoagulability are multi-faceted and controversial. However, at a cellular level, I understand that tumor cells are pro-thrombotic; neoplastic cells are known to activate the clotting system via thrombin and stimulation of mononuclear cells [9]. Tumor cell release of inflammatory cytokines enhances platelet aggregation. Ovarian tumor cells can also activate the clotting cascade via production of tissue factor or tissue factor bearing microparticles [10,11]. Interestingly, ovarian cancer cells can produce a hyperviscosity syndrome [12]. Whether different histologic subtypes of ovarian cancer are more or less prone to cause thrombosis remains undetermined, yet prior studies have suggested those with clear cell histology have the highest risk [13].
The purpose of this clinicopathologic correlation study was 1) to measure the incidence of DVT and PE in patients with different histologic subtypes of epithelial ovarian cancer, and 2) to investigate the yield on radiologic studies aimed at identifying VTE events. I aimed to identify subtypes of epithelial ovarian cancer that pose greater and lesser VTE risk. Considerable time and financial resources are spent investigating VTE risk among ovarian cancer patients. This study may advance my understanding of tumor biology and inform my clinical resource utilization. Furthermore, if a subpopulation of ovarian cancer patients is more or less likely to suffer venous thromboembolic events, safer and better preventive strategies may be employed.
MATERIALS AND METHODS
This study was reviewed, approved, and performed in compliance with the University of Michigan Institutional Review Board. I conducted an in-depth study of a cohort of women who had a diagnosis of epithelial ovarian, fallopian tube, or primary peritoneal adenocarcinoma over a ten-year period, 1999-2009. The University of Michigan Tumor Registry identified 644 cases during this time period. Three were excluded because pathology reports were not available for pathologic confirmation. All 641 evaluable subjects had undergone at least a portion of their treatment at the University of Michigan at which time their pathology was presented at a Tumor Board conference for review. The inpatient and outpatient records for each subject were obtained and reviewed.
The primary exposure of interest was histologic subtype. Histologic subtype was defined as the predominant subtype present in the tumor. If two or more histologic subtypes were present, and no predominant subtype was indicated by the pathologist, the tumor was categorized separately as a mixed epithelial tumor. If the tumor had a second minor subtype present, encompassing less than 10% of the tumor, the tumor was classified as the predominant subtype.
The primary outcome measure was incident DVT or PE within five years of diagnosis. No routine screening occurred, and all events were based on identification of clinically relevant, symptomatic patients confirmed with imaging studies. Superficial thrombophlebitis, ovarian vein thrombosis, and clots related to indwelling catheters were not considered as DVT. Events occurring prior to a diagnosis with cancer were not included.
Clinical covariates collected included: cancer origin (ovarian, fallopian tube, peritoneal), FIGO stage, age, prior history of DVT, prior history of PE, non-white race, parity, menopausal status, body mass index (BMI) at time of diagnosis, smoking status at diagnosis, and number of comorbid conditions. Comorbid conditions abstracted included: hypertension, severe pulmonary disease, atrial fibrillation or cardiac conduction abnormality, coronary artery disease or cardiomyopathy, peripheral vascular disease, stroke, autoimmune disease, and diabetes. Age was categorized into less than 45, 46-55, 56-65, and over 65 years old. BMI was categorized into <25, 25-29.9, and over 30. Smoking was categorized into non-smoker, former smoker in the last 20 years, and current smoker.
Data on tumor pathology, venous thromboembolic events, and patient clinical information were all abstracted independently and in a double blinded fashion. The datasets were then merged for analysis. One-quarter of charts were re-reviewed to ensure intra-observer reliability.
Data were analyzed using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA). Frequencies and bivariate analyses are presented. Chi-square, t-test, and multivariate regressions were performed using a two-sided alpha of 0.05. In the multivariate analyses, logistic regressions were used to predict the occurrence of any venous thromboembolic event (either DVT or PE). A two-stage design was used, first identifying demographic and clinical covariates that predict VTE. The reduced set of variables from a forward selection process that were borderline significant (alpha<0.10) were entered into a second model. The second model examined histologic predictors of VTE, controlling for relevant demographic and clinical covariates from the first model.
RESULTS
Over a ten year span, 641 pathology-confirmed cases of epithelial ovarian, fallopian tube, and primary peritoneal cancers were identified. Of the 641 cases, 192 (30.0%) underwent an imaging test to evaluate for DVT. Among these cases, a diagnosis of DVT was made in 69 (10.8%) subjects. PE testing occurred in 139 (21.7%), and a total of 46 (7.2%) subjects were diagnosed with PE. Twenty-five subjects had both DVT and PE diagnosed. The mean time from cancer diagnosis to VTE was 89 days. Imaging tests were ordered based on clinical suspicion only. A low threshold existed for ordering such an imaging test, as discussed in the discussion.
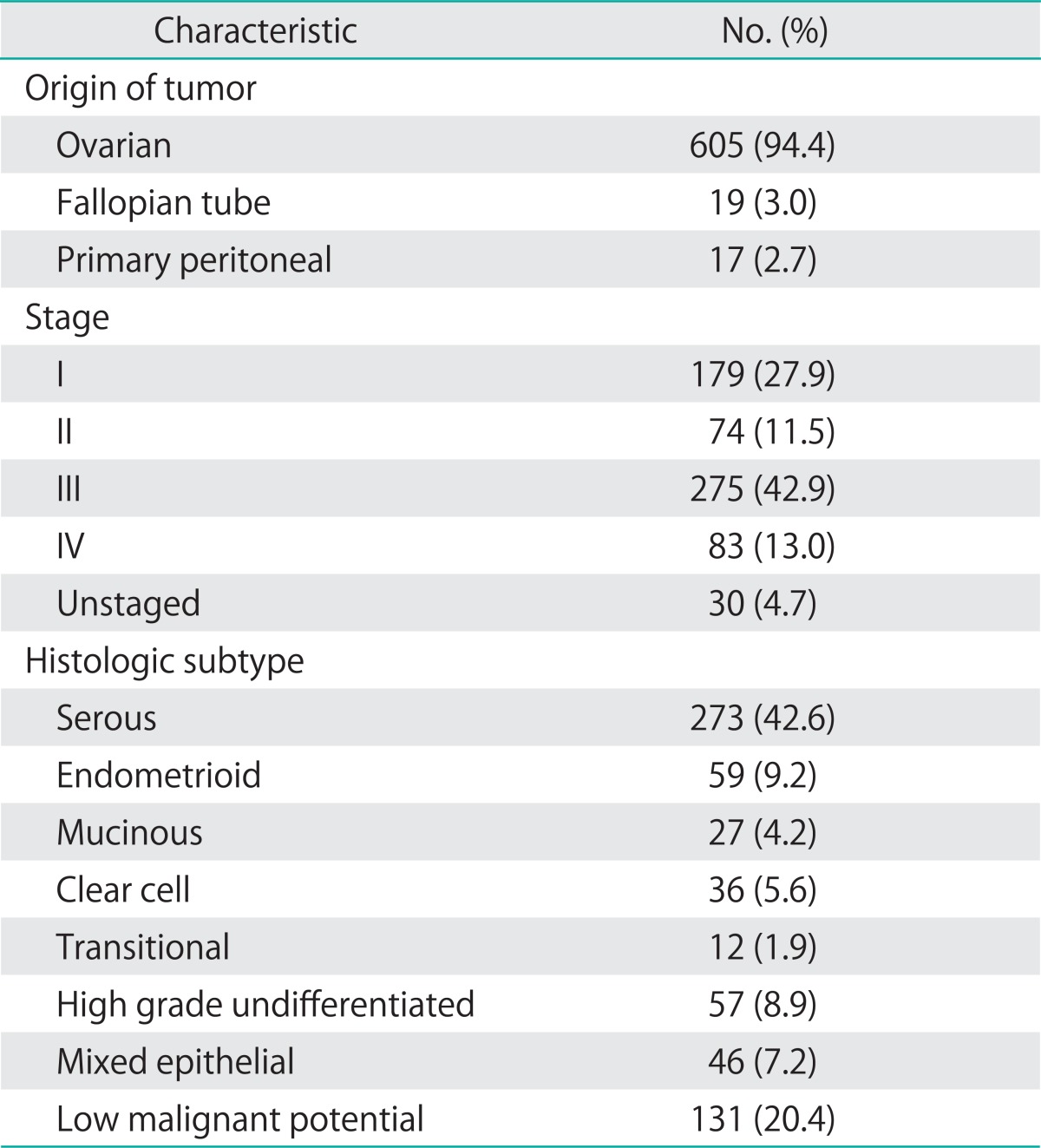
Baseline demographic characteristics of the population are shown in Table 1. The median follow-up time for the cohort was 2.8 years. The mean age for the cohort was 55.2 years, and the mean body mass index was 28.5. Forty percent of subjects had at least one comorbid illness, and 11.8% had at least two comorbid conditions. Pathologic characteristics are given in Table 2. Most cancers were stage III (42.9%). Few cancers (4.7%) were not surgically staged, due to referral from an outside provider who had performed surgery without complete staging, treatment without complete surgical staging, or patient condition or death precluding staging. Serous tumors accounted for the predominant subtype (42.6%). A minority of tumors (7.2%) had mixed epithelial subtypes, where no one subtype predominated. The largest proportion of these mixed epithelial tumors were of mixed serous and clear cell histology (35%), followed by serous and endometrioid histology (27%).
Table 1.
Baseline demographic characteristics of the population
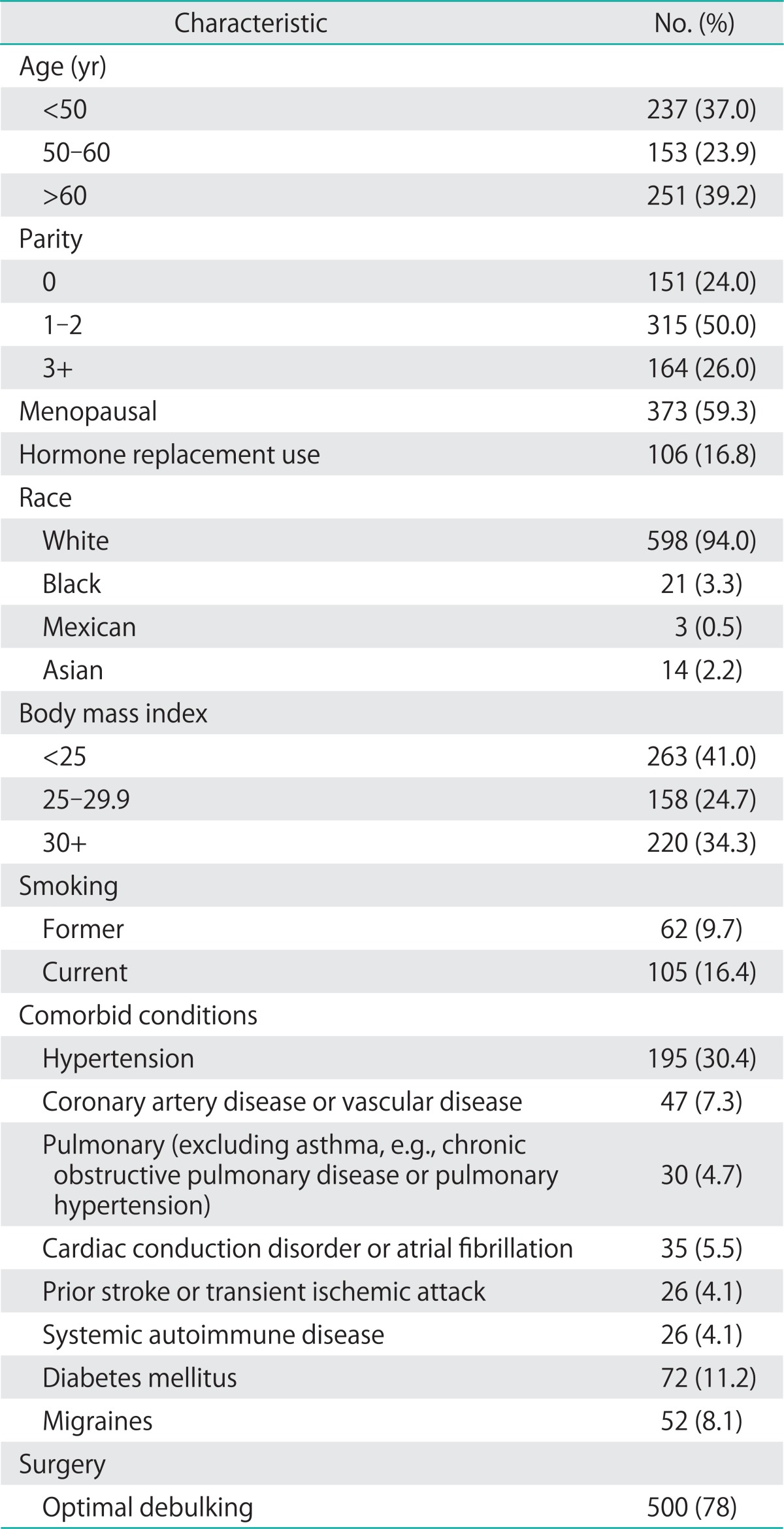
Table 2.
Clinicopathologic characteristics of tumor
1. Evaluation of VTE: clinical correlates
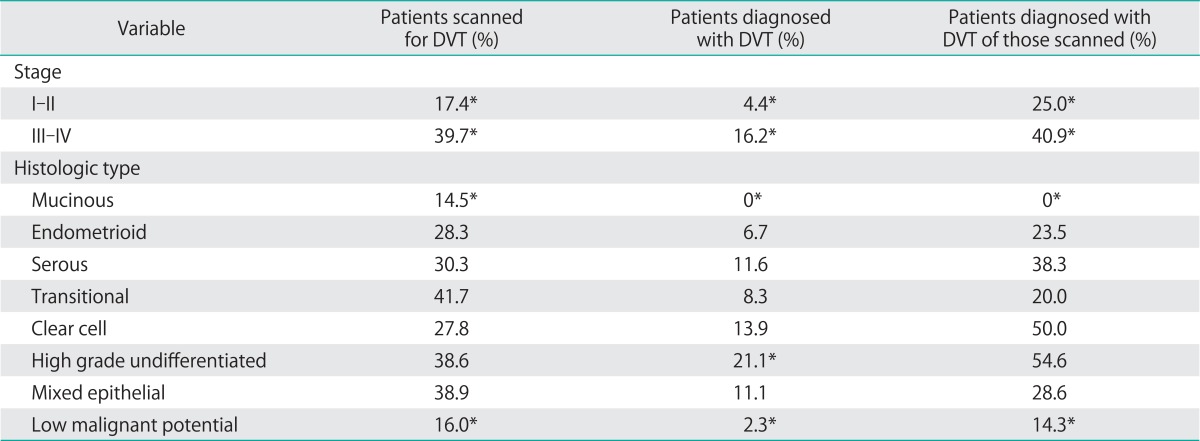
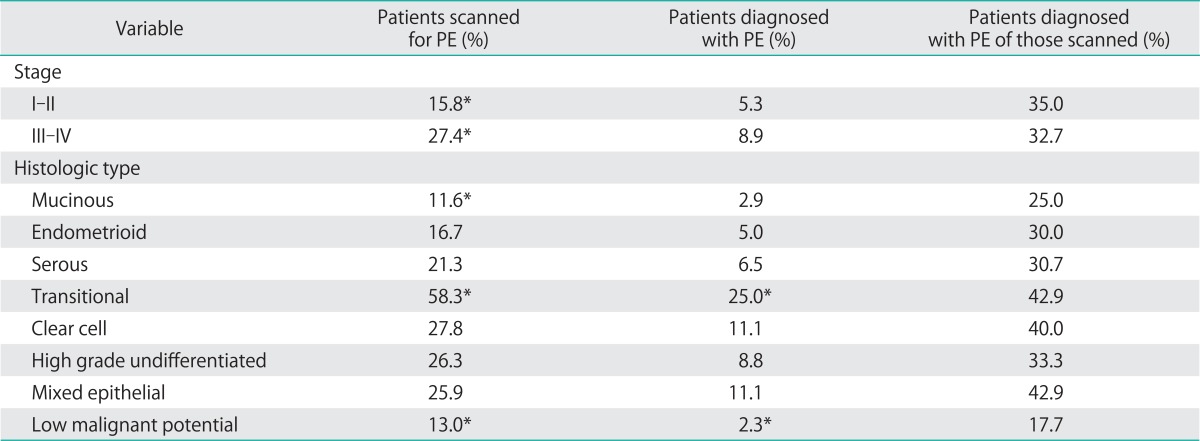
Nearly one third of ovarian cancer patients underwent an imaging test to evaluate for DVT and over 20% underwent testing for PE. The proportions of patients who were scanned and who were diagnosed with DVT are shown (Table 3). Advanced stage and high grade undifferentiated tumors show greater rates of evaluation for and diagnosis of DVT. Mucinous and low malignant potential tumors show lower scanning rates and yield. The proportions of patients who were scanned and who were diagnosed with PE are also shown in Table 4.
Table 3.
Pathologic correlates of deep venous thrombosis (DVT)
*p<0.05.
Table 4.
Pathologic correlates of pulmonary embolism (PE)
*p<0.05.
The likelihood of being scanned for a DVT or PE increased with BMI. Among those with normal BMI, 28.9% were evaluated for a VTE compared to 35.9% of obese individuals (p-trend <0.01). Patients who were overweight or obese, however, were no more likely to be diagnosed with a DVT or PE despite being scanned more often (p-trend: p=0.38 for DVT, p=0.33 for PE). Systemic autoimmune disease was associated with having an evaluation for PE (42.3% vs. 20.8%, p<0.01), although not for DVT (p=0.16). The diagnosis of DVT and PE was not statically more likely (p=0.10 and p=0.06, respectively) among those with autoimmune disease, although small numbers limited this analysis. Age was associated with risk of DVT (14.7% in those over 60 years old vs. 8.9% in those under 50 years old, p-trend=0.03), but older individuals were no more likely to be evaluated for DVT or PE than younger individuals. Hormone replacement was also associated with VTE (p=0.03). No other demographic or clinical covariates were associated with the likelihood of being scanned for VTE or increased rate of VTE. This includes no association with coronary artery disease or peripheral vascular disease.
2. VTE risk: pathologic effect of ovarian tumor pathology
Patients with high-grade undifferentiated tumors had a 38.6% chance of being scanned for DVT and were the most likely of any group to be diagnosed with DVT or PE. Patients with clear cell histology had an 11% chance of thromboembolism. Increased tumor grade and histology correlated with an increased risk of being scanned for and diagnosed with DVT/PE.
Increased tumor stage also showed a strong correlation with both scanning for thromboembolic disease and diagnosis. Patients with stage III/IV disease were more than twice as likely as stage I/II patients to be scanned, and had a four-fold increased risk of being diagnosed with DVT.
Similarly, low grade tumors, tumors of low malignant potential (borderline tumors) and mucinous tumors showed a strikingly low rate of both DVT and PE. Only 2.3% of patients with borderline tumors developed DVT and no patients with mucinous tumors developed DVT. Similarly, only 2.3% of patients with borderline tumors developed a clinically evident PE.
3. Multivariate risk stratification
Using staged multivariate logistic regression modeling, the risk of having any VTE was examined. Among demographic and clinical covariates, tumor stage and BMI were significant predictors of VTE. Surprisingly, history of migraines, known coronary artery or vascular disease, smoking status, menopausal status, optimal debulking and age were not predictors of a venous thromboembolic event in the multivariate setting.
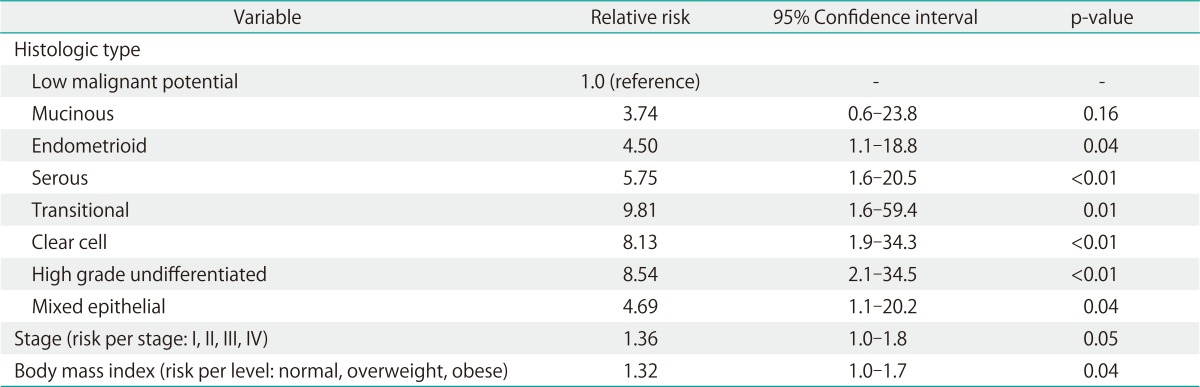
Analyzing demographic and clinical covariates together with pathologic predictors of VTE in my complete model, a significant contribution from the histologic subtype information was seen. The addition of histologic subtype information significantly enhanced the model (likelihood ratio difference, 29.9; degrees of freedom, 7; p<0.001). In fact, the best single predictor of VTE was histologic subtype of the tumor. Mucinous tumors were unlikely to result in a clinically evident VTE, while high-grade undifferentiated and clear cell histology has the greatest relative risk (Table 5).
Table 5.
Multivariate analysis: prediction of any venous thromboembolism
DISCUSSION
The risk for VTE varies substantially among patients with epithelial ovarian carcinomas with differing pathologic subtypes. In this retrospective cohort, I were able to identify both the rate at which ovarian cancer patients are scanned for VTE, and the VTE event rate over a prolonged period. Because I had reporting from hospital, radiology, and outpatient records, I have had a comprehensive look at an important driver of medical costs and morbidity among ovarian cancer patients.
Our study details that nearly one in four women with ovarian cancer develops VTE. This rate is substantially higher than that reported in the literature previously (2.8-16.6%) [2,3,12,14,15]. In my institution, I have a high tolerance (e.g., low threshold) for resident house staff to evaluate for VTE, evident by my finding that nearly 48% of my patients undergo evaluation for VTE at some point in their treatment. This aggressive case finding may contribute to my high incidence, but I do not assess for VTE without signs or symptoms prompting such investigation. Clinical suspicion is the only reason for ordering an imaging test; D-dimer was not used. Furthermore, the high incidence of VTE supports my aggressive testing. An alternative explanation for my high VTE rate may also be that I are able to search complete and comprehensive inpatient, outpatient, and radiology records on each individual. This may account for more accurate assessments.
As expected, I found that VTE assessment was performed more often in the obese. Obesity is a known risk factor for VTE [15] and was a predictor of having VTE in my analysis. Tumor stage also predicted VTE occurrence. Both of these are highly correlated to bed rest, immobility, and prolonged hospital stays. Tumor stage is also related to extent of surgery, and both are correlated with length of time in the operating room. Prior studies have identified extent of surgery, length of operating room time, bed rest, immobility, and length of hospital stay as risk factors for VTE. My study is external validated by consistency with such prior research [16-18]. Surprisingly, both age and smoking were not significant risk factors for VTE. These may not have been independent risks in my multivariate model because age is correlated with stage and there were few smokers in my cohort.
We found very low rates of VTE in mucinous and low malignant potential tumors. My study suggests that in patients with these low grade tumors, aggressive case finding may be unwarranted. Understanding the pretest probability of VTE event can better inform the use of testing.
Conversely, I found elevated VTE risk in those with clear cell and high grade undifferentiated histology; transition cell carcinomas had high risk but represented less than 2% of the total sample. Prior studies have examined the effect of clear cell carcinoma on the risk of VTE in ovarian cancer patients, but no studies have so broadly reported on pathologic correlates to VTE previously [2,13,19,20]. Secondary prevention efforts may be focused on those individuals with the highest risk pathologic subtypes and clinical comorbid conditions. Given the extremely high risks of VTE, in the postoperative setting, vigilant dual prophylaxis with compression devices and pharmacotherapy is warranted. Extended prophylaxis for four weeks after surgery should be strongly considered [21,22]. Furthermore, patients with high risk histologic subgroups may be candidates for low dose warfarin or higher dose anticoagulant therapies. Evaluation trials in such high risk subgroups should be undertaken given the comorbidity and cost associated with thromboembolic events.
Limitations of my study include lack of information on VTE incidence prior to surgery. While none of the patients had a clinical diagnosis of prior VTE, subclinical VTE may have been present. Prior research has noted that subclinical VTE is frequent in cancer patients [13,23], yet routinely not assessed. Data on preoperative VTE prevalence was not available as my data includes only those diagnosed with VTE after the diagnosis of ovarian cancer. Other limitations of my approach include lack of data collection on leg edema, data on outcomes after treatment with heparin or direct thrombin inhibitor, and data on patients who no longer receive care within the health system. My research does capture a wide range of community hospitals and health centers as data inputs, however, so that those evaluated at other institutions for VTE are typically captured in my outpatient record system.
The standard technique for prevention of VTE in my institution during this time period included both sequential compression stockings and subcutaneous heparin in the perioperative and postoperative period. None of my patients is maintained on anticoagulation prophylaxis during chemotherapy as is consistent with published guidelines [24,25].
Our study suggests that more effective thromboprophylaxis, better pretest probability assessment in case finding, and further secondary prevention research strategies are necessary to combat the high incidence of VTE among my patients. Understanding the connections between tumor biology and VTE risk may help stratify patients to conserve medical resources and identify high risk groups.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Black D, Iasonos A, Ahmed H, Chi DS, Barakat RR, Abu-Rustum NR. Effect of perioperative venous thromboembolism on survival in ovarian, primary peritoneal, and fallopian tube cancer. Gynecol Oncol. 2007;107:66–70. doi: 10.1016/j.ygyno.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez AO, Wun T, Chew H, Zhou H, Harvey D, White RH. Venous thromboembolism in ovarian cancer. Gynecol Oncol. 2007;105:784–790. doi: 10.1016/j.ygyno.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Tateo S, Mereu L, Salamano S, Klersy C, Barone M, Spyropoulos AC, et al. Ovarian cancer and venous thromboembolic risk. Gynecol Oncol. 2005;99:119–125. doi: 10.1016/j.ygyno.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SR, Ducruet T, Lamping DL, Arsenault L, Miron MJ, Roussin A, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005;165:1173–1178. doi: 10.1001/archinte.165.10.1173. [DOI] [PubMed] [Google Scholar]
- 5.Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486. doi: 10.18553/jmcp.2007.13.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley CT, Brasel KJ, Miller JJ, Pappas SG. Cost-effectiveness of prolonged thromboprophylaxis after cancer surgery. Ann Surg Oncol. 2010;17:31–39. doi: 10.1245/s10434-009-0671-6. [DOI] [PubMed] [Google Scholar]
- 7.Trousseau A. Clinique médicale de l'Hôtel-Dieu de Paris. Paris: J. B. Bailliere et Fils; 1865. [Google Scholar]
- 8.Svendsen E, Karwinski B. Prevalence of pulmonary embolism at necropsy in patients with cancer. J Clin Pathol. 1989;42:805–809. doi: 10.1136/jcp.42.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prandoni P, Piccioli A, Girolami A. Cancer and venous thromboembolism: an overview. Haematologica. 1999;84:437–445. [PubMed] [Google Scholar]
- 10.Yokota N, Koizume S, Miyagi E, Hirahara F, Nakamura Y, Kikuchi K, et al. Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells. Br J Cancer. 2009;101:2023–2029. doi: 10.1038/sj.bjc.6605406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uno K, Homma S, Satoh T, Nakanishi K, Abe D, Matsumoto K, et al. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer. 2007;96:290–295. doi: 10.1038/sj.bjc.6603552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Tempelhoff GF, Heilmann L, Hommel G, Schneider D, Niemann F, Zoller H. Hyperviscosity syndrome in patients with ovarian carcinoma. Cancer. 1998;82:1104–1111. doi: 10.1002/(sici)1097-0142(19980315)82:6<1104::aid-cncr14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007;97:1053–1057. doi: 10.1038/sj.bjc.6603989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 15.Fotopoulou C, duBois A, Karavas AN, Trappe R, Aminossadati B, Schmalfeldt B, et al. Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel-containing first-line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. J Clin Oncol. 2008;26:2683–2689. doi: 10.1200/JCO.2008.16.1109. [DOI] [PubMed] [Google Scholar]
- 16.Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol. 2007;95:167–174. doi: 10.1002/jso.20625. [DOI] [PubMed] [Google Scholar]
- 17.Peedicayil A, Weaver A, Li X, Carey E, Cliby W, Mariani A. Incidence and timing of venous thromboembolism after surgery for gynecological cancer. Gynecol Oncol. 2011;121:64–69. doi: 10.1016/j.ygyno.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Lim MC, Lee HS, Kang S, Seo SS, Lee BY, Park SY. Minimizing tumor burden by extensive cytoreductive surgery decreases postoperative venous thromboembolism in ovarian clear cell carcinoma. Arch Gynecol Obstet. 2010;281:329–334. doi: 10.1007/s00404-009-1120-2. [DOI] [PubMed] [Google Scholar]
- 19.Duska LR, Garrett L, Henretta M, Ferriss JS, Lee L, Horowitz N. When 'never-events' occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol Oncol. 2010;116:374–377. doi: 10.1016/j.ygyno.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura Y, Robertson G, Marsden DE, Kim SN, Gebski V, Hacker NF. Thromboembolic complications in patients with clear cell carcinoma of the ovary. Gynecol Oncol. 2007;104:406–410. doi: 10.1016/j.ygyno.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Martino MA, Williamson E, Rajaram L, Lancaster JM, Hoffman MS, Maxwell GL, et al. Defining practice patterns in gynecologic oncology to prevent pulmonary embolism and deep venous thrombosis. Gynecol Oncol. 2007;106:439–445. doi: 10.1016/j.ygyno.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Einstein MH, Kushner DM, Connor JP, Bohl AA, Best TJ, Evans MD, et al. A protocol of dual prophylaxis for venous thromboembolism prevention in gynecologic cancer patients. Obstet Gynecol. 2008;112:1091–1097. doi: 10.1097/AOG.0b013e31818b1486. [DOI] [PubMed] [Google Scholar]
- 23.White RH, Chew HK, Zhou H, Parikh-Patel A, Harris D, Harvey D, et al. Incidence of venous thromboembolism in the year before the diagnosis of cancer in 528,693 adults. Arch Intern Med. 2005;165:1782–1787. doi: 10.1001/archinte.165.15.1782. [DOI] [PubMed] [Google Scholar]
- 24.Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 25.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]


