Abstract
Thin films of 1,3-diethylbenzimidazol-2-ylidene (BIEt) were fabricated from THF solution on solid gold substrates and characterised by high-resolution X-ray photoelectron and near-edge X-ray absorption fine structure spectroscopy. The surface-analytical data are in accord with the formation of self-assembled monolayers of BIEt molecules exhibiting an approximately vertical orientation on the substrate. The crystal structure of (BIEt)2 was also determined.
The report of the first stable N-heterocyclic carbene (NHC) in 1991[1] triggered the tremendous development of such singlet carbenes from laboratory curiosities to true workhorses in synthesis and catalysis.[2] Very recently, even applications in the area of functional materials have begun to emerge.[3] NHCs are potent nucleophiles and are widely used as nucleophilic organocatalysts. They are ligands of particularly high σ-donicity and form complexes with almost any metal of the periodic table. In view of this unrivalled versatility in molecular coordination chemistry, we were surprised to find that almost nothing is known about the surface coordination chemistry of NHCs. This prompted us to start an investigation addressing the chemisorption of NHCs on metal substrates, with a view to establish a completely new adsorbate system for the fabrication of self-assembled monolayers (SAMs).[4] The only relevant reports that are related to this issue deal with NHCs interacting with metal nanoparticles.[5–7]
We have studied the chemisorption of 1,3-diethylbenzimidazol-2-ylidene (BIEt) on gold. In comparison to imidazol-2-ylidene-type analogues the benzimidazol-2-ylidene system exhibits an extended π-system, which is expected to be beneficial for lateral interactions in the thin film that forms on the substrate, thus facilitating SAM ordering.[8] BIEt is in equilibrium with its dimer in solution, the process being catalysed by protons and other electrophiles.[2h] Bulkier N-substituents like neopentyl, tert-butyl or 1-adamantyl can prevent dimerisation,[9] but would be detrimental to surface coordination of the divalent carbon atom. Depletive disturbation of this equilibrium by chemisorption of monomeric BIEt is expected to cause additional dimer dissociation according to Le Châtelier’s principle. Furthermore, it seems feasible that chemisorption of (BIEt)2 onto gold affords monomeric BIEt on the surface, in analogy to reactions of enetetraamines with precious metal complexes, which yield the corresponding carbene complexes via cleavage of the central C=C bond.[10]
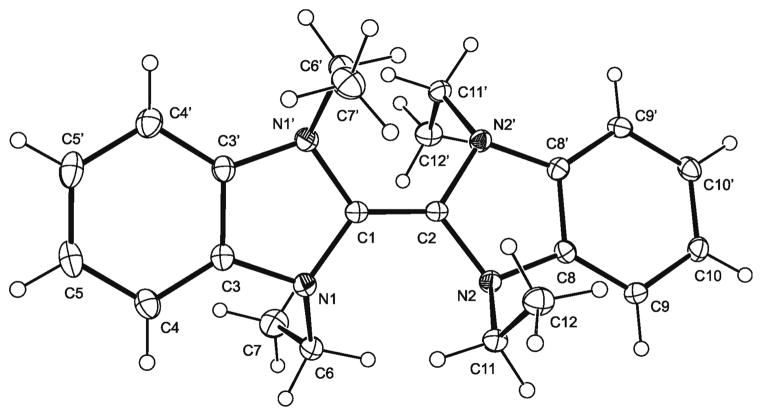
Following a known procedure, the expected mixture of BIEt and (BIEt)2 was obtained from the reaction of 1,3-diethylbenzimidazolium bromide with KOtBu in THF. Since film fabrication was performed with THF solutions, the monomer–dimer equilibrium was investigated in this solvent by variable-temperature NMR spectroscopy, in strict analogy to the study performed with a diglyme solution of this compound.[11] The thermodynamic parameters obtained are ΔH0=14.6±1.9 kcal mol−1 and ΔS0=26.3±3.3 cal mol−1 K−1, which is indistinguishable within experimental error from the values reported for a diglyme solution. As a sideline of our study, we have determined the crystal structure of (BIEt)2 (Figure 1). The central C=C bond has a length of 1.350(3) Å, and the enetetraamine is twisted along this axis with a torsion angle of ca. 15.6°. These values are indistinguishable within experimental error from those reported for (BIiBu)2.[12] The N atoms of (BIEt)2 are pyramidalised, the average sum of angles being ca. 342.5°, which is similar to the value of 348.0° reported for (BIiBu)2.
Fig. 1.
Molecular structure of (BIEt)2 in the crystal. Displacement ellipsoids are shown at the 30% probability level, except for the H atoms, which are shown as circles of arbitrary radius.
A THF solution containing BIEt and its dimer was used for film fabrication on gold. The elemental surface composition before and after BIEt adsorption was obtained from XPS data (Table S1; Accessory Publication). The carbon-to-gold signal ratio (1.3) for the film-covered surface is in agreement with a BIEt monolayer, although the presence of some co-adsorbed (BIEt)2 and remaining surface contaminants cannot be completely ruled out. The film itself consisted of carbon (≈79.2%) and nitrogen (≈16.5%), in line with values expected for a BIEt-based film (84.6% carbon, 16.4% nitrogen). The film also contained 4.3% oxygen, whose origin is not yet clear and needs to be further studied. Carbon 1s high-resolution XPS data indicate C=O film contaminants (≈5%), besides the expected C–C, C=C and C–N species.[13,14] Amide moieties (N–C=O),[15] known to be easily formed by reaction of NHCs with trace amounts of water and oxygen,[16] can be ruled out according to N 1s XP spectra (Figure S1; Accessory Publication). More work needs to be done to determine the amount of adventitious carbon left on the surface after adsorption. Possibly, BIEt reacts with substrate contaminants to a certain degree during film formation, instead of simply removing these loosely bound species from the surface.
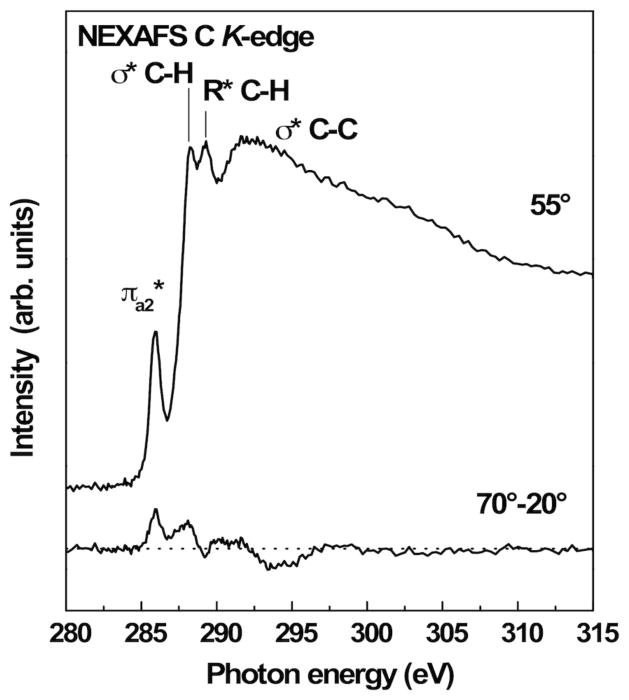
A NEXAFS C K-edge spectrum of the film acquired at an X-ray incidence angle of 55° is presented in Figure 2. At this so-called magic angle the spectra are independent of orientation effects.[17] The spectrum exhibits a distinct feature near 285.9 eV related to the π*a2 orbitals of the ring C=C bonds.[18] Resonances near 288.1 eV and 289.3 eV can be assigned to σ* C–H and Rydberg states, respectively, from the ethyl groups.[17] Broader peaks are related to σ* C–C and σ* C–C′ orbitals.[18–20] The difference of spectra collected at 70° and 20° (Fig. 2) shows a significant angle dependence, pointing to a certain degree of molecular alignment.[17] The positive polarity of the π*a2 dichroism corresponds to a predominantly upright ring orientation.[17] A numerical analysis of the pre-edge π* resonance in the spectra recorded at 70°, 55° and 20° X-ray incidence angles[17] yielded a tilt angle of Θ=30°±6° with respect to the surface normal. This angle is slightly higher than the angles observed for related aromatic thiolate-based SAMs on gold having biphenyl (Θ≈23°), para-terphenyl (Θ≈20°) or anthracene (Θ≈23°) backbones,[21] and is close to the value reported for biphenyl tellurolate on gold (Θ≈28°).[14]
Fig. 2.
C K-edge NEXAFS spectrum of a BIEt-based film on gold acquired at an X-ray incidence angle of 55° (top) along with the difference of spectra taken at 70° and 20° incidence angles (bottom).
In summary, thin films of 1,3-diethylbenzimidazol-2-ylidene (BIEt) were fabricated from THF solution on solid gold substrates. The surface-analytical data are in accord with the formation of SAMs which exhibit an approximately vertical orientation of the BIEt molecules on the surface. In view of the rich coordination chemistry of NHC ligands, there is great potential also for substrates beyond gold with such adsorbate species. Optimisation of the film fabrication procedure is needed to improve the quality of the resulting SAMs and get rid of the oxygen-containing contaminants, whose source and nature is presently unclear. In addition, we are not certain whether film formation is based on the chemisorption of the carbene or its dimer on the gold substrate. These aspects will be addressed in a systematic study that is currently underway.
Supplementary Material
Acknowledgments
This work was funded in part by NESAC-BIO (NIH grant EB-002027). T. W. thanks the Deutsche Forschungsgemeinschaft for a research fellowship. T. W. and J. E. B. thank David G. Castner for support and Daniel Fischer and Cherno Jaye (NIST) for providing them with the experimental equipment for NEXAFS spectroscopy and their help at the synchrotron. NEXAFS studies were performed at the NSLS, Brookhaven National Laboratory, which is supported by the U. S. Department of Energy, Division of Materials Science and Division of Chemical Sciences.
Footnotes
Accessory Publication
The Accessory Publication is available online on the Journal’s website. It contains a description of preparative and crystallographic work, surface-analytical methods, XPS spectra and composition table.
References
- 1.Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:361. [Google Scholar]
- 2.(a) Díez-González S, editor. N-Heterocyclic Carbenes. RSC; Cambridge: 2011. [Google Scholar]; (b) Dröge T, Glorius F. Angew Chem Int Ed. 2010;49:6940. doi: 10.1002/anie.201001865. [DOI] [PubMed] [Google Scholar]; (c) de Frémont P, Marion N, Nolan SP. Coord Chem Rev. 2009;253:862. [Google Scholar]; (d) Hahn FE, Jahnke MC. Angew Chem Int Ed. 2008;47:3122. doi: 10.1002/anie.200703883. [DOI] [PubMed] [Google Scholar]; (e) Glorius F, editor. Top Organomet Chem. Vol. 21. Springer; Berlin: 2007. N-Heterocyclic Carbenes in Transition Metal Catalysis. [Google Scholar]; (f) Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; (g) Nolan SP, editor. N-Heterocyclic Carbenes in Synthesis. Wiley-VCH; Weinheim: 2006. [Google Scholar]; (h) Alder RW, Blake ME, Chaker L, Harvey JN, Paolini F, Schütz J. Angew Chem Int Ed. 2004;43:5896. doi: 10.1002/anie.200400654. [DOI] [PubMed] [Google Scholar]
- 3.(a) Rit A, Pape T, Hepp A, Hahn FE. Organometallics. 2011;30:334. [Google Scholar]; (b) Rit A, Pape T, Hahn FE. J Am Chem Soc. 2010;132:4572. doi: 10.1021/ja101490d. [DOI] [PubMed] [Google Scholar]; (c) Tennyson AG, Norris B, Bielawski CW. Macromolecules. 2010;43:6923. [Google Scholar]; (d) Mercs L, Albrecht M. Chem Soc Rev. 2010;39:1903. doi: 10.1039/b902238b. [DOI] [PubMed] [Google Scholar]; (e) Powell AB, Bielawski CW, Cowley AH. Comments Inorg Chem. 2010;31:75. [Google Scholar]
- 4.(a) Vericat C, Vela ME, Benitez G, Caro P, Salvarezza RC. Chem Soc Rev. 2010;39:1805. doi: 10.1039/b907301a. [DOI] [PubMed] [Google Scholar]; (b) Gölzhäuser A, Wöll C. ChemPhysChem. 2010;11:3201. doi: 10.1002/cphc.201000488. [DOI] [PubMed] [Google Scholar]; (c) Ulman A. Acc Chem Res. 2001;34:885. doi: 10.1021/ar0001564. [DOI] [PubMed] [Google Scholar]; (d) Schreiber F. Prog Surf Sci. 2000;65:151. [Google Scholar]; (e) Flink S, van Veggel FCJM, Reinhoudt DN. Adv Mater. 2000;12:1315. [Google Scholar]
- 5.(a) Scholten JD, Ebeling G, Dupont J. Dalton Trans. 2007:5554. doi: 10.1039/b707888a. [DOI] [PubMed] [Google Scholar]; (b) Ott LS, Campbell S, Seddon KR, Finke RG. Inorg Chem. 2007;46:10335. doi: 10.1021/ic700976z. [DOI] [PubMed] [Google Scholar]; (c) Ott LS, Cline ML, Deetlefs M, Seddon KR, Finke RG. J Am Chem Soc. 2005;127:5758. doi: 10.1021/ja0423320. [DOI] [PubMed] [Google Scholar]
- 6.Hurst EC, Wilson K, Fairlamb IJS, Chechik V. New J Chem. 2009;33:1837. [Google Scholar]
- 7.Vignolle J, Tilley TD. Chem Commun. 2009:7230. doi: 10.1039/b913884f. [DOI] [PubMed] [Google Scholar]
- 8.Frey S, Stadler V, Heister K, Eck W, Zharnikov M, Grunze M, Zeysing B, Terfort A. Langmuir. 2001;17:2408. [Google Scholar]
- 9.(a) Poater A, Ragone F, Guidice S, Costabile C, Dorta R, Nolan SP, Cavallo L. Organometallics. 2008;27:2679. [Google Scholar]; (b) Hahn FE, Wittenbecher L, Boese R, Bläser D. Chem Eur J. 1999;5:1931. [Google Scholar]
- 10.(a) Hahn FE, von Fehren T, Wittenbecher L, Fröhlich R. Z Naturforsch B: Chem Sci. 2004;59:544. [Google Scholar]; (b) Lappert MF. J Organomet Chem. 2005;690:5467. [Google Scholar]; (c) Lappert MF. J Organomet Chem. 1988;358:185. [Google Scholar]; (d) Cardin DJ, Çetinkaya B, Lappert MF. Chem Rev. 1972;72:545. [Google Scholar]
- 11.Liu Y, Lindner PE, Lemal DM. J Am Chem Soc. 1999;121:10626. [Google Scholar]
- 12.Hahn FE, Wittenbecher L, Le Van D, Fröhlich R. Angew Chem Int Ed. 2000;39:541. doi: 10.1002/(sici)1521-3773(20000204)39:3<541::aid-anie541>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Heister K, Zharnikov M, Grunze M, Johansson LSO, Ulman A. Langmuir. 2000;17:8. [Google Scholar]
- 14.Weidner T, Shaporenko A, Müller J, Holtig M, Terfort A, Zharnikov M. J Phys Chem C. 2007;111:11627. [Google Scholar]
- 15.Alang Ahmad S, Hucknall A, Chilkoti A, Leggett GJ. Langmuir. 2010;26:9937. doi: 10.1021/la100438d. [DOI] [PubMed] [Google Scholar]
- 16.(a) Hollóczki O, Terleczsky P, Szieberth D, Mourgas G, Gudat D, Nyulászi L. J Am Chem Soc. 2011;133:780. doi: 10.1021/ja103578y. [DOI] [PubMed] [Google Scholar]; (b) Bonnette F, Kato T, Destarac M, Mignani G, Cossío FP, Baceiredo A. Angew Chem Int Ed. 2007;46:8632. doi: 10.1002/anie.200702288. [DOI] [PubMed] [Google Scholar]
- 17.Stöhr J. NEXAFS Spectroscopy. Springer; Berlin: 1992. [Google Scholar]
- 18.Lehmann JF, Urquhart SG, Ennis LE, Hitchcock AP, Hatano K, Gupta S, Denk MK. Organometallics. 1999;18:1862. [Google Scholar]
- 19.Hitchcock AP, Ennis LE, Lehmann JF, Denk MK. J Electron Spectrosc Relat Phenom. 2001;114:1037. [Google Scholar]
- 20.Urquhart SG, Hitchcock AP, Lehmann JF, Denk M. Organometallics. 1998;17:2352. [Google Scholar]
- 21.Frey S, Stadler V, Heister K, Eck W, Zharnikov M, Grunze M, Zeysing B, Terfort A. Langmuir. 2001;17:2408. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.