Abstract
Aims
As obesity-related cardiovascular mortality, although elevated when compared with normal weight, is lower in females than in males at every body mass index (BMI) level, we aimed to investigate gender-specific differences in left ventricular (LV) hypertrophy in obesity, which themselves have been shown to have varying prognostic value.
Method and results
In total, 741 subjects (female, n = 399) without identifiable cardiovascular risk factors (BMI 15.7–59.2 kg/m2) underwent cardiovascular magnetic resonance (1.5 T) to determine LV mass, end-diastolic volume (EDV, mL), and LV mass/volume ratio (LVM/VR). Across both sexes, there was a strong positive correlation between BMI and LV mass (male r = 0.44, female r = 0.57, both P < 0.001), with males showing a greater LV hypertrophic response (male +2.3 vs. female +1.6 g per BMI point increase, P = 0.001). Concentric hypertrophy was present in both sexes and LVM/VR positively correlated to BMI (male r = 0.45, female r = 0.29, both P < 0.001) on linear regression analysis. However, the degree of concentric hypertrophy was greater in males (male +0.13 vs. female +0.06 LVM/VR increase per BMI point increase, P = 0.001). On the other hand, females showed a greater LV cavity dilatory response (female +1.1 vs. male +0.3 mL per BMI point increase, P < 0.001). Indeed, in contrast to females, where BMI and LV-EDV were positively correlated (r = 0.38, P < 0.001), BMI did not correlate with EDV in men (r = 0.03, P = 0.62).
Conclusion
In the absence of traditional cardiovascular risk factors, obese men show predominantly concentric hypertrophy, whereas obese women exhibit both eccentric and concentric hypertrophy. As concentric hypertrophy is more strongly related to cardiovascular mortality than eccentric hypertrophy, our observations may explain the observed gender difference in obesity-related mortality.
Keywords: Gender, LV hypertrophy, Obesity, Cardiovascular magnetic resonance imaging
Introduction
Obesity-related cardiovascular mortality, although elevated when compared with normal weight, is lower in females than in males at every body mass index (BMI)-level when adjusted for age, physical activity, blood pressure, tobacco consumption, and heart rate.1,2 However, the reasons behind this well-documented pattern remain unknown. Given the fact that at each BMI-level males have less adipose tissue than females,3 the excess mortality in males cannot solely be attributed to the effects of excess adiposity.4
This apparent paradox raises the question whether there are gender-specific cardiac adaptations to excess body fat which predispose males to excess cardiovascular risk. Although obesity was traditionally considered to be a state of chronic volume overload, with early studies reporting an association with eccentric left ventricular (LV) remodelling, it is now becoming evident that both LV cavity size and LV wall thickness are increased in obesity. Indeed, recent studies show a disproportionate wall thickness increase, i.e. concentric LV remodelling.5,6
As different patterns of LV hypertrophy have been shown to have varying prognostic value, with concentric hypertrophy more strongly predictive of cardiovascular mortality than eccentric hypertrophy,7,8 a gender difference in the pattern of LV hypertrophy in response to obesity could explain the observed patterns in obesity-related cardiovascular mortality. On this basis, males would be at higher cardiovascular risk than females if a more concentric pattern of hypertrophy occurs in response to excess fat mass.9
Previous studies investigating LV geometry in obesity5,10,11 have not excluded subjects with obesity-related co-morbidities such as diabetes12 and hypertension, which are known to have independent effects on LV mass,13 and have used 2D echocardiography, which has marked limitations in the setting of obesity.14 Cardiovascular magnetic resonance (CMR) imaging is ideally suited to investigate LV geometry in obesity, as it is not hampered by the need for acoustic windows which are limited in obesity.
As a result, the aim of this study was to use CMR to investigate whether or not there are gender differences in LV adaptation to obesity.
Methods
Ethics and study cohort
The study was approved by the local research ethics committee, and informed written consent was obtained from each patient. In total, 741 subjects (female, n = 399; male, n = 342) without identifiable cardiovascular risk factors were recruited to studies within the University Oxford Centre for Clinical Magnetic Resonance Research (OCMR) [male: normal weight (49%), overweight (30%), obese (21%); female: normal weight (50%), overweight (22%), obese (28%)], and they underwent CMR at 1.5 T for the assessment of LV mass (g), end-diastolic volume (EDV, mL), and LV mass/volume ratio (LVM/VR). Although underweight (BMI <18.5 kg/m2) was not an exclusion criterion, only 8 of the 740 recruits were underweight. These data have been grouped with the normal data in Table 1.
Table 1.
Anthropometric and left ventricular characteristics for the study group separated into WHO body mass index categories, normal weight (body mass index < 25 kg/m2), overweight (25–29.9 kg/m2), and obese (>30 kg/m2)
| Normal weight male (n = 170) | Normal weight female (n = 198) | Overweight male (n = 101) | Overweight female (n = 91) | Obese male (n = 71) | Obese female (n = 110) | |
|---|---|---|---|---|---|---|
| Age | 30 (14) | 30 (18) | 38 ± 13 | 41 ± 15 | 46 ± 13 | 43 ± 11 |
| Body mass index (kg/m2) | 23 ± 2 | 22 ± 2 | 27 ± 1 | 27 ± 1 | 33 (6) | 36 (10) |
| Systolic blood pressure (mmHg) | 120 ± 11 | 115 ± 11 | 124 ± 10 | 119 ± 12 | 128 ± 10 | 123 ± 12 |
| Diastolic blood pressure (mmHg) | 74 ± 9 | 71 ± 8 | 78 ± 8 | 74 ± 9 | 79 ± 9 | 76 ± 8 |
| Left ventricular ejection fraction (%) | 65 ± 6 | 69 ± 7 | 67 ± 6 | 69 ± 6 | 69 ± 7 | 69 ± 6 |
| Left ventricular end-diastolic volume (mL) | 158 ± 29* | 122 ± 20 | 162 ± 28 | 127 ± 25 | 154 ± 31 | 140 ± 23‖,** |
| Left ventricular mass (g) | 128 ± 25* | 87 ± 17 | 137 ± 23# | 101 ± 20† | 155 ± 31‡,§ | 113 ± 20‖,** |
| Left ventricular mass indexed height (g/m) | 71 ± 13* | 53 ± 10 | 77 ± 12# | 61 ± 11† | 86 ± 16‡,§ | 69 ± 12‖,** |
| Left ventricular mass indexed height2.7 (g/m2.7) | 27 ± 5* | 22 ± 4 | 29 ± 4# | 26 ± 5† | 33 ± 6‡,§ | 30 ± 6‖,** |
| Left ventricular mass/volume ratio | 0.82 ± 0.15* | 0.72 ± 0.13 | 0.86 ± 1.5# | 0.81 ± 0.16† | 1.02 ± 0.18‡,§,≠ | 0.82 ± 0.14‖ |
| Fasting cholesterol (mmol/L) | 4.5 ± 1.1 | 4.7 ± 0.9 | 5.0 ± 1.0# | 5.1 ± 0.9† | 5.3 ± 0.9 | 5.1 ± 0.8 |
| Fasting glucose (mmol/L) | 4.9 ± 0.5 | 4.7 ± 0.5 | 5.1 ± 0.5 | 4.9 ± 0.5 | 5.1 ± 0.7 | 5.0 ± 0.6 |
*P < 0.05, normal weight males vs. normal weight females.
#P < 0.05, overweight males vs. normal weight males.
†P < 0.05, overweight females vs. normal weight females.
**P < 0.05, obese females vs. normal weight females.
‡P < 0.05, obese males vs. overweight males.
§P < 0.05, obese males vs. normal weight males.
‖P < 0.05, obese females vs. overweight males.
≠P < 0.05, obese females vs. obese males.
Inclusion criteria
All subjects were screened for the presence of identifiable cardiac risk factors and excluded if they had a history of cardiovascular disease, hypertension, diabetes, smoking, use of prescription medications, or were pregnant. All subjects were normotensive at the time of scanning (taken as an average of three supine measures over 10min under 140/90 mmHg (Model, DINAMAP 1846-SX, Critikon Corp.). Subjects were excluded if they had a history of coronary artery disease, cardiac chest pain, or valvular heart disease and were <18 years of age (age range 18–80 years).
Blood samples
Fasting blood tests for glucose and cholesterol were taken on the day of the scanning and analysed as previously described.15
Body composition analysis
Bio-electrical impedance was used to determine the total body fat mass using Bodystat® 1500 analyser. For the calculation of the waist:hip ratio (WHR), the average of three waist measurements was recorded at (i) the level of the umbilicus and (ii) the level of the greater trochanter of the femur.
Magnetic resonance imaging of the left ventricle
All imaging was prospectively cardiac-gated with a precordial three-lead ECG and acquired during end-expiration breathold. Images were acquired using a steady-state free precession (SSFP) sequence with an echo time of 1.5 ms, a repetition time of 3.0 ms, a temporal resolution of 47.84 ms, and a flip angle of 60° as previously described.14–16 Steady-state free precession cine sequences were used to acquire localization images followed by an SSFP left and right ventricular short-axis stack of contiguous images with a slice thickness of 7 mm and an interslice gap of 3 mm.
Data analysis
Image analysis for LV volumes and mass was performed using the Siemens analytical software (ARGUS®). The short-axis stack was analysed manually, contouring the endocardial borders from base to apex at end-diastole and end-systole. The epicardial border was contoured at end-diastole to yield myocardial mass. Left ventricular mass (g) was calculated as the epicardial volume minus the endocardial volume multiplied by 1.05 (specific gravity of myocardium). The inter-observer and intra-observer coefficients of variation for LV mass measures with this method are excellent, and have been previously reported.16
Statistical analysis
All statistics were analysed using commercial software packages (SPSS 20; SPSS, Chicago, IL, USA; STATA, StataCorp, TX, USA). All data were subjected to Kolmogorov–Smirnov tests to establish normal distribution of the data. All normally distributed results are presented as the mean ± standard deviation; non-normally distributed data are presented as the median (inter-quartile range). Normally distributed data were analysed using ANOVA analysis with the Bonferoni correction; non-normally distributed data sets were analysed using the Kruskal–Wallis tests. Linear regression analysis was used to assess the effect of BMI on LV mass, EDV, and LVM/VR. To compare the coefficient of regression between males and females, dummy variable regression analysis was performed. An additional adjusted regression model accounting for the effects of age and systolic blood pressure was also performed. Values of P < 0.05 were considered as statistically significant.
Results
Anthropomorphic data
Subjects were separated into groups according to gender and World Health Organization BMI categories (Table 1). Age, BMI, systolic blood pressure, and diastolic blood pressure were similar between men and women, stratified by normal, overweight, or obese groups (Table 1). In addition, all subjects were normotensive, normoglycaemic, and normocholesterolaemic on the day of scanning (Table 1).
Left ventricular function and obesity
As LV geometry is altered in the presence of heart failure and cardiomyopathy, and to ensure that all recruits were free of overt cardiovascular disease, LV ejection fraction (LVEF %) was recorded in all subjects. All subjects enrolled into the study had normal LVEF (male: range 55–85%; female: range 56–85%, Table 1), with no significant difference between males and females at any BMI level (Table 1). Of note, there was no correlation between BMI and LVEF in either males (r = −0.03, P = 0.69) or females (r = 0.13, P = 0.83).
Gender differences in left ventricular hypertrophy in obesity
Left ventricular hypertrophy in males
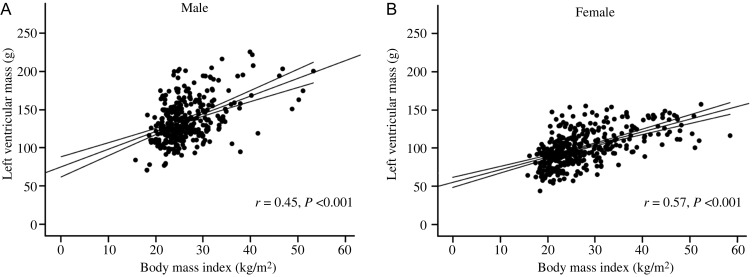
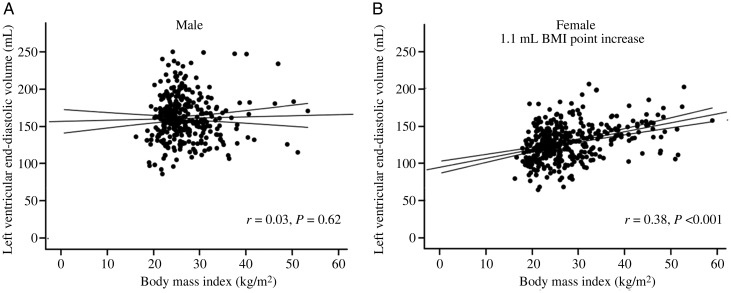
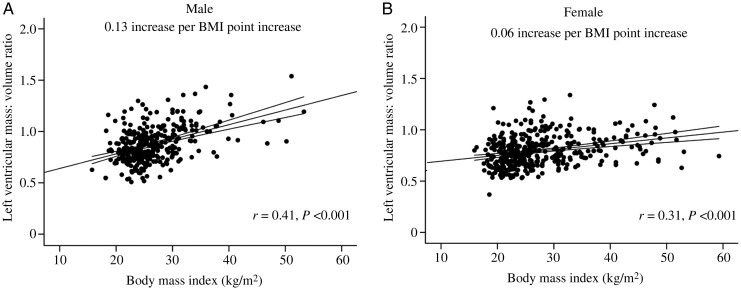
In agreement with previous reports, overweight males had greater LV mass than normal weight males (6%, P < 0.01) and obese males had a greater LV mass than both overweight males and normal weight males (by 11 and 18%, respectively, both P < 0.01, Table 1). As expected, using univariable regression, there was a strong positive correlation between BMI, fat mass and waist:hip ratio, and LV stroke volume (LV-SV) with absolute LV mass (g) in males (BMI r = 0.43, fat mass r = 0.35, WHR r = 0.38, and LV-SV r = 0.52, all P < 0.001, Figure 1A). When LV mass was indexed to height and height2.7, a similar pattern was observed (LV mass/height r = 0.48, P < 0.001; LV mass/height2.7 r = 0.51, P < 0.001). Interestingly, in males, LV-EDV was similar between normal, overweight, and obese males (Table 1), and BMI, fat mass, and WHR were not correlated with LV-EDV (BMI r = 0.06, fat mass r = 0.001, WHR r = −0.005, all P > 0.33, Figure 2A). As a result of this, overweight men had a 5% higher LVM/VR than normal weight men, and obese men a 17% greater LVM/VR than overweight men (Table 1); LVM/VR was positively correlated with BMI, fat mass, and WHR (BMI r = 0.41, fat mass r = 0.38, WHR r = 0.52, all P < 0.001, Figure 3A) and negatively associated with LV-SV (r = 0.29, P < 0.001). Put together, this would suggest that males exhibit a progressive concentric hypertrophic process, without LV cavity dilatation in response to increasing body fat.
Figure 1.
Sex-specific correlations between body mass index and absolute left ventricular mass (A, men; B, women) depicting the steeper relationship between body mass index and left ventricular mass in men. Mean ± 95% CI shown for each graph.
Figure 2.
Sex-specific correlations between body mass index and absolute left ventricular end-diastolic volume (A, men; B, women) depicting the steeper relationship between body mass index (BMI) and left ventricular end-diastolic volume ratio in women. Mean ± 95% CI shown for each graph.
Figure 3.
Sex-specific correlations between body mass index and absolute left ventricular mass/volume ratio (A, men; B, women) depicting the steeper relationship between body mass index (BMI) and left ventricular mass/volume ratio in men. Mean ± 95% CI shown for each graph.
Left ventricular hypertrophy in females
Overweight females had greater LV mass than normal weight females (by 17%, P < 0.01) and obese females a greater LV mass than both overweight and normal weight females (by 13 and 31%, both P < 0.01, Table 1). As expected, using univariable regression, there was again a strong positive correlation between BMI, fat mass, WHR, and LV-SV with absolute LV mass (g) in females (BMI r = 0.58, fat mass r = 0.54, WHR r = 0.20, LV-SV r = 0.60, all P < 0.001, Figure 1B). When LV mass was indexed to height and height2.7, a similar pattern was observed (LV mass/height r = 0.60, P < 0.001; LV mass/height2.7 r = 0.61, P < 0.001). In contrast to males, LV-EDV was similar between normal and overweight groups, but obese females had an 11% greater EDV than overweight and a 14% greater EDV than normal weight females (Table 1). As a result, both BMI and fat mass positively correlated with LV-EDV (BMI r = 0.36, fat mass r = 0.39, both P < 0.001 Figure 2B). Overweight females had a 13% higher LVM/VR than normal weight females. As a result of this, obese females had similar LVM/VR to overweight females, but 13% greater LVM/VR than normal weight females (Table 1). Interestingly, despite the LV cavity dilatation in obese females, LVM/VR was still positively correlated with BMI, fat mass, and WHR (BMI r = 0.31, fat mass r = 0.24, WHR r = 0.19, all P < 0.001, Figure 3B) and negatively correlated with stroke volume (r = 0.19, P < 0.001). This suggests that, although cavity dilatation occurs along with elevated LV mass in female obesity (i.e. eccentric hypertrophy), an initial degree of concentric hypertrophy is still present in overweight females with increased LVM/VR.
Comparing gender-specific hypertrophy in obesity
Left ventricular mass
When comparing the coefficient of regression between BMI and LV mass in males and females, males showed a greater LV hypertrophic response to increasing BMI (male LV mass increase + 2.3 g per BMI point increase vs. female, +1.6 g per BMI point increase, P = 0.001). However, LV mass was also positively correlated with age and systolic blood pressure, fat mass and waist:hip ratio (Table 2). When LV mass was indexed to height and height2.7, a similar pattern was seen (data not shown). Given this association, an adjusted model accounting for these variables was performed. This revealed that, when adjusting for age and systolic blood pressure, fat mass and waist:hip ratio, LV mass remained positively correlated with BMI in both males and females; in addition, the steeper relationship between LV mass and BMI observed in males remained present (male 2.2 g vs. female 1.4 g per BMI point increase, P = 0.01).
Table 2.
Gender differences in linear regression for left ventricular mass, end-diastolic volume, and left ventricular mass/volume ratio
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| r2 | β | P-value | r2 | β | P-value | |
| LV mass (g) | ||||||
| Body mass index (kg/m2) | 0.18 | 2.2 | <0.001 | 0.32 | 1.6 | <0.001 |
| Age (years) | 0.03 | 0.1 | 0.38 | 0.3 | 0.3 | <0.001 |
| Systolic blood pressure (mmHg) | 0.04 | 0.5 | 0.01 | 0.11 | 0.6 | <0.001 |
| Diastolic blood pressure (mmHg) | 0.03 | 0.5 | <0.001 | 0.05 | 0.6 | <0.001 |
| Fat mass (kg) | 0.14 | 0.86 | <0.001 | 0.29 | 0.67 | <0.001 |
| Waist:hip ratio | 0.14 | 0.09 | <0.001 | 0.04 | 0.05 | <0.001 |
| LV-EDV (mL) | ||||||
| Body mass index (kg/m2) | 0.01 | 0.19 | 0.5 | 0.15 | 1.2 | <0.001 |
| Age (years) | 0.06 | −0.5 | <0.001 | 0.001 | −0.06 | 0.52 |
| Systolic blood pressure (mmHg) | 0.001 | 0.01 | 0.94 | 0.002 | 0.25 | 0.012 |
| Diastolic blood pressure (mmHg) | 0.02 | −0.44 | 0.02 | 0.001 | 0.07 | 0.62 |
| Fat mass (kg) | 0.02 | 0.11 | <0.001 | 0.16 | 0.52 | <0.001 |
| Waist:hip ratio | 0.00 | −0.01 | 0.94 | 0.002 | 0.002 | 0.49 |
| LV mass/volume ratio | ||||||
| Body mass index (kg/m2) | 0.19 | 0.014 | <0.001 | 0.09 | 0.005 | <0.001 |
| Age (years) | 0.1 | 0.004 | <0.001 | 0.06 | 0.003 | <0.001 |
| Systolic blood pressure (mmHg) | 0.04 | 0.003 | 0.001 | 0.07 | 0.003 | <0.001 |
| Diastolic blood pressure (mmHg) | 0.1 | 0.006 | <0.001 | 0.06 | 0.004 | <0.001 |
| Fat mass (kg) | 0.13 | 0.005 | <0.001 | 0.06 | 0.002 | 0.02 |
| Waist:hip ratio | 0.15 | 0.006 | <0.001 | 0.03 | 0.03 | 0.01 |
β, the coefficient of regression.
Left ventricular end-diastolic volume
In contrast to females, where LV cavity size increased with increasing BMI (+1.1 mL per BMI point increase, P < 0.001), in males there was no relationship between LV end-diastolic cavity size and increasing BMI (+0.3 mL per BMI point increase, P = 0.39, Figure 2A and B). This again suggests that LV hypertrophy seen in males in response to increased BMI is not eccentric, i.e. not related to cavity dilatation, but instead due to concentric hypertrophy. As with LV mass, LV-EDV was associated with age and systolic blood pressure (Table 2). Again, an adjusted model was performed to account for this. This showed that, when adjusting for the effects of age and systolic blood pressure, LV-EDV remained positively correlated with BMI in females (r = 0.14, β = 1.1, P < 0.001). In addition, LV-EDV was positively correlated with fat mass in females (0.6 mL per kg fat mass increase) but not in men (P = 0.6), again suggesting that fat mass is related to cavity dilatation in females but not males. Interestingly, as LV-EDV was correlated with waist:hip ratio neither in males (r = −0.005, P = 0.94) nor in females (r = 0.04, P = 0.49), this suggests that it is the total fat mass, not the relative distribution of central fat that is important in determining cavity size in women.
Left ventricular mass/volume ratio
As expected, LVM/VR ratio was greater in both normal weight and obese males than normal weight and obese females (both P < 0.001, Table 1). However, interestingly, LVM/VR was similar in overweight males and overweight females (P = 0.46).This would suggest that, unlike in males, increasing BMI in females is not related to progressive concentric hypertrophy but appears to be related to initial concentric hypertrophy in overweight, followed by eccentric hypertrophy in overt obesity. Although present in both sexes, on comparison of coefficient of regression, the degree of concentric hypertrophy was greater in males, with a steeper regression coefficient observed (males +0.13 vs. females +0.06 LVM/VR increase per BMI point increase, P < 0.001, Figure 3A and B). In addition to this, in males and females, LVM/VR was associated with age and systolic and diastolic blood pressure (Table 2). When adjusted for age and systolic blood pressure, LVM/VR remained positively correlated with BMI and the relationship remained steeper for males than females. This pattern of a steeper relationship in males was seen again with both fat mass (males +0.05 vs. females +0.02 LVM/VR per kg fat mass increase, P = 0.02) and waist:hip ratio (males +0.05 vs. females +0.029 LVM/VR per 0.1 increase in waist:hip ratio, P = 0.047).
Taken together, these results suggest that the greater degree of concentric hypertrophy seen in obesity in response to increasing BMI, fat mass, and waist:hip ratio is not only independent of the effects of age or systolic blood pressure (making an obesity-specific mechanism more likely) but is also greater in males than females.
Discussion
Obesity-related mortality, irrespective of additional risk factors, is greater in males than in females at all BMI levels above normal.1 The reasons for this are unclear, but, given the fact that at the same BMI obese males have less fat mass than obese females,17 it is difficult to explain this mortality difference purely on the basis of the effects of excess fat. This study has shown that in response to obesity per se, in the absence of traditional cardiovascular risk factors, males exhibit a greater concentric hypertrophic response than women, where a mixed eccentric and hypertrophic response is observed. Given the link between concentric hypertrophy and mortality, this observation provides a plausible explanation for the mortality differences between men and women in response to obesity.
Gender differences in left ventricular hypertrophy in response to increasing body mass index
Left ventricular hypertrophy in obesity has been the subject of numerous previous studies with concentric hypertrophy, concentric remodelling, and eccentric hypertrophy,18,19 all being reported. In addition, previous studies have reported gender-specific differences in the adaptation of the LV to the combination of hypertension and obesity, with post-menopausal females being reported to be more susceptible to concentric hypertrophy, suggesting a gender difference in response to varying hypertrophic stimuli.19 However, as mentioned earlier, the majority of previous studies investigating gender differences in LV hypertrophy in response to obesity have focused on populations with obesity-related co-morbidities such as hypertension.
The Multi-Ethnic Study of Atherosclerosis (MESA) group, using CMR, has previously published that obesity is associated with concentric LV hypertrophy without change in ejection fraction, and also that males exhibit a greater degree of concentric hypertrophy with a lesser degree of cavity dilatation than females.5 However, as acknowledged by the authors, interpreting the effects of obesity per se in this study is difficult given the high prevalence of hypertension, diabetes, hypercholesterolaemia, and history of smoking, and merely 1.8% of the MESA population was obese without related co-morbidities. To date, this is the largest study to investigate the gender-specific effects of obesity alone on LV geometry.
This study is in direct conflict with many previous studies investigating the so-called uncomplicated obesity which have not reported an association between obesity and LV hypertrophy and have suggested that, without the co-morbidities of hypertension and diabetes, obesity has little effect on LV mass.11,12,14 An explanation for this may be that the previous studies have used 2D echocardiography, a technique which can have limitations in image quality, being hampered by the need for acoustic windows which are limited in obesity, and the need for geometric assumptions to generate 3D data (i.e. LV mass and LV-EDV) from 2D data. Using CMR imaging, in a large group of subjects free of co-morbidities, this study provides the strongest evidence to date that obesity per se produces an LV hypertrophic response, independent of blood pressure, age, and diabetes.
Concentric hypertrophy and mortality
It is well known that, when compared with normal weight females, normal weight males have a greater LV mass.20 We again show that both LV mass and LVM/VR are higher in normal weight males than females. Interestingly, however, when comparing overweight men and women, LVM/VR was similar between men and women.
We have previously reported, in females, that an increase in BMI from normal to overweight results in concentric LV hypertrophy, without the expected volume-dependent change in LV dilatation and that this is potentially mediated by the associated increase in insulin, leptin, and other hypertrophy-generating adipokines.21 This early concentric hypertrophy would provide an explanation for the equalization of the LVM/VR ratio seen in this study in overweight females and males. As male obesity was associated with elevated LV mass without cavity dilatation, this explains the continuing increase in LVM/VR. In contrast, when BMI increased from overweight to obese in females, the LV mass increase was accompanied by a similar degree of cavity dilatation. The reasons for this differential response are unknown but it is likely that the greater degree of adipose tissue in obese females is playing a role. As adipose tissue is known to expand circulating volume,22 the subsequent increase of LV-SV is believed to result in eccentric hypertrophy. This would then provide an explanation for the gender difference in hypertrophy seen in obesity. In support of this, we have shown that increased LV-SV is positively related to absolute LV mass, but negatively related to LV mass:volume ratio. This then suggests that the overall increase in LV mass:volume ratio that occurs in male and, to a lesser extent, female obesity is not likely to be related to stroke volume changes.
Sex hormones and gender differences in left ventricular mass
In addition to the obvious gender differences in LV size, the changes in LV mass in response to hypertrophic stimuli, such as hypertension and aortic stenosis, are also greater in men than in women23 and are even exaggerated in women >50 years old where sex hormone levels have reduced. Given the fact that oestrogen is believed to inhibit cardiac hypertrophy and testosterone promotes LV hypertrophy,24 endogenous sex hormone differences have been an attractive, although never fully proved, hypothesis to account for gender difference in LV mass. However, explaining the difference in LV mass changes that occurs in response to obesity by the variation in endogenous sex hormone concentrations is complicated by the fact that male obesity is linked to lower levels of free testosterone,25 and fat mass itself increases serum oestrogen levels in post-menopausal females but paradoxically decreases oestrogen levels in pre-menopausal women.26
The renin–angiotensin–aldosterone system and gender differences in left ventricular mass
A more likely mechanism to explain gender differences in LV hypertrophy in response to obesity seen in this study is obesity-related modulation of the renin–angiotensin–aldosterone system. There is now recent evidence that, in C57BL/6J mice, which are susceptible to diet-induced obesity, angiotensin-converting enzyme (ACE) inhibition reduces food intake, body weight, insulin resistance, and markers of inflammation.27 In addition, human obesity is related to increased angiotensin II levels, and to cardiac hypertrophy in male Wistar rats, which is abolished by chronic angiotensin II blockade.28 When put together with data demonstrating a gender difference in the expression of ACE in the murine heart with greater cardiac ACE levels seen in male animals compared with females,29 this suggests that ACE inhibition, which is known to have beneficial effects on LV concentric hypertrophy above and beyond its effects on blood pressure,28 may have a role in reversing obesity-induced concentric LV hypertrophy in human male obesity, even in the absence of hypertension.
Clinical relevance
Concentric LV hypertrophy develops in response to a chronically increased LV afterload and is associated with increased cardiovascular events and progression to systolic dysfunction, mainly as a result of myocardial infarction.30,31 As males were seen to have more pronounced concentric hypertrophy in response to obesity, this provides a potential explanation for the excess cardiovascular risk in male obesity. Although sex hormone differences do not provide an adequate explanation for these findings in obesity, differences in the renin–angiotensin–aldosterone system provide a viable explanation for the observed differences. This raises the possibility that pharmacological agents known to have beneficial effects in concentric LV hypertrophy, such as ACE inhibitors,28 may also have beneficial effects in male obesity without co-morbidities. As a result of this, this study suggests that an adverse concentric pattern of LV remodelling occurs in male obesity and that that there may, in the future, be a role for a clinical trial investigating the effect of ACE-inhibition on male obesity-related LV remodelling and, as a consequence, obesity-related mortality.
Limitations
Although LV remodelling patterns have been linked to mortality, this study is not designed or powered to investigate the effects of obesity-related LV remodelling on cardiovascular mortality in this healthy population. As such, further large-scale studies looking at mortality in obesity are needed.
Conclusion
In a large age- and blood pressure-matched population of males and females, free of cardiovascular risk factors, increasing BMI is strongly related to increasing LV mass. In addition, gender-specific LV adaptation to obesity exists. Whereas LV hypertrophy is more commonly seen in this study in females than in males, the greater degree of concentric hypertrophy is greater in males, with females exhibiting a combination of eccentric and concentric LV hypertrophy. Given the fact that concentric hypertrophy is prognostically worse than eccentric hypertrophy, this may partly explain the gender differences in obesity-related cardiovascular mortality.
Funding
The study was supported by a grant from the Wellcome Trust and by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health's NIHR Biomedical Research Centres' funding scheme. S.N. acknowledges support from the Oxford BHF Centre of Research Excellence.
Conflict of interest: none declared.
Acknowledgements
We would like to thank Professor Bernard Fingleton, PhD (Aberystwyth), MPhil, PhD (Cantab), CStat, AcSS, for his help with the statistical analysis.
References
- 1.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2011;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas F, Bean K, Pannier B, Oppert JM, Guize L, Benetos A. Cardiovascular mortality in overweight subjects: the key role of associated risk factors. Hypertension. 2005;46:654–659. doi: 10.1161/01.HYP.0000184282.51550.00. [DOI] [PubMed] [Google Scholar]
- 3.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy AP, Shea JL, Sun G. Comparison of the classification of obesity by BMI vs. dual-energy X-ray absorptiometry in the Newfoundland population. Obesity (Silver Spring) 2009;17:2094–2099. doi: 10.1038/oby.2009.101. [DOI] [PubMed] [Google Scholar]
- 5.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rider OJ, Francis JM, Ali MK, Byrne J, Clarke K, Neubauer S, Petersen SE. Determinants of left ventricular mass in obesity: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2009;11:9. doi: 10.1186/1532-429X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerdts E, Cramariuc D, de Simone G, Wachtell K, Dahlof B, Devereux RB. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study) Eur J Echocardiogr. 2008;9:809–815. doi: 10.1093/ejechocard/jen155. [DOI] [PubMed] [Google Scholar]
- 8.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 9.Liao Y, Cooper RS, Mensah GA, McGee DL. Left ventricular hypertrophy has a greater impact on survival in women than in men. Circulation. 1995;92:805–810. doi: 10.1161/01.cir.92.4.805. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, Leonetti F. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res. 2002;10:767–773. doi: 10.1038/oby.2002.104. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan R, Becker RJ, Beighley LM, Lopez-Candales A. Impact of body mass index on markers of left ventricular thickness and mass calculation: results of a pilot analysis. Echocardiography. 2005;22:203–210. doi: 10.1111/j.0742-2822.2005.03138.x. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G, Ribaudo MC, Zappaterreno A, Vecci E, Tiberti C, Di Mario U, Leonetti F. Relationship of insulin sensitivity and left ventricular mass in uncomplicated obesity. Obes Res. 2003;11:518–524. doi: 10.1038/oby.2003.73. [DOI] [PubMed] [Google Scholar]
- 13.Patel DA, Srinivasan SR, Chen W, Berenson GS. Influence of the metabolic syndrome versus the sum of its individual components on left ventricular geometry in young adults (from the Bogalusa Heart Study) Am J Cardiol. 2009;104:69–73. doi: 10.1016/j.amjcard.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G. True uncomplicated obesity is not related to increased left ventricular mass and systolic dysfunction. J Am Coll Cardiol. 2004;44:2257. doi: 10.1016/j.jacc.2004.09.012. author reply 8. [DOI] [PubMed] [Google Scholar]
- 15.Rider OJ, Tayal U, Francis JM, Ali MK, Robinson MR, Byrne JP, Clarke K, Neubauer S. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18:2311–2316. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 16.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 17.Pasco JA, Nicholson GC, Brennan SL, Kotowicz MA. Prevalence of obesity and the relationship between the body mass index and body fat: cross-sectional, population-based data. PLoS One. 2012;7:e29580. doi: 10.1371/journal.pone.0029580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavie CJ, Milani RV, Patel D, Artham SM, Ventura HO. Disparate effects of obesity and left ventricular geometry on mortality in 8088 elderly patients with preserved systolic function. Postgrad Med. 2009;121:119–125. doi: 10.3810/pgm.2009.05.2011. [DOI] [PubMed] [Google Scholar]
- 19.Kuch B, Muscholl M, Luchner A, Doring A, Riegger GA, Schunkert H, Hense HW. Gender specific differences in left ventricular adaptation to obesity and hypertension. J Hum Hypertens. 1998;12:685–691. doi: 10.1038/sj.jhh.1000689. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Devereux RB, Roman MJ, Ganau A, Chien S, Alderman MH, Atlas S, Laragh JH. Gender differences in left ventricular anatomy, blood viscosity and volume regulatory hormones in normal adults. Am J Cardiol. 1991;68:1704–1708. doi: 10.1016/0002-9149(91)90333-g. [DOI] [PubMed] [Google Scholar]
- 21.Rider OJ, Petersen SE, Francis JM, Ali MK, Hudsmith LE, Robinson MR, Clarke K, Neubauer S. Ventricular hypertrophy and cavity dilatation in relation to body mass index in females with uncomplicated obesity. Heart. 2011;97:203–208. doi: 10.1136/hrt.2009.185009. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull. 1962;1:39–44. [PubMed] [Google Scholar]
- 23.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 24.Hayward CS, Webb CM, Collins P. Effect of sex hormones on cardiac mass. Lancet. 2001;357:1354–1356. doi: 10.1016/S0140-6736(00)04523-2. [DOI] [PubMed] [Google Scholar]
- 25.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, Dandona P. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–1192. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicennati V, Ceroni L, Genghini S, Patton L, Pagotto U, Pasquali R. Sex difference in the relationship between the hypothalamic-pituitary-adrenal axis and sex hormones in obesity. Obesity (Silver Spring) 2006;14:235–243. doi: 10.1038/oby.2006.30. [DOI] [PubMed] [Google Scholar]
- 27.Premaratna SD, Manickam E, Begg DP, Rayment DJ, Hafandi A, Jois M, Cameron-Smith D, Weisinger RS. Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice. Int J Obes. 2005;36:233–243. doi: 10.1038/ijo.2011.95. [DOI] [PubMed] [Google Scholar]
- 28.du Toit EF, Nabben M, Lochner A. A potential role for angiotensin II in obesity induced cardiac hypertrophy and ischaemic/reperfusion injury. Basic Res Cardiol. 2005;100:346–354. doi: 10.1007/s00395-005-0528-5. [DOI] [PubMed] [Google Scholar]
- 29.Freshour JR, Chase SE, Vikstrom KL. Gender differences in cardiac ACE expression are normalized in androgen-deprived male mice. Am J Physiol. 2002;283:H1997–H2003. doi: 10.1152/ajpheart.01054.2001. [DOI] [PubMed] [Google Scholar]
- 30.Milani RV, Drazner MH, Lavie CJ, Morin DP, Ventura HO. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol. 2011;108:992–996. doi: 10.1016/j.amjcard.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamoorthy A, Brown T, Ayers CR, Gupta S, Rame JE, Patel PC, Markham DW, Drazner MH. Progression from normal to reduced left ventricular ejection fraction in patients with concentric left ventricular hypertrophy after long-term follow-up. Am J Cardiol. 2011;108:997–1001. doi: 10.1016/j.amjcard.2011.05.037. [DOI] [PubMed] [Google Scholar]