Abstract
Activation of mitosis promoting factor (MPF) drives mitotic commitment1. In human cells active MPF appears first on centrosomes2. We show that local activation of MPF on the equivalent organelle of fission yeast, the spindle pole body (SPB), promotes Polo kinase activity at the SPBs long before global MPF activation drives mitotic commitment. Artificially promoting MPF or Polo activity at various locations revealed that this local control of Plo1 activity on G2 phase SPBs dictates the timing of mitotic commitment. Cytokinesis of the rod shaped fission yeast cell generates a naïve “new” cell end. Growth is restricted to the experienced old end until a point in G2 phase called “New End Take Off” (NETO) when bipolar growth is triggered3. NETO coincided with MPF activation of Plo1 on G2 phase SPBs4. Both MPF and Polo activities were required for NETO and both induced NETO when ectopically activated at interphase SPBs. NETO promotion by MPF required polo. Thus, local MPF activation on G2 SPBs directs polo kinase to control at least two distinct and temporally separated, cell cycle transitions at remote locations.
Keywords: Polo kinase, Centrosome, S. pombe, NETO, MPF, Cdk1, Mitosis
Mutations in the SPB component Cut12 display a reciprocal relationship with mutations in the MPF activating phosphatase Cdc25. The loss of function mutation cdc25.22 is suppressed by gain of function mutations in cut12, such as cut12.s115,6. Conversely, increased Cdc25 levels suppress the loss of function mutant cut12.17. The polo kinase Plo1 associates with the SPB towards the end of G2 phase. The timing of this recruitment is advanced in cut12.s11 cells4,8. The antibody MPM2 recognizes SPBs in a Plo1 dependent manner8 and so offers an ideal tool with which to assess the local activity of Plo1 on the SPB. Unlike bulk in vitro kinase assays, MPM2 assessment of Plo1 activity on SPBs is independent of any changes in Plo1 activities at any other locations. MPM2 SPB staining indicates that the Plo1 that associates with SPBs during G2 phase of both wild type and cut12.s11 cells is active8. Prompted by the fact that the enhancement of Plo1 activity that accompanies mitotic commitment is driven by MPF activation9, we now address the function of this SPB associated Plo1 in mitotic control.
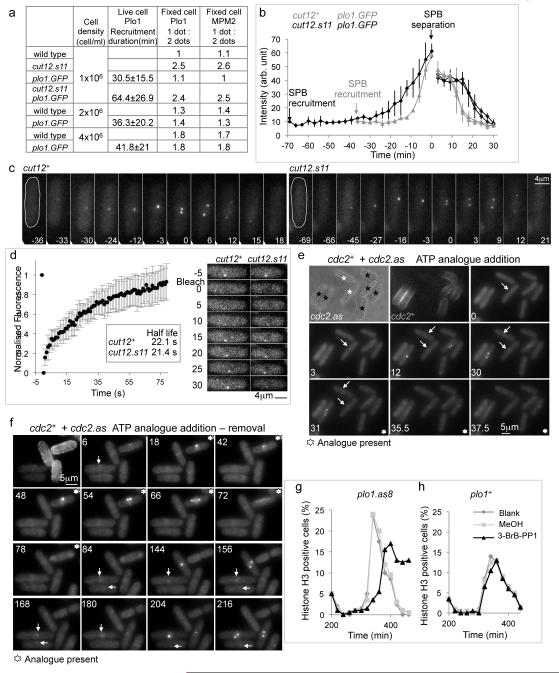
MPM2 or Plo1 immunofluorescence staining reveals single (late G2 phase), or paired foci (mitotic) of SPB staining. The ratio of one:two foci (G2:Mitotic) in cell populations gives an indication of the point in G2 at which Plo1 is recruited to (Plo1 staining), or activated at (MPM2), SPBs. Staining patterns in an established plo1.GFP strain10 revealed that the fusion of GFP sequences to the carboxyl terminus of Plo1 did not alter the timing or activation of Plo1 at SPBs (Figure 1a). Time lapse imaging revealed that Plo1 was initially recruited around 30 minutes before mitosis (Figure 1a-c). This initial recruitment was advanced by a further 30 minutes in cut12.s11 cells (Figure 1a-c; Supplementary movie 1). Fluorescence recovery after photobleaching (FRAP) revealed a rapid turnover of Plo1.GFP (half-life 21-22 sec) at the SPBs of both cut12+ and cut12.s11 cells (Figure 1d; Supplementary movie 2).
Figure 1. MPF activity controls Plo1.GFP recruitment to the SPB in late G2 phase.
a) Timing of Plo1.GFP recruitment before SPB separation (living cells) and the ratios of 1 dot (G2, early mitotic SPB) to two dots (mitotic SPB) MPM2 staining. b) Plots of the intensity of the Plo1.GFP signals averaged for three cells. In each case the two plots of different shading after SPBs split indicate the signals arising from two individual SPBs that emerge from this single focus of paired SPB signals in G2. intensity of the two (see also Supplementary movie 1). (c) Time lapse images of plo1.GFP and cut12.s11 plo1.GFP cells. (d) FRAP of the signal from a late G2 Plo1.GFP SPB in wild type cells (left). The FRAP profile of a cut12.s11 plo1.GFP strain was indistinguishable from this wild type plot (right; see also Supplementary movie 2). e, f) Wild type (TRITC-lectin pre-treated) and analogue sensitive (cdc2.as) cells (no coating) recorded side by side in the same field of view at 25°C. The timing the addition of 25μM 1NA-PP1 is indicated in the panel. Control cdc2+ cells advanced through mitosis irrespective of the presence of analogue with Plo1.GFP on their SPBs while Plo1.GFP signal left the SPBs of neighbouring cdc2.as mutant cells following analogue addition. White arrows indicate Plo1.GFP signals on SPBs. See also Supplementary movies 3, 4. g, h) Size selected cultures of either plo1.as8 (g) or plo1+ (h) cells were split into three following completion of the first synchronous division after size selection and treated as indicated in the legend to the graphs before processing for immunofluorescence with phospho-histone H3 serine 10 antibodies. The analogue 3-BrB-PP1 was added to a final concentration of 20 μM.
To assess the impact of MPF activity on Plo1 SPB recruitment we exploited the cdc2.as mutation in the catalytic subunit of MPF that renders it sensitive to inhibition by ATP analogues11. plo1.GFP cdc2+ cells were labeled by transient immersion in fluorescent lectin12 and mixed with plo1.GFP cdc2.as cells for live cell imaging. Addition of the ATP analogue 1NA-PP1 prompted the immediate loss of Plo1.GFP from G2 SPBs of cdc2.as cells while it persisted on the G2 SPBs of neighbouring cdc2+ cells (Figure 1e, f; Supplementary Movie 3, 4). Restoration of MPF activity by removal of the analogue promoted a rapid return (Figure 1f; Supplementary Movie 4), indicating that MPF activity is continuously required for Plo1 association with the late G2 SPB.
We wanted to extend the analogue sensitive approach to assess the contribution of Plo1 activity to mitotic control in unperturbed cell cycles. In strains harbouring the plo1.as1, plo1.as2, plo1.as3 or plo1.as4 mutations that are predicted to confer sensitivity13 addition of analogue had little impact upon the most sensitive read out of Plo1 function, spindle formation14. However, septation was compromised in the first division after the addition of 40 μM 3MB-PP1 to plo1.as3 cells (data not shown), indicating a modest degree of sensitization14. We therefore studied the structure of AMP-PNP bound human Plk115 to seek notable differences between the structure of this mammalian kinase that can be sensitized to ATP analogue inhibition and its refractory fission yeast counterpart, Plo1. Plk1 harbours a phenylalanine at position 170 at the bottom of the ATP binding pocket that is replaced by methionine in Plo1. Mutation of this methionine to phenylalanine in a plo1.as3 backbone created the plo1.as8 allele that responded to 3MB-PP1 or 3-BrB-PP1 addition by arresting mitotic progression with the monopolar spindle phenotype that is a characteristic of severely compromised Plo1 function14 (Supplementary Figure 1a,b). Addition of 20 μM 3-BrB-PP1 to G2 plo1.as8 cells delayed the phosphorylation of histone H3 serine 10 that accompanies mitotic commitment by 40 minutes (Figure 1g, h).
Plo1 comprises a catalytic domain and two polo boxes that direct the kinase to target molecules that are either direct substrates or platforms from which neighbouring substrates can be phosphorylated16-18 (Supplementary Figure 1c). We reasoned that Plo1 could be directed to a site of choice by replacement of the polo boxes with a surrogate targeting sequence. We replaced the polo box domain of plo1.as8 with sequences encoding the GFP binding protein, GBP19-21, in a transgene that was expressed from an ectopic location. We incorporated mutations conferring constitutive activation8 into the catalytic domain of this hybrid molecule in order to over-ride the controls that normally co-ordinate Plo1 activity with cell cycle progression (Supplementary Figure 1c). Ectopic expression of this Plo1.RL chimera in the presence of inhibitory ATP analogue targets a restrained kinase to any location that hosts GFP (Supplementary Figure 2a). Subsequent analogue removal releases sustained Plo1 kinase activity at the target site. This approach enabled us to uncouple kinase activation from its normal cell cycle context and ask which events are triggered by forced Plo1 activation at a particular location?
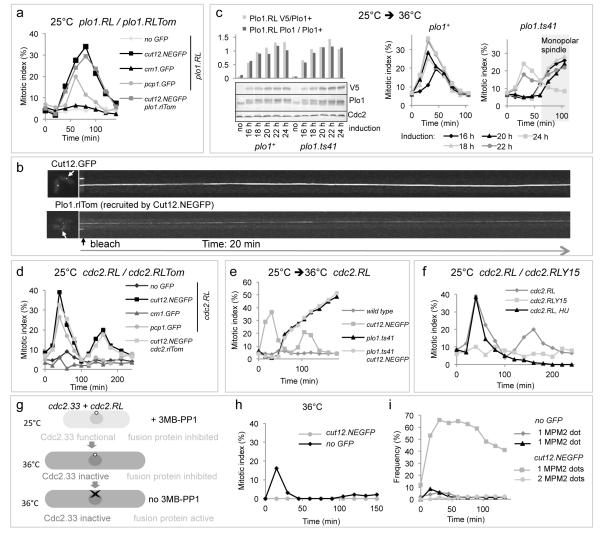
Plo1.RL activation at SPBs via recruitment to Cut12.NEGFP promoted mitotic commitment (Figure 2a). A lower level of mitotic induction arose from recruitment to the SPB components Pcp1.GFP and Sid4.GFP while none followed targeting to any other location (Figure 2a, Supplementary Figure 2b). Bleaching the fluorescence signal derived from either the host molecule Cut12.NEGFP or the associated Plo1.RLTom chimeric kinase revealed no turnover of the Cut12.NEGFP/Plo1.RLTom complex at SPBs (Figure 2b). We therefore asked whether the anchored Plo1.RL chimeric kinase relied upon the endogenous, mobile, Plo1 to promote mitotic commitment. plo1.ts41 cells (Supplementary Figure 1e,f) harbouring Cut12.NEGFP anchored Plo1.RL were shifted from 25°C to 36°C to inhibit Plo1 function as the inhibitory analogue was removed. Different induction times produced different levels of Plo1.RL at this point of release. Levels of Plo1.RL (18 or 20 h induction) that were competent to induce mitosis when the endogenous Plo1 kinase was a functional wild type molecule were unable to do so when it was the incapacitated Plo1.ts41 (Figure 2c).
Figure 2. Ectopic activation of either Plo1 or MPF on G2 SPBs promotes mitotic commitment.
a) 3-MB-PP1 removal from strains hosting a de-repressed plo1.RL allele (see also Supplementary Figure 2b). crn1+ encodes the actin binding protein coronin, pcp1+, pericentrin. b) Photobleaching of Cut12.NEGFP and Cut12.NEGFP anchored Plo1.RLTom. c) Expression of plo1.RL was induced in cut12.NEGFP plo1+ or cut12.NEGFP plo1.ts41 backgrounds by removal of thiamine at t=0. Levels of the endogenous and chimeric Plo1 molecules were assessed by Plo1 polyclonal antibodies while V5 monoclonal antibodies detected Plo1.RL alone. Cells were shifted from 25°C to 36°C at t=0 immediately after filtration into analogue free medium. Tubulin immunofluorescence revealed the mitotic index. d) 3-MB-PP1 removal from strains hosting a de-repressed cdc2.RL allele (for recruitment to other locations see Supplementary Figure 3b). e) 3-MB-PP1 removal from cdc2.RL expressing strains indicated that MPF activation relied upon Plo1 function to drive mitotic commitment. The accumulation of mitotic plo1.ts41 cells from 60 mins reflected the block to mitotic progression that arose from Plo1 inactivation (Supplementary Figure 1e, f). f) 3-MB-PP1 removal from a cut12.NEGFP strains hosting either a de-repressed cdc2.RL or cdc2.RLY15 chimera. For cdc2.RL HU the analogue free medium that was substituted at t=0 to release the kinase activity contained 10 mM hydroxyurea to block DNA synthesis. g) A cartoon depicting the scheme used to generate the mitotic index (h) and MPM2 staining data in i. h) MPF activation via cdc2.RL expression and analogue release promoted a modest level of mitotic commitment in cdc2.33 cells at 36°C when it was free to diffuse throughout the cell (cut12+), but not when restricted to the SPB (cut12.NEGFP). i) MPM2 staining demonstrating that MPF activation on cdc2.33 SPBs at 36°C triggered Plo1 activation on SPBs even though cells did not enter mitosis.
As MPF associates with G2 SPBs22,23 and controls SPB recruitment of Plo1 (Figure 1e-f), we asked whether it was also responsible for generating the pro-mitotic Plo1 signal at SPBs. We tested this possibility with a Cdc2/GBP fusion protein, Cdc2.RL (cdc2. F84GY15F Supplementary Figure 1c) that is insensitive to either Wee1 or Mik1 inhibition24 and sensitive to ATP analogues11. Activation of Cdc2.RL by analogue removal from strains in which the chimeric kinase had been recruited to a variety of locations (Supplementary Figure 3a) mirrored the Plo1.RL data: SPB recruitment via Cut12.NEGFP gave a strong induction of mitosis, weaker levels followed SPB recruitment with either Pcp1.GFP or Sid4.GFP and no induction was seen following recruitment to any other location (Figure 2d, Supplementary Figure 3b). Bleaching Cut12.NEGFP anchored Cdc2.RLTom revealed a static association with the SPBs (Supplementary Figure 3c). MPM2 reactivity of SPBs showed that the wave of mitosis that arose from activation of Cut12.NEGFP anchored Cdc2.RL was preceded by Plo1 activation (Supplementary Figure 3d). Consistently, this ability of Cut12.NEGFP anchored Cdc2.RL to induce mitosis relied upon Plo1 activity (Figure 2e).
Three further Cut12.NEGFP/Cdc2.RL experiments were informative. 1) When the cdc2 encoding sequence retained tyrosine 15 (Cdc2.RLY15, Supplementary Figure 1c) mitosis was not induced (Figure 2f), indicating that the balance of Wee1 and Cdc25 activities normally determines the timing at which SPB bound MPF activity is enhanced to trigger mitosis. 2) Mitotic induction by SPB tethered Cdc2-RL was blocked in a cdc2.33 background in which the bulk population of MPF within the nucleus22,23 was inactivated (Figure 2g, h), even though Plo1 activity was promoted on the SPBs of these interphase cells (Figure 2i). Thus, division is not driven from the SPB, rather MPF activation at the SPB serves as trigger event that promotes conversion of the bulk population MPF throughout the cell22,23 to the active state. 3) The second wave of mitosis after analogue removal was abolished by the inclusion of hydroxyurea in the replacement, analogue free, medium (Figure 2f), indicating that the checkpoint remains intact.
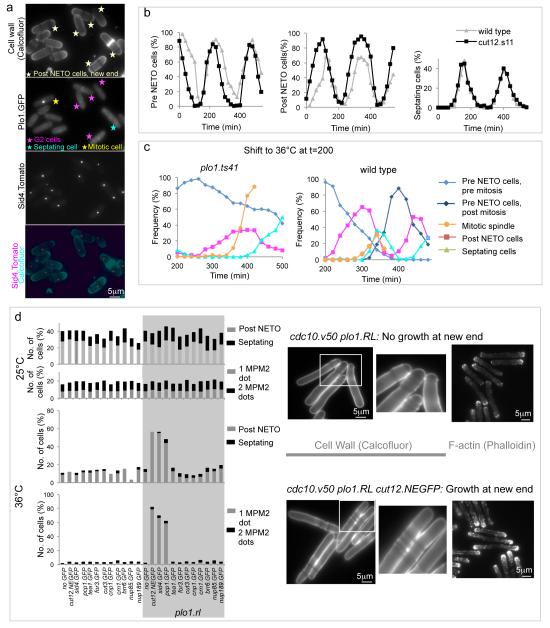
The point in G2 phase at which Plo1 was initially recruited to G2 SPBs is strongly influenced by cell density (Figure 1a). Consistently, nutrient provision and TOR signaling is closely tied to SPB recruitment of Plo1 in mitotic control25-28. This context dependency is highly reminiscent of the way by which the timing of the G2 event NETO changes in response to changes in nutrient supply3. We therefore examined the relationship between Plo1 recruitment to the G2 SPB and NETO. Strikingly, the two events were coincident: in counts of 100 cells, every post-NETO G2 cell displayed a Plo1.GFP signal at its SPB while neighbouring pre-NETO cells had none (Figure 3a). The correlation between NETO and Plo1 recruitment was even maintained when Plo1 SPB recruitment was advanced by 30 minutes by cut12.s11 (Figure 3b).
Figure 3. NETO is triggered by Plo1 activation/recruitment on the G2 SPB.
a) Fluorescence imaging of cell wall signals (calcofluor, top), Plo1.GFP and Sid4.Tom of living cells of the indicated strains. Arrows identify cells with Plo1.GFP signals on SPBs. b) Size selected small G2 cells were fixed and scored for NETO status and septation index as they transited the cell division cycle. c) Cultures in which cell cycle progression had been synchronized by the selection of small cells at t=0 were allowed to transit one cell cycle (Supplementary Figure 1g) before the temperature was shifted to 36°C and the frequency of NETO and mitotic spindle index scored in fixed cells. As indicated in Figure 1g, inactivation of Plo1 delayed mitotic commitment in the plo1.ts41 panel. Despite this delay, over 60% of cells failed to undergo NETO by the time the mitotic index rose sharply as cells accumulate with monopolar spindles (Supplementary Figure 1f). d) Mutants harbouring cdc10.v50 and the indicated GFP fusion genes were grown to early log phase at 25°C before the plo1.RL allele was induced by removal of thiamine in the presence of 40 μM 3MB-PP1. After dilution and a further 24 hours at 25°C the early log phase cells were filtered into analogue and thiamine free medium and split into two, one half being kept at 25°C, the other being shifted to 36°C (lower). 5 hours later cells were fixed and the frequency of NETO scored (left). The panels on the right show examples of the wild type control (upper) and cut12.NEGFP (lower) cdc10.v50 strains after incubation at 36°C stained with Calcofluor to highlight cell wall material or TRITC phalloidin to stain F-actin.
To ask whether there was a direct causal relationship between Plo1 activity and NETO, NETO was monitored when small G2 plo1.ts41 cells (Supplementary Figure 1e,f) were shifted from 25°C to 36°C. NETO execution was severely compromised by Plo1 inactivation (Figure 3c, Supplementary Figure 1g). To determine whether it was the SPB associated pool of Plo1 that triggered NETO, we targeted Plo1.RL to various locations in cdc10.v50 cells. At 36°C, cdc10.v50 arrests cell cycle progression in G1 phase before NETO3. NETO was triggered when Plo1.RL was activated at the SPBs of cdc10.v50 cells but nowhere else, including recruitment to the cell end itself through either Tea1.v5GFP or For3.GFP fusion proteins (Figure 3d; Supplementary Figure 2a).
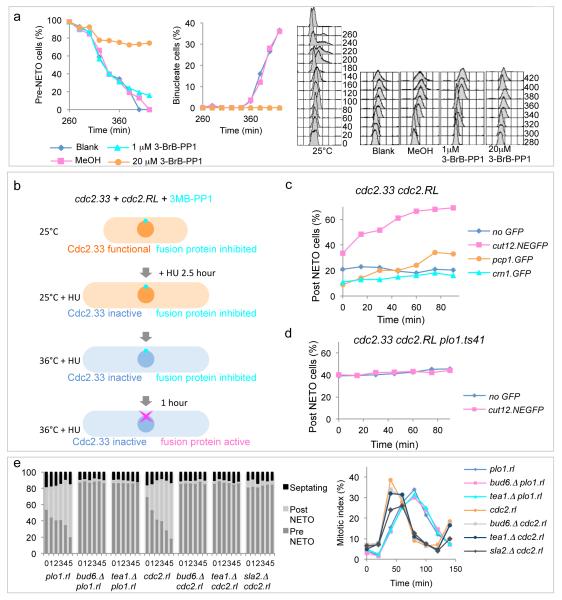
The observation that G2 arrested cdc2.33 cells undergo NETO3, yet NETO can be triggered by Plo1 activation on SPBs (an MPF dependent event (Figure 1e-f)), prompted us to re-examine the relationship between NETO and Cdc2 with the cdc2.as allele. Addition of 1 μM BrB-PP1 to G2 cdc2.as cells mimicked temperature mediated inactivation of cdc2.33 in having no impact on NETO yet arresting cell cycle progression in G2. However, a stronger level of inhibition (20 μM BrB-PP1) did block NETO (Figure 4a, Supplementary Figure 4), indicating that the threshold of MPF activity required for NETO was lower than that for mitotic commitment.
Figure 4. MPF activation on the SPB triggers NETO in a Plo1 dependent manner.
a) Cell cycle progression was synchronized by the selection of small, G2, cdc2.as cells at 25°C. After transit of one cell cycle at 25°C (Supplementary Figure 4a) the culture was split into four equal aliquots which were all shifted to 32°C. Either 1 or 20 μM 3-BrB-PP1, solvent alone or nothing were added before NETO was scored in fixed samples at the indicated intervals. Samples were also processed for FACS analysis to monitor DNA content (right). For a repeat of this FACs analysis see Supplementary Figure 4c. b) The scheme used to assess whether analogue removal from strains harbouring cdc2.RL and the indicated GFP targeting proteins was able to induce NETO when the bulk population of MPF in the cell was inactivated by incubation of cdc2.33 cells at 36°C. c, d) While NETO was induced when Cdc2.RL was targeted to the SPB by Cut12.NEGFP (c), no induction occurred when Plo1 kinase was inhibited (d). e) Left cut12.NEGFP cells containing the indicated additional mutations were treated as for Figure 3d except that cells were maintained at 25°C throughout. The time interval is in hours. Activation of Plo1 or Cdc2 on the SPB did not induce NETO in the polarity mutants bud6.Δ, tea1.Δ or sla2.Δ despite the fact that processing of the cut12.NEGFP mutant strains as described for Figure 2a, revealed that none of them compromised the ability of activation of either kinase on SPBs to induce mitosis (right).
The contribution of SPB associated MPF to the promotion of NETO cannot be assessed by the Cdc2.RL approach in wild type cultures because the immediate induction of mitotic commitment (Figure 2d) obscures any impact upon NETO. We therefore blocked the propagation of the mitotic commitment signal with the cdc2.33 mutation (Figure 2h) before assessing NETO induction. Hydroxyurea treatment enriched the pool of S phase arrested (pre-NETO3) cells prior to temperature shift from 25°C to 36°C for 1 hour to inactivate Cdc2.33 before analogue removal activated the SPB associated Cdc2.RL (Figure 4b). NETO was triggered by Cdc2.RL activation on the SPB, but at no other location. Recruitment to Cut12 had a greater impact than to Pcp1 or Sid4 (Figure 4c, Supplementary Figure 3e). Critically, this ability of Cut12.NEGFP anchored Cdc2.RL to induce NETO was reliant on Plo1 activity (Figure 4d). It also required the polarity factors Tea1, Bud6 and Sla2 (Figure 4e).
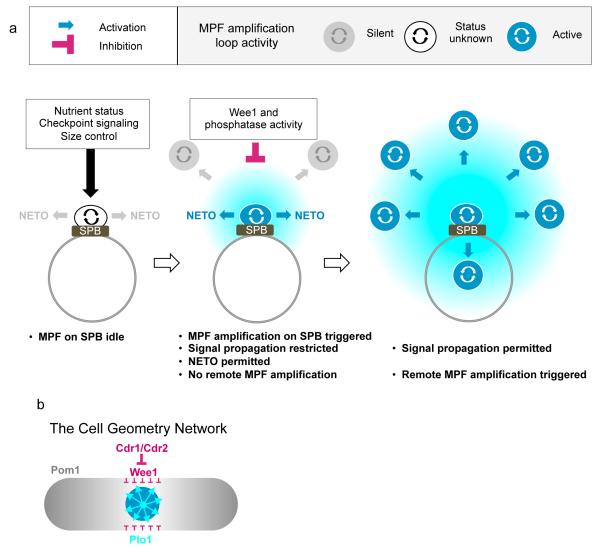
We propose that Plo1 recruitment to SPBs in G2 phase both triggers the cell cycle dependent morphogenetic switch to bipolar growth and constitutes a priming event that licenses the cell to regulate the timing of mitotic commitment. The MPF dependency and activity of the Plo1 recruited to these G2 SPBs suggests a local activation of the feedback loops that will later be employed at remote locations to drive the global cell cycle during mitotic commitment (Figure 5a). Because NETO happens as soon as the feedback loop is running on the SPB the target/s for Plo1 in NETO control probably differ from those in G2/M commitment control. The fact that it is only constitutively active forms of MPF that can override the normal controls to induce mitosis and NETO when targeted to the SPB (Figure 2f, Supplementary Figures 2d, e, 3f) indicates that Wee1 activity blocks the propagation of the mitotic commitment signal from the SPB. Once the restraining activity of Wee1 is removed/overcome cells can execute mitosis. Given the relative location of the different signaling components, it would seem likely that the anti-mitotic activity of the equatorial belt of inhibitory Cdr1/Cdr2/Wee1 nodes that encircle the nucleus blocks the propagation of the signals emanating from the SPB (Figure 5b)29,30.
Figure 5. Model of Feedback Loop activation on the SPB and its subsequent propogation throughout the cell to promote mitotic commitment.
a) The absence of a readout for MPF activity on the SPB in early G2 means that it is not possible to propose activity at this site before activation/enhancement promotes Plo1 turnover in late G2 (left). MPF feedback loop enhancement/activation on the G2 SPB subsequently triggers the pathway that ultimately leads to NETO, yet this level of G2 activation is insufficient to trigger mitotic commitment (centre). Mitosis is triggered later when there has either been a reduction in the level of inhibition by Wee1 or phosphatases such as PP2A and the Cdc14 homologue Flp1/Clp1 or loop activity is enhanced to exceed an inhibitory threshold, or a combination of the two (right). It is likely that the different pathways that impact upon the timing of mitotic commitment will target different parts of this switch. (b) The spatial organization of Plo1 activation on the SPB relative to the Pom1/Cdr1/Cdr2 pathway that couples tip extension to mitotic commitment29,30. Note that the close relationship between the position of the Cdr1/Cdr2/Wee1 nodes and the position of the nucleus that is established by the cycles of nuclear import/export of Mid1 places this inhibitory network directly over the SPB, putting it in the optimal location to dampen/neuter the pro-mitotic signal emerging from the SPB.
The well documented ability of TOR signaling to influence the timing of mitotic commitment in fission yeast relies upon its ability to promote Plo1 recruitment to the SPB25-28, indicating that, in some situations, enhancing the level of Plo1 signaling from the SPB is sufficient to advance the timing of mitotic commitment. Perhaps mitotic commitment is also finally triggered when Plo1 activity exceeds a critical threshold in unperturbed cell cycles.
Because NETO ensures that each daughter cell will receive an experienced “old” cell tip that will be optimal for growth in the subsequent cell cycle, Plo1 recruitment to G2 SPBs could be seen as the first phase of mitotic commitment. However, the question remains as to how a signal that emanates from the cell equator triggers an event at the cell extremity (NETO). A recent study may hold the clue31. The Cdc42 activation that drives tip growth is not a persistent signal at one end. Rather it alternates between the two cell tips throughout interphase. Thus, Cdc42 is activated at new ends every 3 minutes throughout early G2 up to NETO. However, NETO is only triggered once global Cdc42 activity exceeds a critical threshold level of global Cdc42 activation. A general signal emanating from the SPB could set the level of this general threshold by targeting Cdc42 or its regulators globally.
Given the impact of TOR and stress pathways upon SPB recruitment of Plo126,27,32 and the co-ordination of cytokinetic ring formation and function by a second cell cycle regulatory network on the same SPBs (the Septum Initiation Network33), we propose that the SPB (and by implication the centrosome) acts as a platform at which signals from diverse signaling pathways are integrated to generate, coordinated and coherent signals to control cell cycle progression.
Supplementary Material
Acknowledgements
We thank: Ben Hodgson for technical assistance. Valerie Doye (Insitut Jacques Monod, Paris), Daniel Mulvihill (University of Kent, UK), Ursula Fleig (Düsseldorf, Germany) and Dorota Feret (Paterson Institute) for strains; Keith Gull (Oxford University, UK) and Hiro Ohkura (Edinburgh University, UK) for antibodies; Dorota Feret (Paterson Institute), Gislene Pereira (DKFZ) and Tony Carr (GDSC, Sussex, UK) for plasmids; Danny Bitton (Paterson Institute) for discussions during the design of plo1.as8. This work was supported by Cancer Research UK [CRUK] grant number C147/A6058. VS and AK were supported by Swiss National Science Foundation and EPFL.
Footnotes
Contribution statement
IMH conceived the study. AK and VS generated cdc2.as. AP generated the conditional polo kinase alleles, including Plo1.RL. VAT and KYC did the imaging work in figure 1. AG performed the remainder of the experiments with occasional assistance from AP and KYC for more complex experiments and cloning. IMH wrote the manuscript with input and discussions from all authors.
References
- 1.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 2.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–8. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 3.Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- 4.Mulvihill DP, Petersen J, Ohkura H, Glover DM, Hagan IM. Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe. Mol Biol Cell. 1999;10:2771–2785. doi: 10.1091/mbc.10.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson JD, Feilotter H, Young PG. stf1: non wee mutations epistatic to cdc25 in the fission yeast Schizosaccharomyces pombe. Genetics. 1990;126:309–315. doi: 10.1093/genetics/126.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridge AJ, Morphew M, Bartlett R, Hagan IM. The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev. 1998;12:927–942. doi: 10.1101/gad.12.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallada VA, Bridge AJ, Emery PE, Hagan IM. Suppression of the S. pombe cut12.1 cell cycle defect by mutations in cdc25 and genes involved in transcriptional and translational control. Genetics. 2007;176:73–83. doi: 10.1534/genetics.107.072090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIver FH, Tanaka K, Robertson AM, Hagan IM. Physical and functional interactions between polo kinase and the spindle pole component Cut12 regulate mitotic commitment in S. pombe. Genes Dev. 2003;17:1507–23. doi: 10.1101/gad.256003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka K, et al. The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe. EMBO Journal. 2001;20:1259–1270. doi: 10.1093/emboj/20.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bähler J, et al. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J Cell Biol. 1998;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dischinger S, Krapp A, Xie L, Paulson JR, Simanis V. Chemical genetic analysis of the regulatory role of Cdc2p in the S. pombe septation initiation network. J Cell Sci. 2008;121:843–53. doi: 10.1242/jcs.021584. [DOI] [PubMed] [Google Scholar]
- 12.May J, Mitchison JM. Length growth in fission yeast cells measured by two novel techniques. Nature. 1986;322:752–754. [Google Scholar]
- 13.Zhang C, et al. A second-site suppressor strategy for chemical genetic analysis of diverse protein kinases. Nature Methods. 2005;2:435–41. doi: 10.1038/nmeth764. [DOI] [PubMed] [Google Scholar]
- 14.Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase Plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 15.Kothe M, et al. Structure of the catalytic domain of human polo-like kinase 1. Biochemistry. 2007;46:5960–71. doi: 10.1021/bi602474j. [DOI] [PubMed] [Google Scholar]
- 16.May KM, Reynolds N, Cullen F, Yanagida M, Ohkura H. Polo boxes and Cut23 (Apc8) mediate an interaction between polo kinase and the anaphase-promoting complex for fission yeast. J Cell Biol. 2002;156:23–28. doi: 10.1083/jcb.200106150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–75. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 18.Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThrbinding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–31. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 19.Rothbauer U, et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nature Methods. 2006;3:887–9. doi: 10.1038/nmeth953. [DOI] [PubMed] [Google Scholar]
- 20.Rothbauer U, et al. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Prot: MCP. 2008;7:282–9. doi: 10.1074/mcp.M700342-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Bertazzi DT, Kurtulmus B, Pereira G. The cortical protein Lte1 promotes mitotic exit by inhibiting the spindle position checkpoint kinase Kin4. J Cell Biol. 2011;193:1033–48. doi: 10.1083/jcb.201101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body populations of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- 23.Decottingnies A, Zarzov P, Nurse P. in vivo localisation of fission yeast cyclin-dependent kinase cdc2p and cyclin B cdc13p during mitosis and meiosis. J Cell Sci. 2001;114:2627–2640. doi: 10.1242/jcs.114.14.2627. [DOI] [PubMed] [Google Scholar]
- 24.Gould KL, Nurse P. Tyrosine Phosphorylation Of the Fission Yeast Cdc2+ Protein-Kinase Regulates Entry Into Mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 25.Petersen J, Nurse P. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat Cell Biol. 2007;9:1263–72. doi: 10.1038/ncb1646. [DOI] [PubMed] [Google Scholar]
- 26.Halova L, Petersen J. Aurora promotes cell division during recovery from TOR-mediated cell cycle arrest by driving spindle pole body recruitment of Polo. J Cell Sci. 2011;124:3441–9. doi: 10.1242/jcs.083683. [DOI] [PubMed] [Google Scholar]
- 27.Petersen J, Hagan IM. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature. 2005;435:507–12. doi: 10.1038/nature03590. [DOI] [PubMed] [Google Scholar]
- 28.Hartmuth S, Petersen J. Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J Cell Sci. 2009;122:1737–46. doi: 10.1242/jcs.049387. [DOI] [PubMed] [Google Scholar]
- 29.Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature. 2009;459:857–60. doi: 10.1038/nature08074. [DOI] [PubMed] [Google Scholar]
- 30.Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–6. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]
- 31.Das M, et al. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science. 2012;337:239–43. doi: 10.1126/science.1218377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen J. TOR signalling regulates mitotic commitment through stressactivated MAPK and Polo kinase in response to nutrient stress. Biochem Soc Trans. 2009;37:273–7. doi: 10.1042/BST0370273. [DOI] [PubMed] [Google Scholar]
- 33.Simanis V. Events at the end of mitosis in the budding and fission yeasts. J Cell Sci. 2003;116:4263–4275. doi: 10.1242/jcs.00807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.