Abstract
Background
The IL-17 axis is implicated in pathogenesis of chronic rejection following human lung transplantation. Using a murine model of obliterative airway disease (OAD), we recently demonstrated that Abs to MHC class I antigens can induce immune responses to self-antigens that contributes to immunopathogenesis of chronic rejection. Using a murine model of OAD, we determined the role of IL-17 family members in induction of autoimmunity leading to OAD following ligation of MHC class I.
Methods
Anti-MHC class I or control antibodies (Abs) were administered intrabronchially to wild-type (WT) and IL-17a knock out (IL-17A−/−) C57BL/6.
Results
By day 30, anti-MHC I administered endobronchially in IL-17A−/− mice demonstrated significant reduction in cellular infiltration, a 36.8% reduction in CD4+ T cells, 62.7% in CD11b+ macrophages, 37.5% in degree of fibrosis, 1.94 fold and 2.17 fold decrease in anti-KAT and anti-Col-V respectively when compared to wild type mice. Analysis of lung infiltrating cells in anti-MHC I WT revealed increase in IL-17A (KAT:92+21,Col-V:103+19spm) and IL-17F (KAT:5.03%,Col-V:2.75%) secreting CD4+ T cells. However, administration of anti-MHC I in IL-17A−/− demonstrated increase only in IL-17F for KAT (13.70%) and Col-V (7.08%). Anti-IL-17(A-F) mAb administration following anti-MHC I abrogated OAD in both WT and IL-17A−/−.
Conclusion
Our findings indicate that IL-17A and IL-17F secreted by CD4+Th17 cells specific to lung self-antigens are critical mediators of autoimmunity leading to the pathogenesis of OAD.
Introduction
Bronchiolitis Obliterans Syndrome (BOS) is an obstructive lung disease that leads to constriction of small airways (terminal bronchioles) by inflammation and fibrosis. BOS is clinically diagnosed with a progressive decline in forced expiratory volume in 1 second (FEV1) of >20% from baseline (1). Various etiological factors including acute rejection, respiratory viral infections, gastro esophageal reflux have been implicated as independent risk factors for the development of BOS following human lung transplantation (LTx) (2). Studies from our lab and others have shown that development of antibodies to mismatched donor HLA correlates with the development of chronic rejection following transplantation (3, 4). A recent study from our lab has also demonstrated that development of anti-MHC precede development of autoimmune responses and contribute to development of BOS (5). Several reports have demonstrated that antibodies (Abs) and T cell mediated responses to donor mismatched HLA antigens and self-antigens in the lung, K-α-1 tubulin (KAT) and Collagen-V (Col-V) contribute to immunopathogenesis of BOS (6–11).
Using a murine model of obliterative airway disease (OAD), we recently demonstrated that Abs to MHC class I antigens can induce immune responses to self-antigens that contributes to immunopathogenesis of chronic rejection (12). Intrabronchial administration of Abs to MHC class I resulted in cellular infiltration surrounding bronchioles, epithelial hyperplasia, fibrosis and luminal occlusion. Analysis of lung infiltrating lymphocytes (LIL) revealed predominance of IL-17 secreting CD4+T cells reactive to KAT and Col-V. It has been previously demonstrated that pro-inflammatory Th17 cells secrete not only IL-17A but also IL-17 B-F, IL-21, IL-22, IL-23 (13, 14). Among the IL-17 family members secreted by CD4 T cells, IL17F bears close structural homology (~60%) to IL-17A and play a significant role in neutrophil recruitment and chemokine secretion (14). Therefore, in addition to IL-17A, IL-17F may also play an important role in induction of OAD.
Members of the IL-17 family (IL17 A-F) share a similar protein structure with four cysteine residues that are critical for their three dimensional shape (15). IL-17 has been shown to induce the production of various cytokines (IL-6, GM-CSF, IL-1b, TGF-b), chemokines (IL-8, Gro-1, MCP-1) and prostaglandins (16) and facilitate infiltration of various cells including neutrophils, playing a critical role in airway remodeling, autoimmune disease and allograft rejection (17, 18). Overexpression of IL-17F gene in murine airways is associated with inflammation through increased airway neutrophilia, induction of cytokines, increased airway hyperreactivity, and hypersecretion of mucus (19). Several studies using IL-17A and IL-17F deficient animals have demonstrated that IL-17A and IL-17F play distinct roles in humoral immunity, inflammatory responses and immunity to bacterial infections (19). Studies in the literature have also demonstrated that deficiency in one member leads to compensation by other members (19) .
Results
Decreased induction of cellular infiltration around bronchioles and vessels, epithelial hyperplasia and fibrosis in IL-17A−/− following administration of MHC Class I Abs
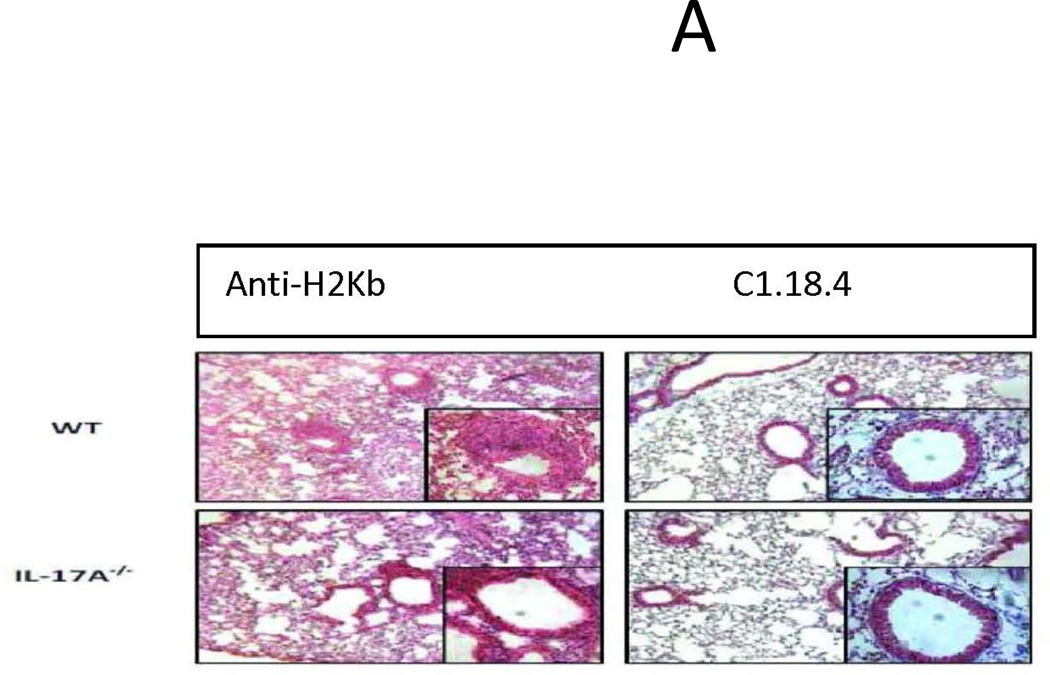
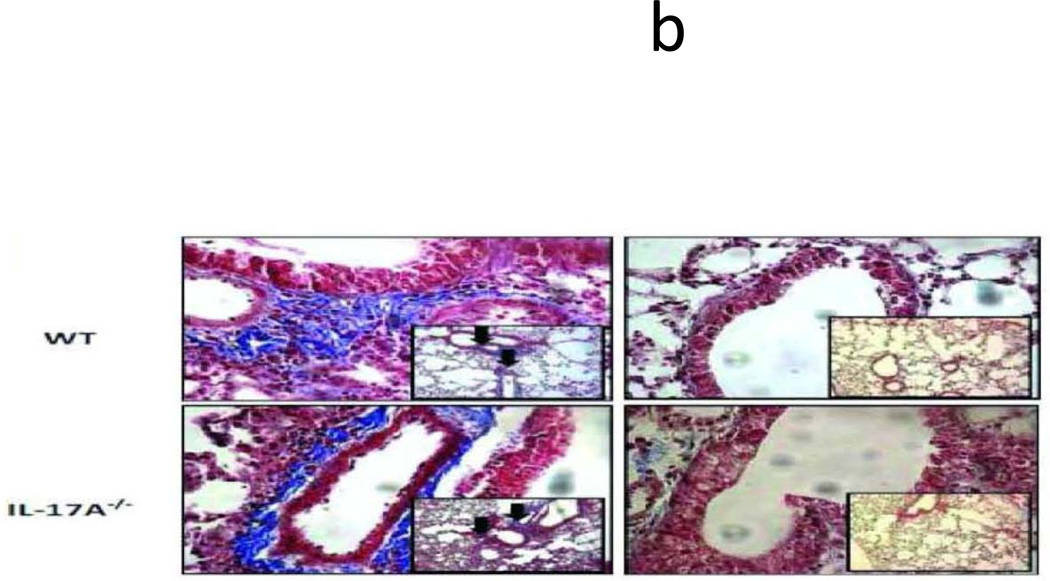
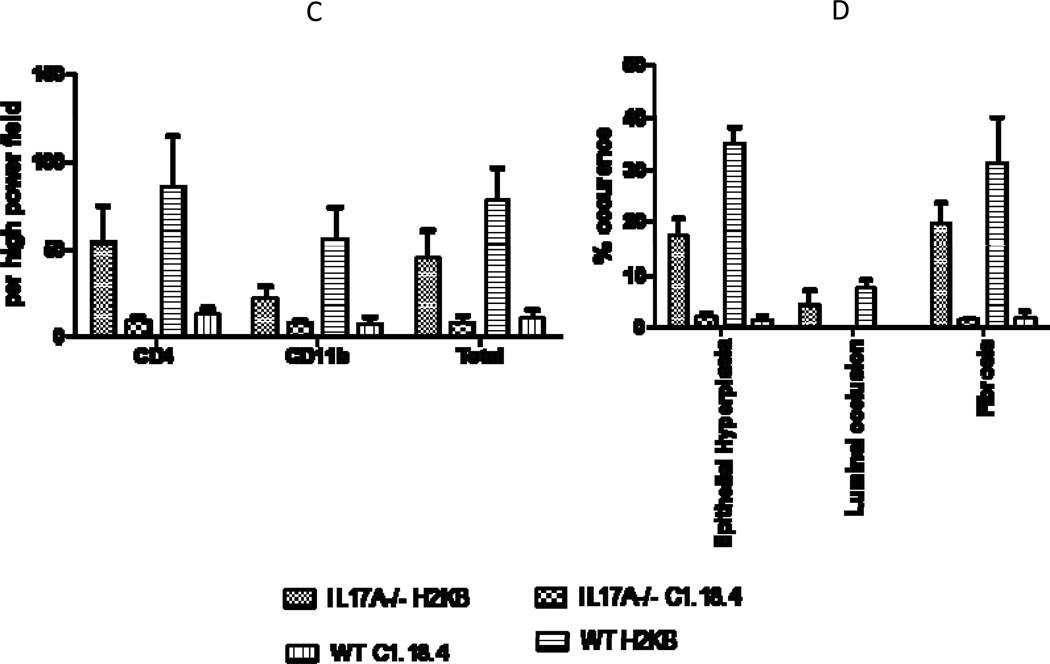
To identify the role of individual IL-17 family cytokines in pathogenesis of anti-MHC class I mediated autoimmunity and OAD, anti-H2Kb or control C1.18.4 Abs were administered intrabronchially in IL-17A−/− and WT C57BL/6 mice. H&E (Figure 1a) and trichrome staining (Figure 1b) of the lungs demonstrated that administration of anti-MHC resulted in a significantly lower injury in the IL-17A−/− when compared to the wild type mice. Anti-H2Kb administered IL-17A−/− mice compared to WT demonstrated a 36.8% reduction in frequency of CD4+ T cell, 62.7% reduction in frequency of CD11b+ macrophage and 37.5% reduction in fibrosis around bronchioles by day 30 (Figure 1c). These findings suggest that IL-17A may play a significant role in induction of OAD following anti-H2Kb administration into the lung. However, IL-17A−/− were not resistant to development of OAD following anti-H2Kb administration, as evidenced by significant peribronchial cell infiltrates (44.8%vs7.5%), fibrosis (19.5%vs1.4%) and epithelial hyperplasia (17.3%vs2%) compared to control treated IL-17A−/− animals (Figure 1d). These findings indicate the possible involvement of other IL-17 family members besides IL-17A in pathogenesis of OAD.
Figure 1. Significant reduction in induction of cellular infiltration around bronchioles and vessels, epithelial hyperplasia and fibrosis following ligation of anti-MHC class I Abs in IL17A−/− mice.
Anti-H2Kb or control C1.18.4 Abs were administered intrabronchially in IL-17A deficient (IL-17A−/−) and WT C57BL/6 mice. A: H&E staining of the lungs. Serial administration of anti-H2Kb Abs resulted in cellular infiltration around bronchioles and vessels, epithelial changes including hyperplasia, fibrosis and luminal occlusion in both IL-17A deficient and WT by day 30 compared to controls treated with C1.18.4 respectively. B: Trichrome staining of the lungs. Collagen deposition around the bronchioles and vessels are stained blue in color. C: Semiquantitative analysis of the cellular infiltrates in the lungs. D: Morphometric analysis of the lungs. Semiquantitative analysis of the slides demonstrated less significant lesions in IL-17A −/− mice compared to WT mice with respect to cellular infiltration, epithelial hyperplasia, fibrosis and luminal occlusion.
Loss of CD4+CD25+Foxp3+ Tregs is accompanied by development of Abs to KAT and Col-V in lungs of IL-17A−/− and WT mice following anti-MHC class I administration
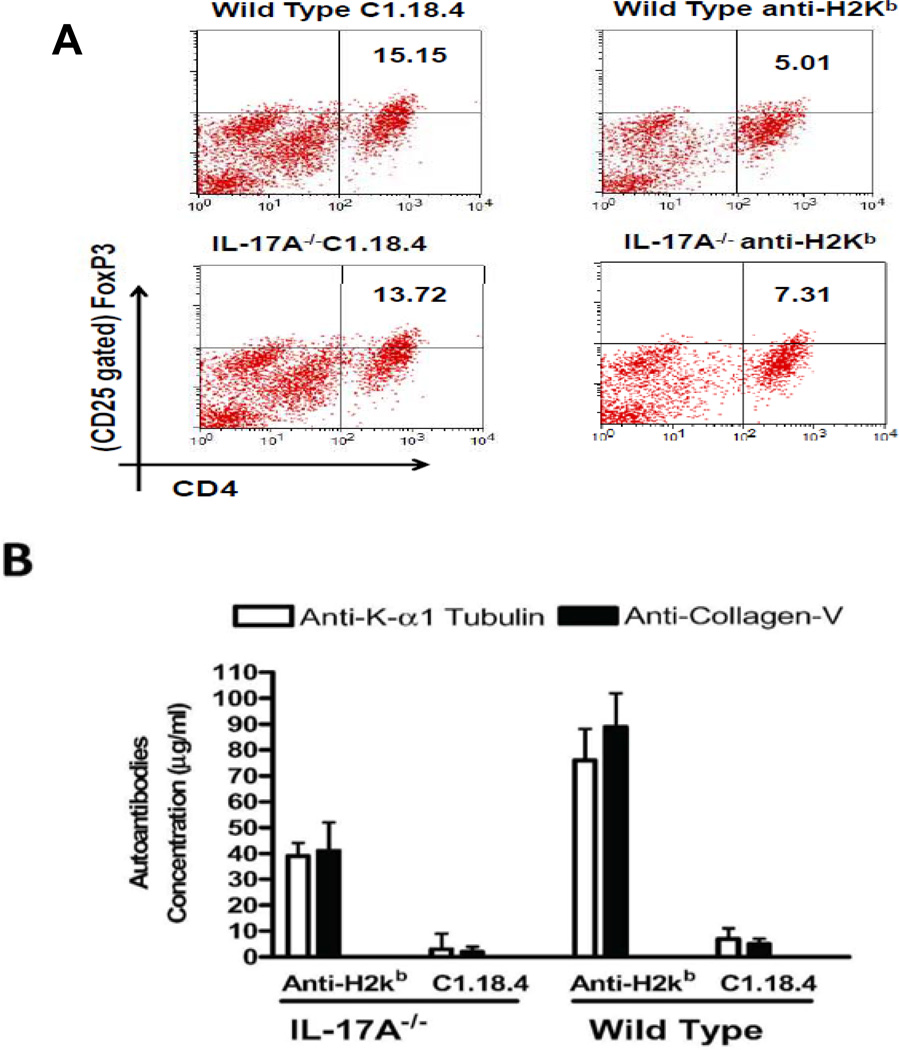
Flow cytometric analysis of frequency of Tregs in lungs of anti-H2kb treated mice revealed a 3 fold reduction in WT mice (anti-H2kb:5.01%; C1.18.4:15.15%, p=0.02) and 2 fold reduction in IL-17A−/− mice (anti-H2kb:7.31%; C1.18.4:13.72%, p=0.03, Figure 2A). Anti-H2Kb administered IL-17A−/ demonstrated 1.94 fold and 2.17 fold reduction in Abs to KAT and Col-V respectively compared to WT mice in BAL (Figure 2B). However, levels of anti-KAT (antiH2kb:39+5mcg/ml, C1.18:3+6mcg/ml) and anti-Col-V (anti-H2kb:41+11mcg/ml, C1.18:2+2mcg/ml) Abs in antiH2kb treated IL-17A−/ animals were significantly higher than control animals. A decrease in the percentage of Tregs in lung infiltrating lymphocytes (LIL) accompanied with an increase in autoantibodies in BAL in both WT and IL-17A−/− mice treated with anti-H2Kb suggests that other IL-17 family members may be involved in the development of autoimmunity in OAD.
Figure 2. Loss of CD4+CD25+Foxp3+ regulatory T cells and Induction of autoAbs to pulmonary self antigens, KAT and Col-V following ligation of anti-MHC class I Abs.
(A) Flow cytometric analysis of the frequency of Tregs in the lungs of anti-H2kb treated mice revealed a significant decline in the both IL-17A−/− (anti-H2kb- 7.31; C1.18.4–13.72) and WT mice (anti-H2kb5.01; C1.18.4–15.15) compared to control mice. However, the percentage decline in the frequency of Tregs was higher in WT (10.14%) compared to IL-17A−/− (6.41%) treated with anti-H2kb. (B) Levels of antibodies against the self antigens KAT and Col-V in BAL were measured by ELISA. The titers of the antibodies are presented as mean ± SD (µg/ml). A significant increase in the titer of Abs to both KAT and Col-V was observed in anti-H2kb treated IL-17A−/− and WT mice.
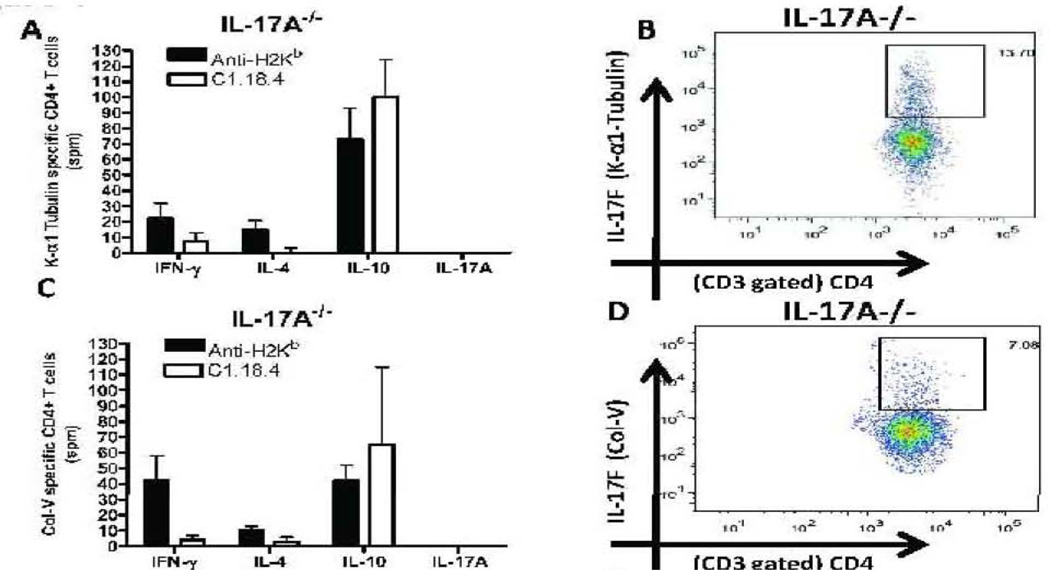
Induction of autoreactive CD4+ T cells which predominantly secrete IL-17A and IL-17F following anti-MHC class I administration
Lung infiltrating cells were analyzed for frequency of CD4+ T cell secreting IFN-γ, IL-4, IL-17A and IL10 specific to KAT and Col-V by ELISPOT (Fig.3A,C,E,G). The frequency of IL-17F secreting CD4+ T cells specific to self-antigens was determined by flow cytometry (Figure 3B,D,F,H). A significant increase in frequency of IL-17A secreting CD4+ T cells specific to KAT (92+21spm) and Col-V (103+19spm) was observed in lungs of anti-H2kb treated WT mice compared to control animals. An increased frequency of IL-17F secreting CD4+ T cells specific to KAT (IL-17A−/−:13.70% vs. WT:5.03%) and Col-V (IL-17A−/−:7.08% vs. WT:2.75%) was noted in anti-H2Kb treated IL-17A−/− mice compared to WT mice. No statistically significant difference was seen in levels of IFN-γ, and IL-4 secreting CD4+ T cells between IL-17A−/− and WT administered with anti-H2kb. Administration of anti-MHC in the wild type mice resulted in a decrease in the frequency of IL-10 secreting T cells when compared to the isotype administered mice, whereas in the case of IL-17A −/− mice there was no statistically significant difference in the frequency of IL-10 secreting T cells between the anti-MHC and isotype ab administered mice. Overall, these results indicate that IL-17A and IL-17F secreted by autoreactive CD4+ lung infiltrating cells play significant role in induction of Abs to KAT and Col-V following administration of anti-MHC class I.
Figure 3. Induction of autoreactive CD4+ T cells which predominantly secrete IL-17A and IL-17F following administration of anti-MHC Class I Abs.
Lung infiltrating T cells were isolated from the lungs of IL17A −/− and wild type mice treated with anti-H2Kb or control antibody (n=5 each, the figures shown are a representative of 5 mice). The frequency of T cells secreting IFN-γ, IL-4, IL-17A and IL-10 specific to KAT and Col-V were measured by ELISPOT (Figure 3, A,C,E,G) and presented as means ± SD. The frequency of IL-17F secreting CD4+ T cells specific to self antigens were determined by flow cytometry (Figure 3, B,D,F,H). A significant increase in the frequency of IL-17A secreting CD4+ T cells specific to KAT and Col-V was observed in the lungs of anti-H2kb treated WT mice compared to control animals. An increased frequency of IL17F secreting CD4+ T cells specific to KAT (IL-17A−/−:13.70% vs WT: 5.03%) and Col-V (IL-17A−/:7.08% vs WT: 2.75%) was noted in anti-H2Kb treated IL-17A−/− mice compared to WT mice. No significant difference was seen in the levels of IFN-γ, IL-4 and IL-10 secreting CD4+ T Cells specific to KAT and Col-V was seen between IL-17A−/− and WT administered with anti-H2kb.
Increased induction of IL-17A and IL-17F mediated inflammatory cascades following anti-MHC class I administration
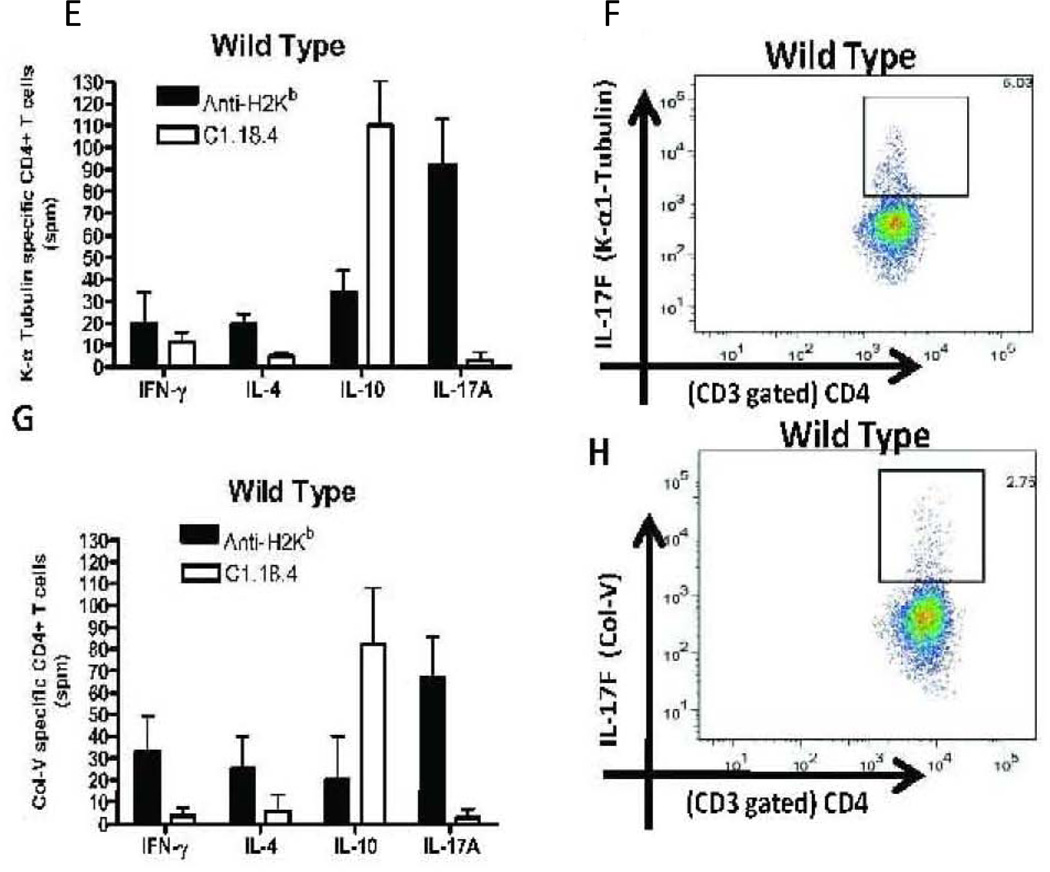
To investigate the potential role of known Th17 associated inflammatory cytokines in induction of autoimmunity following MHC class I ligation, a RT2 profiler PCR array for Th17 inflammation and autoimmunity was performed using total RNA extracted from whole lung RNA from WT and IL-17A−/ mice following administration of anti-H2Kb or isotype control Abs. Anti-H2Kb administration in IL-17A−/− resulted in significant increase in levels of IL-17F (7.80 fold, p=0.002) alone but not IL-17A (−6.4 fold), IL-17B (−0.28 fold), IL-17C (−0.23 fold), IL-17D (−0.70 fold), IL-17E (−0.49 fold), IL-6 (−4.01 fold), IL-21(−3.84 fold) and IL-23(−6.18 fold) compared to WT (Figure 4A). Anti-H2Kb administered IL-17A−/− demonstrated a significant increase in levels of IL-17RC (3.2 fold) and TRAF-6 (2.3 fold) but not IL-17RA (−2.70 fold). Anti-H2Kb administered IL17A−/− demonstrated a significant increase in the levels of CXCL1 (5.2 fold), CXCL2 (3.2 fold) and significant reduction in levels of CXCL12 (−2.10 fold), CCL7 (4.24 fold) and CX3CL1 (−3.12 fold) (Figure 4b). Anti-H2Kb administered IL-17A−/− demonstrated differential induction of MMP-3 (6.2 fold), MMP-9 (−3.1 fold) and MMP-13 (−2.8 fold) (Figure 4c). These results indicate that increased expression of IL-17F, its receptor, IL-17RC and downstream signal transducer TRAF-6 in IL-17A−/− was accompanied by induction of chemokines CXCL1, CXCL2 and MMP-3 following administration of anti-MHC class I Abs in the lung. No significant difference in expression of other cytokines, chemokines and MMPs analyzed in this panel were identified in both WT and IL-17A−/− between anti-H2kb and C1.18.4 treated animals. This indicates that IL-17A and IL-17F may result in upregulation of proinflammatory mediators that mediate OAD.
Figure 4. Increased induction of IL-17A and IL-17F mediated inflammatory cascades following anti-MHC class I administration.
Total RNA from the lungs of anti-H2KB treated WT and IL-17A−/− were extracted using Trizol reagent and used for the expression profile of the signaling cascades intermediates in the autoimmunity pathway using autoimmunity PCR array. The samples were normalized using housekeeping gene expression and the results are expressed as fold expression observed in IL17A −/− mice over expression observed in WT mice. Anti-H2Kb administration in IL-17A−/− resulted in significant increase in levels of IL-17F (7.80 fold) but not IL-17A (−6.4 fold), IL-17B (−0.28 fold), IL-17C (−0.23 fold), IL-17D (0.70 fold), IL-17E (−0.49 fold), IL-6 (−4.01 fold), IL-21(−3.84 fold) and IL-23(−6.18 fold) compared to WT. Anti-H2Kb administered IL-17A−/− demonstrated a significant increase in levels of IL-17RC (3.2 fold) and TRAF-6 (2.3 fold) but not IL-17RA (−2.70 fold). Anti-H2Kb administered IL-17A−/ demonstrated a significant increase in the levels of CXCL1 (5.2 fold), CXCL2 (3.2 fold) and a significant reduction in the levels of CXCL12 (−2.10 fold), CCL7 fold (−4.24 fold) and CX3CL1 (−3.12 fold). Anti-H2Kb administered IL-17A−/− demonstrated differential induction of MMP-3 (6.2 fold), MMP-9 (−3.1 fold) and MMP-13 (−2.8 fold).
Neutralization of IL-17F in IL-17A−/− mice following administration of anti-MHC class I results in abrogation of OAD lesions and autoimmunity to lung self-antigens, KAT and Col-V
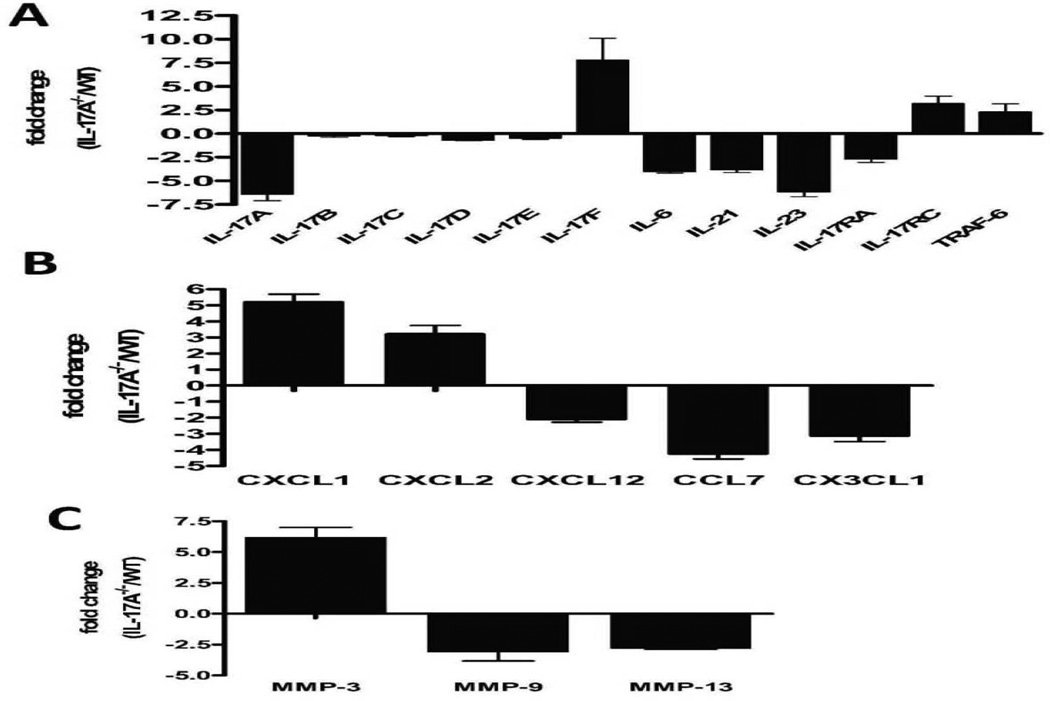
Neutralizing pan specific mAbs to IL-17(A-F) or control Abs (50µg i.v) were administered immediately following anti-H2kb in IL-17A−/− C57BL/6 mice on days 1,5,12 and 26. Administration of pan specific Abs to IL-17(A-F) following anti-H2kb administration in IL-17A−/− resulted in significant reduction of cell infiltrates around bronchioles and vessels accompanied by marked reduction in levels of Abs to KAT (4.5 fold) and Col-V (3.6 fold) in BAL fluid by day 30 (Figure 5A,B).
Figure 5. Neutralization of IL-17F following administration of anti-MHC class I Abs results in abrogation of OAD lesions and autoimmune responses to lung self antigens, KAT and Col-V in IL17A−/− mice.
In order to identify a role for IL-17 cytokine family in development of autoimmunity and OAD, neutralizing pan specific Abs reactive to IL-17A-F or control Abs (50µg i.v) were administered immediately following anti-H2kb in IL-17A−/− mice on days 1,5,12 and 26. (A ,B) administration of pan specific Abs to IL-17A-F following administration in IL-17A−/− resulted in reduction of cell infiltrates around bronchioles and vessels accompanied by (C) marked reduction in the levels of Abs to KAT (4.5 fold) and Col-V (3.6 fold) in the BAL fluid by day 30.
Discussion
There is increasing evidence that Abs to mismatched MHC is a critical mediator in immunopathogenesis of chronic rejection following transplantation (8, 20). Studies from our lab has also demonstrated that development of antibodies to MHC precede the development of autoantibodies to self-antigens and correlate with the development of BOS (5). The ligation of Abs to MHC class I antigens expressed on epithelial and endothelial cells has been shown to induce the activation of signaling cascades leading to upregulation of MHC class II molecules, secretion of inflammatory cytokines, chemokines, fibrogenic growth factors and adhesion molecules (21, 22). The anti-MHC class I Abs induced activation of inflammatory cascades in the allograft leads to neutrophil and mononuclear infiltration (21). The ensuing inflammatory milieu often leads to exposure of previously sequestered immunogenic domains of self-antigens (23). Inflammation following GERD, respiratory viral infection can also predispose to development of BOS following human LTx (24). Recent studies have suggested a critical role for Abs and T-cell mediated responses to pulmonary self-antigens, KAT and Col-V in the pathogenesis of chronic rejection following human LTx (10, 11). A recent longitudinal study of post-LTx recipients demonstrated that increased incidence and severity of BOS is associated with strong Col-V specific responses dependent on IL-17 (10). IL-17A, a prototype member of IL-17 family, which also includes IL-17B, IL-17C, IL-17D, IL-17E and IL-17F play a crucial role in the pathogenesis of inflammatory and autoimmune diseases (25–27).
In this study, we determined the role of IL-17 family members in the induction and progression of autoimmunity induced by anti-MHC class I Abs leading to OAD. Histopathological analysis of WT lungs treated with anti-H2Kb but not isotype control Abs revealed a significant CD4+ and CD11b+ cellular infiltration around bronchioles and vessels, epithelial hyperplasia and fibrosis (Figure 1a, b, c &d). Importantly, we demonstrated the induction of autoimmunity to pulmonary self-antigens, KAT and Col-V following ligation of Anti-H2Kb in lung. This was noted by the detection of a high frequency of CD4+ T cells infiltrating the lungs specific to KAT and Col-V and a high titer of Abs to KAT and Col-V in the serum and BAL fluid of mice. Regulatory T cells play a crucial role in regulation of IL-17 responses against self-antigens (28). The loss of regulatory T cells precedes the development of immune responses against self-antigens and BOS development in LTx (29). In this study we observed a 3 fold reduction in WT mice and 2 fold reduction in IL-17A−/− mice (Figure 2A). A decrease in the percentage of Tregs in LIL accompanied with an increase in autoantibodies in BAL in both WT and IL-17A−/− mice treated with anti-H2Kb suggests that other IL-17 family members may be involved in the development of autoimmunity in OAD.
IL-17F is a member of the IL-17 family of cytokines with significant homology to IL-17A and is secreted by CD4+ Th17 cells. Several studies have identified IL-17F to mediate inflammation in disease conditions commonly associated with IL-17A, by forming a biologically active heterodimer complex, IL-17A/F (30, 31). The CD4+ T cell infiltrates in the lung specific to KAT or Col-V predominantly secreted both IL-17A and IL-17F. Administration of pan specific neutralizing Abs to IL-17 following anti-MHC class I administration resulted in abrogation of peribronchial cell infiltrates and significant decline in auto-Abs to KAT and Col-V. However, IL-17A−/− mice administered with anti-H2Kb were not completely resistant to development of autoimmunity to KAT and Col-V and demonstrated peribronchial cell infiltrates comprising of CD4+ T cells and CD11b+ macrophages. This was accompanied by a 36.8% reduction in CD4+ cells, 61.6% decrease in CD11b+ infiltrates and 37.5% reduction in fibrosis in IL-17A −/− compared to wild type administered with anti-MHC class I Abs (Figure 1). Interestingly, IL-17A−/− mice revealed a 7.81 fold increase in IL-17F mRNA levels but not IL-17A, IL-17B, IL-17C, IL-17D, IL-17E following anti-H2Kb administration. IL-17A−/− mice demonstrated CD4+ T cells secreting IL-17F, reduced frequencies of IFN-γ and no change in IL-4 secreting CD4+ T cells. These observations suggest that IL-17F along with other cytokines and chemokines could play a role in the induction of these lesions in the IL-17A−/− mice following administration of anti-MHC antibodies.
IL-17 signals through heteromeric single phase type I transmembrane receptors, IL-17RA and IL-17RC which are widely distributed in the lung (30). A significant increase in IL-17RA and IL-17RC levels was noted in both WT and IL-17A animals administered with anti-H2Kb (data not shown). Lack of IL-17A resulted in increased expression of IL-17F, IL-17RC and its downstream adapter, TRAF-6. However, the decreased levels of Th17 related inflammatory cytokines, IL-6, IL-21, IL-23 and chemokines (CXCL12, CCL7 and CX3CL1) in the lungs of IL-17A−/− mice suggested a significant difference in the relative potencies of IL-17A and IL-17F. IL-17 has been shown to induce auto-Abs formation through CXCL12 (31). Lack of IL-17A resulted in 2.10 fold reduction in CXCL12 levels which was associated with 1.94 and 2.17 fold reduction in levels of auto-Abs to KAT and Col-V respectively following anti-MHC class I Ab administration. These findings indicate that IL-17A and IL-17F exhibit overlapping functions in the induction of autoimmunity following ligation of MHC class I molecules in the lung. Furthermore, neutralization of all IL-17 members in IL-17A−/− following administration of anti-H2Kb resulted in diminution of cell infiltrates around bronchioles and importantly, decreased auto-Ab titers to KAT (4.5 fold) and Col-V (3.6 fold) (Figure 5). Overall, these findings are consistent with our hypothesis that ligation of MHC class I in the lung induces autoimmunity in a Th17 dependent manner, that results in fibro-obstructive airway diseases including chronic lung allograft rejection.
In summary, our findings indicate that IL-17A and IL-17F secreted by CD4+Th17 cells specific to lung self-antigens are critical mediators of autoimmunity leading to fibro-obstructive airway diseases including OAD. Although less potent in the induction of auto-Abs to pulmonary self-antigens following anti-MHC class I administration, IL-17F functions differently compared to IL-17A by activating a distinct profile of chemokine and MMP expression in the lung. The neutralization of both IL-17A and IL-17F using pan specific mAbs represent an effective and viable therapeutic strategy to prevent the induction and development of de novo autoimmunity to pulmonary self-antigens leading to fibro-obstructive airway diseases including OAD following LTx.
Materials and Methods
Murine model of Anti-MHC Class I Ab induced OAD
Six to eight week old, male, WT C57BL/6 mice were purchased from Jackson laboratories. IL-17A knockout mice (IL-17A−/−) were a gift from Dr. John Russell at Washington University. Anti-MHC class I Abs (anti-H2Kb, 200µg, clone AF6.88.5, BioXcell Inc. West Lebanon, NH) or matched isotype control Abs (C1.18.4, 200µg) were administered intrabronchially (12). 200µg of endotoxin free anti-H2Kb or control Ab, C1.18.4 was administered on days 1, 2, 3, and 6, and weekly thereafter (n=5 each). Animals were sacrificed on days 15, 30 and lungs, bronchoalveolar lavage (BAL) fluid, serum and spleen were harvested. All experiments were performed in compliance with guidelines of Institutional Laboratory Animal Care and Use Committee of Washington University School of Medicine.
Histopathological and immunohistochemical analysis
Lung sections were evaluated for peribronchial cellular infiltration, epithelial abnormalities, luminal occlusion of bronchioles and fibrosis following H/E and Masson’s Trichrome staining. Morphometric analysis of percentage cellular infiltration was calculated by dividing number of affected bronchioles by total number of bronchioles in a low power field (×100). Degree of epithelial abnormalities was assessed by dividing number of abnormal epithelial cells divided by total number of epithelial cells in a low power field (×100). Percentage fibrosis was calculated using Optimas software version 6.5.172 (Media Cybernetics). Immunohistochemical analysis for CD4, CD8, CD11b and CD19 was performed on tissue sections obtained from lung embedded in Freez Tissue Matrix (OCT) as described earlier (12).
Isolation of lung infiltrating lymphocytes
Lung infiltrating lymphocytes (LILs) were isolated from Abs administered mice on days 15 and 30 as described previously (12). LILs (2×106 cells/ml) were stimulated with either KAT (1.0µg/ml) or Col-V (20.0µg/ml) or media alone for 3–5 days in a humidified 5%CO2 incubator at 37°C. CD4+ T cells were enriched (>98% data not shown) using negative selection kit (Stem cell Technologies, Canada). CD4 depleted LILs were irradiated at 3000 rads and used as antigen presenting cells (APCs) for performing CD4+ T cell based immunoassays.
Flow cytometry
Anti-mouse FITC-CD4, PE-CD25, PerCP-Foxp3 and corresponding isotype control Abs (BD Pharmingen), Alexa Fluor 488 anti-mouse IL-17F (eBioscience) were obtained. For IL-17F staining, CD4+ T cells were stimulated with either KAT (1.0µg/ml) or Col-V (20.0µg/ml). Monensin (2µM, eBioscience) was added during the last 5 hrs of in vitro activation of CD4+ cells. Phytohemagglutinin (1µg/ml) served as positive control and cells incubated in medium alone served as negative controls. Following FcR blocking with CD16/CD32 (eBioscience) cells were stained with FITC-CD4 and PE-CD25. Cells were resuspended in 100µl of permeabilization buffer (eBioscience) containing 20µl of flurochrome labeled Abs and analyzed in BD LSR II analyzer (BD Biosciences).
ELISPOT
ELISPOT assays were performed as described previously to determine the frequency of CD4+ LILs secreting either IFN-γ, IL-4, IL-10 or IL-17 specifically to self-antigens, KAT and Col-V (12). Phytohemagglutinin (1µg/ml) was used as positive control and cells in medium alone were used as negative controls. Spots were analyzed in an ImmunoSpot Series I analyzer (Cellular Technology).
ELISA for detection of Abs to KAT and Col-V
Abs titers to KAT and Col-V in BAL samples were measured using ELISA (13). Concentration of Abs was expressed as µg/ml based on a standard curve using the binding of known concentration of anti-KAT or anti-Col V Abs (Santa Cruz Biotechnology).
Quantitative real time PCR array for gene expression
To determine the gene expression profile of 84 different cytokines, cytokine receptors, chemokines, matrix metalloproteinases, and signaling intermediates associated with induction of autoimmunity, total RNA extracted from LILs and lung tissues of anti-H2Kb or isotype controls were analyzed by RT2 Profiler PCR Array for mouse Th17 for autoimmunity and inflammation (SAbiosciences).
Neutralization Experiments
To determine the role of IL-17 cytokine family members in autoimmunity following anti-H2kb administration, we administered 50µg of pan-specific mAb to IL-17(R&D Systems, MN) or isotype goat IgG on days 1, 5, 12 and 26 to WT and IL-17A−/− mice treated with anti- H2Kb.
Acknowledgments
The authors would like to thank Billie Glasscock for her help in submitting this manuscript.
This work was supported by an award from the National Institutes of Health/National Heart Lung Blood Institute/National Institute Allergy Infectious Diseases HL092514 (TM), NIH Training Grant T32 HL07776 (DSN), and Barnes-Jewish Hospital Foundation (TM), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
ABBREVIATIONS
- Abs
antibodies
- APCs
antigen presenting cells
- BOS
Bronchiolitis Obliterans Syndrome
- BAL
bronchoalveolar lavage
- Col-V
Collagen-V
- KAT
K-α-1-Tubulin
- LIL
lung infiltrating lymphocytes
- LTx
Lung transplantation
- OAD
Obliterative Airway Disease
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship:
HB, SR, AP and TM participated in research design; _HB, SR and TM_ participated in the writing of the paper; HB, MT, VT, DN, NB participated in the performance of the research; _ HB, VT, DN, SR and TM participated in data analysis.
The authors have no financial conflicts of interest.
References
- 1.Lama VN, Murray S, Lonigro RJ, et al. Course of FEV(1) after onset of bronchiolitis obliterans syndrome in lung transplant recipients. Am J Respir Crit Care Med. 2007;175(11):1192. doi: 10.1164/rccm.200609-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo A, Smith MA, Phelan D, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplant Proc. 1999;31(1–2):185. doi: 10.1016/s0041-1345(98)01495-x. [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67(8):1155. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 5.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(6):624. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Seminars in thoracic and cardiovascular surgery. 2008;20(2):173. doi: 10.1053/j.semtcvs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Lu KC, Jaramillo A, Mendeloff EN, et al. Concomitant allorecognition of mismatched donor HLA class I- and class II-derived peptides in pediatric lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22(1):35. doi: 10.1016/s1053-2498(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 8.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1129. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 10.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. The Journal of clinical investigation. 2007;117(11):3498. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha 1 tubulin-specific Abs: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine & growth factor reviews. 2003;14(2):155. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Angkasekwinai P, Dong C, Tang H. Structure and function of interleukin-17 family cytokines. Protein & cell. 2011;2(1):26. doi: 10.1007/s13238-011-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hymowitz SG, Filvaroff EH, Yin JP, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. The EMBO journal. 2001;20(19):5332. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6(11):1133. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Annals of the New York Academy of Sciences. 2008;1143:188. doi: 10.1196/annals.1443.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. The Journal of experimental medicine. 2008;205(5):1063. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaramillo A, Fernandez FG, Kuo EY, Trulock EP, Patterson GA, Mohanakumar T. Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant. 2005;9(1):84. doi: 10.1111/j.1399-3046.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris PE, Bian H, Reed EF. Induction of high affinity fibroblast growth factor receptor expression and proliferation in human endothelial cells by anti-HLA antibodies: A possible mechanism for transplant atherosclerosis. J Immunol. 1997;159(11):5697. [PubMed] [Google Scholar]
- 22.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum. Immunol. 2003;64(5):521. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 23.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177(8):5631. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 24.Hachem RR. Lung allograft rejection: diagnosis and management. Curr Opin Organ Transplant. 2009;14(5):477. doi: 10.1097/MOT.0b013e32832fb981. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Ota N, Peng I, et al. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. Journal of immunology. 2010;184(8):4307. doi: 10.4049/jimmunol.0903614. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi Y, Fujio K, Shoda H, et al. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. Journal of immunology. 2007;179(10):7128. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. Journal of leukocyte biology. 2002;71(1):1. [PubMed] [Google Scholar]
- 28.Bharat A, Fields RC, Mohanakumar T. Regulatory T cell-mediated transplantation tolerance. Immunol Res. 2005;33(3):195. doi: 10.1385/IR:33:3:195. [DOI] [PubMed] [Google Scholar]
- 29.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6(8):1799. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 30.Wright JF, Bennett F, Li B, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. Journal of immunology. 2008;181(4):2799. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 31.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature immunology. 2008;9(2):166. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]