Abstract
Lay health advisers (LHAs) are increasingly used to deliver tobacco dependence treatment, especially with low-socioeconomic status (SES) populations. More information is needed about treatment adherence to help interpret mixed evidence of LHA intervention effectiveness. This study examined adherence to behavioral counseling and nicotine patches in an LHA intervention with 147 Ohio Appalachian female daily smokers. Participants were randomly selected from clinics and randomized to the intervention condition of a randomized controlled trial. Overall, 75.5% of participants received all seven planned LHA visits, 29.3% used patches for >7 weeks and approximately half received high average ratings on participant responsiveness. Depressive symptoms and low nicotine dependence were associated with lower patch adherence while high poverty-to-income ratio was associated with high responsiveness. Compared with those with fewer visits, participants who received all visits were more likely to be abstinent (22.5 versus 2.8%, P = 0.026) or have attempted quitting (85.0 versus 47.4%, P = 0.009) at 3 months. High participant responsiveness was associated with 12-month abstinence. LHA interventions should focus on improving adherence to nicotine patches and managing depression because it is an independent risk factor for low adherence.
Introduction
Tobacco use causes 435 000 premature deaths annually in the United States, representing 18.1% of all deaths [1]. Furthermore, there are persistent socioeconomic disparities in smoking rates. In 2009, almost one-third of adults living below poverty were current smokers compared with less than one-fifth of all other adults [2]. To impact these disparities within the upcoming decades, it is necessary to help low-socioeconomic status (SES) smokers quit smoking [3]. Although there is conflicting evidence about whether low-SES smokers are less likely than higher SES smokers to attempt quitting [4, 5], low-SES smokers are less likely to successfully quit smoking [4–6]. Evidence-based treatments such as behavioral counseling and nicotine replacement therapy (NRT) double a smoker’s chances of quitting successfully and are recommended for all smokers, including low-SES populations [7]. However, lack of knowledge about effectiveness and limited availability of cessation aids are common barriers to use in this population [8].
Lay health advisers (LHAs) are one promising strategy for increasing the use of evidence-based tobacco dependence treatments among low-SES smokers [9]. LHAs do not have formal or professional education but are trained on intervention-specific approaches and protocols [10]. They also have a personal connection to the community in which they work through their place of residence, disease status, race/ethnicity or other shared characteristics [11]. For these reasons, LHAs are assumed to be able to reach and connect with so-called hard-to-reach populations more effectively than traditional health care providers [12]. LHAs have been an integral part of tobacco dependence interventions with urban and rural African-American churches, women living in public housing, Spanish-speaking populations and rural Appalachian communities [13–18].
Unfortunately, evidence of the effectiveness of LHA interventions to treat tobacco dependence has been mixed. Community-based LHA interventions are clearly feasible and successful at recruiting and training LHAs as well as identifying participants who want to quit smoking from community-based settings [13–16, 19]. An early study found participants randomized to group cessation classes led by LHAs achieved cessation rates equivalent to those in classes led by doctoral students with counseling experience [9]. However, with two exceptions, cessation outcomes for community-based LHA interventions have not been significant [14, 16].
Because community-based LHA interventions reach hard-to-reach populations, it is important to identify factors that may explain their limited effectiveness. Two factors examined in this study include adherence and participant responsiveness to the intervention. Adherence refers to the content, coverage, frequency and duration of intervention components [20]. Adherence is important because interventions are not always delivered as intended [21] and participants often do not comply with prescribed activities or treatments even if they are delivered as planned [22, 23]. Even when intervention outcomes are positive, adherence must be documented to determine whether the prescribed treatment caused the observed effect [24] and which components of a multi-component intervention were most effective [10]. Participant responsiveness, defined as levels of enthusiasm for and engagement with an intervention, is another factor that may influence the extent to which an intervention leads to hypothesized outcomes. Responsiveness is frequently included in broader implementation fidelity frameworks and may influence outcomes directly or indirectly through adherence [20, 25].
Treatment adherence—but not participant responsiveness—has been evaluated for many tobacco dependence interventions delivered in clinical research or healthcare settings. For example, the proportion of study participants who used a specified percentage of NRT has ranged from about one-third to two-thirds in recent studies [22, 23, 26, 27]. Adherence to behavioral counseling sessions is typically higher at ∼70% [23, 28]. More intensive behavioral interventions have been associated with higher NRT use while higher nicotine dependence has predicted lower use [26, 27]. At least two studies found that higher education levels were associated with higher rates of counseling adherence [23, 28]. A limited number of studies assessing associations between depressive symptoms and adherence to behavioral counseling or NRT use have shown mixed results [28–30]. Higher NRT use and participation in counseling sessions have been consistently and positively associated with both short- and long-term cessation outcomes [22, 23, 26, 27]. However, these types of treatment adherence measures have not been reported for any LHA interventions to treat tobacco dependence. In addition, participant responsiveness has not been examined in any setting.
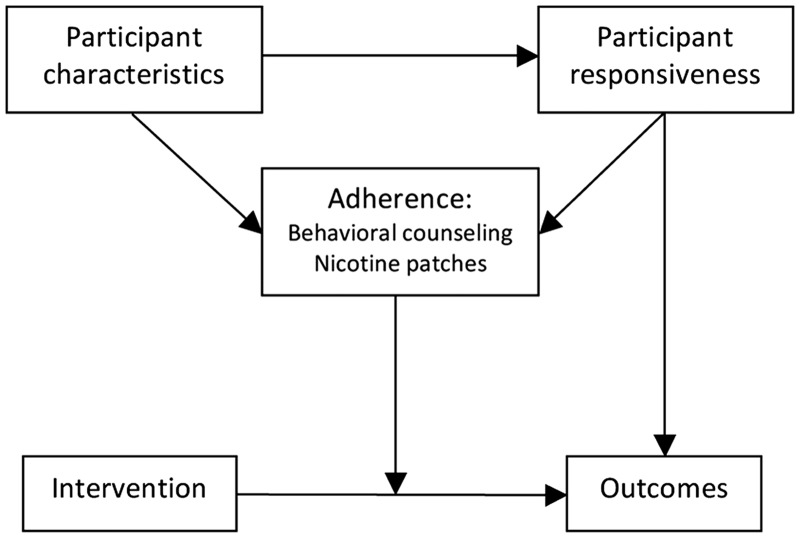
This study examined treatment adherence and participant responsiveness in a randomized, controlled trial of a tobacco dependence treatment intervention delivered by LHAs in a community setting. Figure 1 illustrates hypothesized relationships between participant characteristics, treatment adherence, participant responsiveness and the link between the intervention and desired outcomes. The study aimed to: (i) describe adherence to two primary intervention components (behavioral counseling and nicotine patches) and participant responsiveness to the intervention; (ii) identify participant characteristics associated with adherence and responsiveness; (iii) determine whether responsiveness was associated with adherence and (iv) determine whether adherence and responsiveness were associated with cessation-related outcomes.
Fig. 1.
Conceptual framework for tobacco dependence treatment adherence.
Methods
Research design
The LHA intervention trial used a randomized controlled longitudinal design. Data collection occurred at baseline (prior to randomization) and 3, 6 and 12 months after randomization. The study design and data collection procedures have been described in detail [31]. This study used baseline and 3-month follow-up data from participants in the intervention condition as well as additional data collected by LHAs at each counseling session. LHAs were required to record specific information about each visit (e.g. date, topics covered). LHAs also recorded participants’ responses to several questions (e.g. problems with patch use) and their own ratings about the visit (e.g. participant understanding). The study was reviewed and approved by the University’s Institutional Review Board.
Research participants
Participants were adult women living in Ohio Appalachian counties. This population was selected because the smoking rate was 30.4% among Ohio Appalachian adults in 2008 [32]. In addition, adults living in Appalachian Ohio have disproportionately low rates of education (20.6% less than high school), income (45.8% below 200% of federal poverty level) and health insurance (18.4%) [32], all of which may limit access to evidence-based cessation treatment. Participants were limited to women because this was part of a larger study addressing cervical cancer. Participants were randomly selected from women who received care within the past 2 years at 1 of 14 health clinics and were daily smokers. The study was conducted from 2005–2008. Selected participants at each clinic were invited to enroll with letters and telephone calls. After baseline data collection, participants were individually randomized to intervention or control conditions.
Tobacco dependence treatment intervention
The LHA intervention protocol has been described elsewhere [31]. Briefly, the intervention condition received a 10-week intervention delivered by an LHA, including seven individuals, face-to-face behavioral counseling sessions and 8 weeks of free 21 mg nicotine patches. Control participants received a letter from their personal clinic physician advising them to quit smoking, encouraging them to schedule an appointment with their provider and providing information about efficacious methods for quitting smoking such as counseling and medication. Intervention participants had higher quit rates than control participants at 3, 6 and 12 months, but the difference was not significant at 12 months [31]. This study only examined participants in the intervention condition (n = 147); detailed adherence measures were not collected in the control condition.
Measures
Adherence to behavioral counseling
Number of visits
LHAs recorded each visit completed with each participant. The total number of visits was calculated for each participant (0–7 visits). This was the primary adherence measure for behavioral counseling and was dichotomized into all seven versus fewer visits based on the distribution of visits among participants.
Length of intervention
Dates of the first and last visits were subtracted to calculate the total length of the intervention for each participant.
Intervention content
The LHA protocol included specific topics to discuss at each visit, most of which were accompanied by one- to two-page handouts for participants. LHAs recorded which topics were covered at each visit. The proportion of prescribed topics covered at each visit was calculated.
Adherence to nicotine patches
Number of patches used
At each visit, the LHA recorded the number of nicotine patches provided to the participant, the number returned unused by the participant and the participant’s self-reported number of patches used since the last visit. Patches provided and returned were summed for each participant across visits; the difference was the estimated number of patches used by each participant, the primary adherence measure for nicotine patches. Five participants had negative numbers from this calculation; therefore, the sum of self-reported patches used was substituted for these participants. Participants who did not receive any LHA visits were assigned a value of zero for patch use. Patch adherence was dichotomized into 7 weeks or less versus >7 weeks to distinguish between participants who received close to the recommended 8 weeks of patches from those who did not.
Reasons for discontinuing patches
If participants reported prematurely ending patch use during a visit, the LHA-recorded reasons using pre-determined categories; open-ended responses were also categorized.
Participant responsiveness
After each visit, the LHA rated the participant on three measures of responsiveness (rapport, understanding and interest in quitting) (1 = poor, 2 = fair, 3 = good and 4 = excellent). Ratings for each measure were averaged across visits and summed to create a composite measure of responsiveness (α = 0.93). Average ratings for each measure were categorized as follows: poor (1–1.5); fair (1.6–2.5); good (2.6–3.5) or excellent (3.6–4.0). The composite measure was dichotomized ( > 10.5 versus ≤ 10.5) for logistic regression models to distinguish between participants who received an average of excellent ratings across all responsiveness measures from those who did not. Participants who did not receive any LHA visits were not assigned any values for responsiveness variables.
Cessation-related outcomes
Point prevalence abstinence
7-Day abstinence with biochemical validation at 3 and 12 months post-enrolment were the primary cessation outcomes. Self-reported abstinence was validated with salivary cotinine; non-responders were classified as smokers based on intent-to-treat principles [31].
Quit attempt
Making at least one quit attempt during the intervention was a secondary outcome. At 3 months, participants were asked how many times they had gone without smoking for at least 24 hours in a serious attempt to quit since the beginning of the intervention. Participants who reported at least one quit attempt were categorized as having made a quit attempt.
Baseline participant characteristics
Demographics
Variables included age (18–30, 31–50 and 51 + years), education (lower than high school, high school diploma/General Educational Development and more than high school) and employment status (full- or part-time versus all others).
Health insurance status
Any type of private or public (e.g. Medicare or Medicaid) health insurance versus none.
Poverty-to-income ratio
Ratio of midpoint of observed family income category to official poverty threshold for a family of the same size for the same calendar year.
Nicotine dependence
Score ≥ 6 (high) on the Fagerstrom test of nicotine dependence versus < 6 (low) (α = 0.67) [33, 34].
Depressive symptoms
Score ≥ 16 on the Center for Epidemiologic Studies Depression Scale (yes) versus < 16 (no) (α = 0.92) [35].
Perceived stress scale
The perceived stress scale (PSS) is a continuous scale based on the sum of responses to 14 questions about the degree to which participants found their lives unpredictable, uncontrollable and overloaded in the past week (range 0–40; higher score reflects higher stress) (α = 0.88) [36]. This measure expands the limited psychosocial predictors of adherence examined in previous studies and was selected because participants with higher PSS levels may have found it difficult to prioritize attending behavioral counseling sessions.
Data analysis
Descriptive statistics were used to describe levels of adherence and responsiveness. Logistic regression was used to examine multiple associations between participant characteristics, adherence, participant responsiveness and/or cessation outcomes. Bivariate associations were examined first; independent variables with at least marginal significance (P < 0.20) were included in multivariate models. Other covariates were included as appropriate to control for confounding [37].
Results
Adherence to LHA visits
On average, participants received 5.7 (SD = 2.4) visits. Sixteen participants (10.9%) did not receive any visits while 75.5% received all seven visits. Among those receiving at least one visit, the intervention was delivered during an average of 8.4 weeks (SD = 2.0), ranging from 1.1 to 11.3 weeks. Among participants who received all visits, the average length was 9.1 weeks (SD = 0.6), ranging from 7.7 to 11.3 weeks.
The average proportion of prescribed topics covered during visits 1–5 ranged from 60.2 to 94.1% across visits. Adherence to prescribed content was highest for the first visit and lowest for the fifth visit. Topics covered during the last two visits were not prescribed; topics depended on each participant’s needs. The most common topics discussed during the last two visits were stress management (41.1%), help for slips (32.7%), substitute activities (19.4%), exercise (16.5%), reasons to quit (13.7%), muscle relaxation (13.7%) and support people (13.3%).
Adherence to nicotine patches
Approximately one-fourth (25.9%) of participants did not use any patches (including those who did not receive any visits) while 18.4% used patches for 8 or more weeks. Almost one-third (29.3%) used patches for > 7 weeks (primary adherence measure). The most common reasons for not using patches were the participant did not try to quit or relapsed (n = 19), felt she did not need the patch (n = 15) or was concerned about side effects (n = 15).
Participant responsiveness
Approximately half of participants received excellent average ratings from LHAs for rapport (55.0%), interest in quitting (47.3%) and understanding (55.7%). Few participants received poor or fair average ratings for rapport (n = 2), understanding (n = 4) or interest (n = 9).
Association between participant characteristics and adherence/responsiveness
No participant characteristics were significantly associated with adherence to behavioral counseling (data not shown). Depressive symptoms, nicotine dependence and age were associated with patch use in bivariate models (Table I). In the full model, not having depressive symptoms was associated with significantly higher odds of using the patch for > 7 weeks and high nicotine dependence was associated with almost three times higher odds of high patch use. Age was not retained in the full model.
Table I.
Participant characteristics associated with treatment adherence and participant responsiveness
| Adherence to nicotine patchesa |
Participant responsivenessb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % High | Unadjusted OR (95% CI) | Adjustedc OR (95% CI) | n | % High | Unadjusted OR (95% CI) | Adjustedc OR (95% CI) | |||
| Age | ||||||||||
| 18–30 | 65 | 20.0 | 1.00 | 56 | 55.4 | 1.00 | ||||
| 31–50 | 63 | 39.7 | 2.63* (1.19–5.80) | 58 | 50.0 | 0.81 (0.39–1.68) | ||||
| 51+ | 19 | 26.3 | 1.43 (0.44–4.69) | 17 | 58.8 | 1.15 (0.38–3.46) | ||||
| Education | ||||||||||
| Less than HS | 24 | 29.2 | 1.00 | 19 | 42.1 | 1.00 | ||||
| HS or GED | 48 | 29.2 | 1.00 (0.34–2.94) | 45 | 62.2 | 2.27 (0.76–6.75) | ||||
| More than HS | 75 | 29.3 | 1.01 (0.37–2.77) | 67 | 50.8 | 1.42 (0.51–3.96) | ||||
| Employment status | ||||||||||
| Not employed | 66 | 28.8 | 1.00 | 59 | 49.2 | 1.00 | ||||
| Full/part-time | 81 | 29.6 | 1.04 (0.51–2.13) | 72 | 56.9 | 1.37 (0.69–2.73) | ||||
| Health insurance | ||||||||||
| No | 34 | 29.4 | 1.00 | 27 | 74.1 | 1.00 | ||||
| Yes | 111 | 28.8 | 0.97 (0.42–2.26) | 102 | 49.0 | 0.34 (0.13–0.87) | ||||
| PIR | ||||||||||
| <1.00 | 54 | 24.1 | 1.00 | 48 | 39.6 | 1.00 | ||||
| 1.00–1.99 | 36 | 33.3 | 1.58 (0.62–4.01) | 33 | 63.6 | 2.67* (1.07–6.67) | 2.67* (1.07–6.67) | |||
| ≥2.00 | 43 | 32.6 | 1.52 (0.62–3.72) | 38 | 63.2 | 2.62* (1.09–6.29) | 2.62* (1.09–6.29) | |||
| Depressive symptoms | ||||||||||
| Yes | 77 | 20.8 | 1.00 | 71 | 49.3 | 1.00 | ||||
| No | 70 | 38.6 | 2.39* (1.15–4.97) | 2.48* (1.14–5.39) | 60 | 58.3 | 1.44 (0.72–2.88) | |||
| PSS | 147 | — | 0.99 (0.94–1.04) | 131 | — | 1.00 (0.95–1.05) | ||||
| Nicotine dependence | ||||||||||
| Low | 101 | 22.8 | 1.00 | 90 | 56.7 | 1.00 | ||||
| High | 40 | 42.5 | 2.51* (1.15–5.47) | 2.83* (1.26–6.36) | 35 | 48.6 | 0.72 (0.33–1.58) | |||
HS, high school and GED, General Educational Development. aThe outcome for the logistic regression model was using nicotine patches for >7 weeks. bThe outcome for the logistic regression model was having a score >10.5 on a 12-point composite scale of lay health advisors’ ratings of rapport, participant understanding and participant interest at each visit. cAdjusted for other variables that remained in the final model, if any, *P < 0.05.
Two participant characteristics were significantly associated with participant responsiveness in bivariate models: poverty-to-income ratio (PIR) and health insurance status (Table I). Only PIR remained significant in the full model. The odds of having high responsiveness were more than twice as high for participants with higher PIRs compared with those in the lowest PIR category.
Association between responsiveness and adherence
Participants with high responsiveness were more likely to complete all visits than those with low responsiveness (91.4 versus 77.1%, P = 0.028) and remained significant after controlling for PIR, which was related to responsiveness in Table I (AOR = 3.33, 95% confidence interval [CI]: 1.05, 10.62). There was no association between responsiveness and patch use.
Association between adherence/responsiveness and cessation outcomes
Completing all LHA visits was the only adherence measure significantly associated with 7-day point abstinence at 3 months (Table II). Completing all LHA visits and having high responsiveness were significantly associated with having made at least one quit attempt at 3 months in bivariate models (Table II). In the multivariate model, PIR was included as a covariate due to its previously described association with responsiveness. Although responsiveness and PIR were no longer statistically significant in this model, the coefficient for LHA visits changed by ∼16%, providing some evidence of confounding. Therefore, both non-significant variables were retained. In the final model, completing all LHA visits was associated with seven times higher odds of attempting to quit by 3 months compared with not completing all visits. High responsiveness was associated with 12-month abstinence even after controlling for PIR (21.4 versus 4.9%; Adjusted odds ratio (AOR) = 4.4; 95% CI: 1.2, 16.4). Associations between adherence to counseling or patch use and 12-month abstinence were not significant.
Table II.
Associations between adherence/responsiveness and 3-month cessation outcomes
| Variable | Cessation outcome |
||||||
|---|---|---|---|---|---|---|---|
| 7-Day point prevalence |
At least one quit attempt |
||||||
| n | % | Unadjusted OR (95% CI) | n | % | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | |
| Counseling adherence | |||||||
| Low | 36 | 2.8 | 1.00 | 19 | 47.4 | 1.00 | 1.00 |
| High | 111 | 22.5 | 10.17* (1.33–78.02) | 93 | 85.0 | 6.27* (2.16–18.19) | 7.01* (1.64–30.00) |
| Patch adherence | |||||||
| Low | 104 | 14.4 | 1.00 | 76 | 73.7 | 1.00 | |
| High | 43 | 25.6 | 2.04 (0.85–4.90) | 36 | 88.9 | 2.86 (0.90–9.10) | |
| Responsiveness | |||||||
| Low | 61 | 13.1 | 1.00 | 47 | 66.0 | 1.00 | 1.00 |
| High | 70 | 25.7 | 2.29 (0.92–5.73) | 58 | 91.4 | 5.47* (1.83–16.39) | 3.17 (0.95–10.51) |
aAdjusted for PIR. *P < 0.05.
Discussion
This is the first study to examine treatment adherence in a tobacco dependence treatment intervention delivered by LHAs. LHAs are increasingly being used to address health disparities in underserved populations, with mixed evidence of effectiveness. Little is known about which intervention components are essential in LHA interventions with successful outcomes. Alternatively, poor outcomes may be due in part to implementation failure as opposed to inadequate intervention design.
The investigation of adherence was important in part because significant short-term cessation outcomes were achieved from the intervention. Documenting adherence strengthens the argument that outcomes resulted from the intended intervention. Adherence to LHA visits was relatively high, with 75% of participants completing all seven visits. This is slightly higher than counseling adherence reported for tobacco dependence treatment interventions delivered by professionally trained providers, especially because the strictest possible definition of adherence was used in this study (i.e. all planned visits were completed) [23, 28]. This is notable because about half of study participants had less than or equal to high school diploma and previous studies have found less education to be associated with lower counseling adherence [23, 28]. Also consistent with previous studies, participants who completed all LHA visits were more likely to be abstinent or have made a quit attempt at 3 months.
Results were somewhat different for adherence to nicotine patches. Less than one-fifth of participants used nicotine patches for the recommended 8 weeks; less than one-third used patches for > 7 weeks. Although NRT adherence rates have varied substantially for non-LHA tobacco dependence treatment interventions, rates in this study were on the low end of the reported range. Rates of 50% or higher have been reported for interventions delivered in clinical settings [26] or those requiring intensive data collection activities such as patch use diaries [22]. Other studies have reported rates similar to this study [23, 27]. However, it is challenging to compare NRT adherence across studies because of inconsistent definitions. For example, the highest reported adherence rate (68.2%) was based on using patches for at least 20 of the first 21 days, [22] which is a dramatically different measure than using patches for 7–8 weeks. In another study, adherence was based on self-reported use at a 7-week follow-up visit; fewer than one-third of participants reported using ‘all of the patches’ and another half used ‘most or some’ patches [27]. Many NRT adherence studies have reported dose–response relationships between patch use and cessation outcomes [22, 27]. This association was not statistically significant in this study, but was in the hypothesized direction; 25.7% of high patch users were abstinent at 3 months compared with 14.4% of other participants. Future LHA interventions should explore additional strategies to help participants use nicotine patches for the recommended duration. Reasons for discontinuing NRT use were similar to those reported in population-based samples, including resuming smoking, side effects and perceptions that NRT did not help with quitting [38].
Study results add to the mixed existing evidence about depressive symptoms and adherence [28–30]. The association between depressive symptoms and patch use was important especially because depressive symptoms were previously associated with lower odds of 12-month abstinence in this study population [31]. Patch use may mediate the relationship between depressive symptoms and cessation because depressed individuals may smoke cigarettes partially to self-medicate with nicotine [39, 40] and patches do not produce the same level of symptom control [40]. Thus, these smokers may be more likely to stop patch use early and return to smoking to relieve depressive symptoms. Alternatively, smokers with depressive symptoms may resume smoking due to different smoking expectancies than other smokers [41] or environmental factors associated with both smoking and depressive symptoms [39]. Tobacco dependence treatment interventions may need to simultaneously address depressive symptoms, especially among low-SES women. This would not necessarily require extensive training for LHAs or more face-to-face counseling for participants. Instead, it might be accomplished by linking participants to existing evidence-based telephone [42] or Internet [43, 44] counseling for depression. Only one randomized trial has evaluated an intervention using LHAs to treat depression more directly; a collaborative stepped-care model implemented in India resulted in significant decreases in mental health conditions including depression after 12 months [45] and could potentially be adapted for use in tobacco dependence treatment programs.
An unexpected finding was the relationship between poverty and participant responsiveness. Specifically, participants in the highest poverty category received lower responsiveness ratings from LHAs. One concern was whether the propensity of individual LHAs to give higher or lower responsiveness ratings confounded this relationship. The intervention was implemented in four regions of Ohio Appalachia with a different LHA in each region. Average responsiveness ratings did vary significantly between LHAs in different regions (data not shown), but poverty level did not vary by region. Thus, regionally assigned LHAs did not explain the observed association. In addition, the relationship between poverty and participant responsiveness was in the same direction for each region (i.e. LHA).
In contrast to poverty, education was not related to responsiveness. Therefore, the association between poverty and responsiveness was likely not due to limited literacy or understanding of intervention materials. Because most visits were conducted in the home, the environment may have been more chaotic for lower income participants or the homes may have offered fewer opportunities to conduct the visit away from other family members or responsibilities. This may have prevented these participants from fully engaging in the intervention or may have negatively influenced LHA’s perceptions of responsiveness. Alternatively, there may be social class differences in responsiveness which led a middle-class LHA to misinterpret behaviors or responses of a person living in poverty as a lack of responsiveness.
This study has several limitations. Most measures were self-reported by participants or LHAs. Program implementation, including participant responsiveness, is typically measured by self-reports from providers or independent third-party observations [21, 25]. Although there is some evidence that third-party observations are more strongly associated with intervention outcomes due to less social desirability influence, such measures were not available for this study. However, some measures used in this study—especially adherence to counseling visits—did not depend on recall or subjective ratings.
The participant responsiveness measure did depend on subjective ratings by LHAs. Although reliability was not tested for the three items comprising this scale, the composite measure had content validity based on accepted definitions of participant responsiveness [25, 46]. In addition, LHAs completed ratings for each visit before knowing whether a participant would complete the next visit. And, responsiveness was not associated with patch use; therefore, LHAs’ knowledge of whether participants used patches prior to a given session did not appear to influence their ratings for the session. Finally, mean responsiveness ratings did not increase over time (i.e. visits) (data not shown), therefore it was appropriate to average ratings across visits as described.
The measure of patch adherence also had some limitations. Five participants had implausible (i.e. negative) values for the number of patches given minus those returned. These participants self-reported using small numbers of patches (i.e. 0, 1, 1, 4 and 18). The LHA likely made multiple attempts to help these participants initiate patch use or recover from slips, which may have complicated patch accounting procedures. Regardless of the reason for reporting errors, the exact numbers of patches used by these participants would not influence results because they were not close to the cut-point used in this study. Self-reported length of patch use was also collected at 3-month follow-up interviews, but was not analyzed here due to concerns about participant recall bias and missing follow-up data.
Finally, results may not generalize to all LHA-based interventions for treating tobacco dependence. The current intervention was intensive, requiring seven, individual, face-to-face behavioral counseling sessions for participants. In contrast, LHAs sometimes provide brief interventions more informally to individuals in their social networks [12, 47]. Individuals who agree to participate in an intensive intervention likely differ from participants in brief interventions. In addition, the definition and importance of treatment adherence for brief LHA interventions likely differ from this study.
Conclusion
In an LHA-led tobacco dependence treatment intervention for Ohio Appalachian women, adherence to behavioral counseling was relatively high and positively associated with cessation outcomes. Counseling adherence was consistent across participant demographic characteristics. Thus, LHAs appear to be a feasible strategy for addressing smoking disparities in a population with numerous barriers to healthcare access and socioeconomic opportunities. However, a majority of participants did not use nicotine patches provided by the study, at no cost, for the recommended 8 weeks. Future LHA-based interventions should focus on more nuanced strategies to help quit attempters comply with evidence-based medications. Participants with depressive symptoms may need additional support or referrals to achieve recommended doses. Examination of treatment adherence provides insight into effective components of LHA interventions and opportunities for improvements. Details about adherence and responsiveness may also help practitioners or researchers replicate the intervention in other settings.
Funding
National Cancer Institute (P50 CA105632); the Behavioral Measurement Shared Resource at The Ohio State University Comprehensive Cancer Center (P30 CA016058) from the National Cancer Institute, and the General Clinical Research Center at The Ohio State University from the National Center for Research Resources (M01 RR00034). Funding for open access charge: xxxx.
Conflict of interest statement
None declared.
Acknowledgements
The authors thank study participants, research staff and clinics in each county for their involvement and commitment to the study.
References
- 1.Mokdad A, Marks J, Stroup D, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged >or = 18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1135–40. [PubMed] [Google Scholar]
- 3.Levy D, Cummings K, Hyland A. A simulation of the effects of youth initiation policies on overall cigarette use. Am J Public Health. 2000;90:1311–14. doi: 10.2105/ajph.90.8.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94:269–78. doi: 10.2105/ajph.94.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid JL, Hammond D, Boudreau C, et al. Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12:S20–33. doi: 10.1093/ntr/ntq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal A, Sartor C, Pergadia ML, et al. Correlates of smoking cessation in a nationally representative sample of U.S. adults. Addict Behav. 2008;33:1223–6. doi: 10.1016/j.addbeh.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 8.McMenamin S, Halpin H, Bellows N. Knowledge of medicaid coverage and effectiveness of smoking treatments. Am J Prev Med. 2006;31:369–74. doi: 10.1016/j.amepre.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Lando HA. Lay facilitators as effective smoking cessation counselors. Addict Behav. 1987;12:69–72. doi: 10.1016/0306-4603(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 10.Lewin S, Dick J, Pond P, et al. Lay health workers in primary and community health care. Cochrane Db Syst Rev. 2009 doi: 10.1002/14651858.CD004015.pub2. Issue 3, Article Number: CD004015. pp. 1–206. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care. 2010;48:792–808. doi: 10.1097/MLR.0b013e3181e35b51. [DOI] [PubMed] [Google Scholar]
- 12.Eng E, Parker E, Harlan C. Lay health advisor intervention strategies: a continuum from natural helping to paraprofessional helping. Health Educ Behav. 1997;24:413–7. doi: 10.1177/109019819702400402. [DOI] [PubMed] [Google Scholar]
- 13.Lacey L, Tukes S, Manfredi C, Warnecke RB. Use of lay health educators for smoking cessation in a hard-to-reach urban community. J Community Health. 1991;16:269–82. doi: 10.1007/BF01320335. [DOI] [PubMed] [Google Scholar]
- 14.Voorhees CC, Stillman FA, Swank RT, et al. Heart, body, and soul: impact of church-based smoking cessation interventions on readiness to quit. Prev Med. 1996;25:277–85. doi: 10.1006/pmed.1996.0057. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Bristow Z, Sias JJ, Urquidi UJ, Feng C. Tobacco cessation services through community health workers for Spanish-speaking populations. Am J Public Health. 2006;96:211–3. doi: 10.2105/AJPH.2005.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schorling JB, Roach J, Siegel M, et al. A trial of church-based smoking cessation interventions for rural African Americans. Prev Med. 1997;26:92–101. doi: 10.1006/pmed.1996.9988. [DOI] [PubMed] [Google Scholar]
- 17.Becker DM, Yanek LR, Johnson WR, et al. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation. 2005;111:1298–304. doi: 10.1161/01.CIR.0000157734.97351.B2. [DOI] [PubMed] [Google Scholar]
- 18.Conway TL, Woodruff SI, Edwards CC, et al. Intervention to reduce environmental tobacco smoke exposure in Latino children: null effects on hair biomarkers and parent reports. Tob Control. 2004;13:90–2. doi: 10.1136/tc.2003.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaneda H, Nichter M, Nichter M, Muramoto M. Enabling and sustaining the activities of lay health influencers: lessons from a community-based tobacco cessation intervention study. Health Promot Pract. 2010;11:483–92. doi: 10.1177/1524839908318288. [DOI] [PubMed] [Google Scholar]
- 20.Carroll C, Patterson M, Wood S, et al. A conceptual framework for implementation fidelity. Implement Sci. 2007;2:40. doi: 10.1186/1748-5908-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol. 2008;41:327–50. doi: 10.1007/s10464-008-9165-0. [DOI] [PubMed] [Google Scholar]
- 22.Shiffman S, Sweeney CT, Ferguson SG, et al. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10 week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30:1852–8. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Okuyemi K, Zheng H, Guo H, Ahluwalia JS. Predictors of adherence to nicotine gum and counseling among African-American light smokers. J Gen Intern Med. 2010;25:969–76. doi: 10.1007/s11606-010-1386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71:S52–63. [PubMed] [Google Scholar]
- 25.Dane A, Schneider B. Program integrity in primary and early secondary prevention: are implementation effects out of control. Clin Psychol Rev. 1998;18:23–45. doi: 10.1016/s0272-7358(97)00043-3. [DOI] [PubMed] [Google Scholar]
- 26.Alterman A, Gariti P, Cook T, Cnaan A. Nicodermal patch adherence and its correlates. Drug Alcohol Depend. 1999;53:159–65. doi: 10.1016/s0376-8716(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 27.Cooper T, DeBon M, Stockton M, et al. Correlates of adherence with transdermal nicotine. Addict Behav. 2004;29:1565–78. doi: 10.1016/j.addbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Patterson F, Jepson C, Kaufmann V, et al. Predictors of attendance in a randomized clinical trial of nicotine replacement therapy with behavioral counseling. Drug Alcohol Depend. 2003;72:123–31. doi: 10.1016/s0376-8716(03)00194-7. [DOI] [PubMed] [Google Scholar]
- 29.Catley D, Ahluwalia J, Resnicow K, Nazir N. Depressive symptoms and smoking cessation among inner-city African Americans using the nicotine patch. Nicotine Tob Res. 2003;5:61–8. [PubMed] [Google Scholar]
- 30.Curtin L, Brown R, Sales S. Determinants of attrition from cessation treatment in smokers with a history of major depressive disorder. Psychol Addict Behav. 2000;14:134–42. doi: 10.1037//0893-164x.14.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wewers ME, Ferketich AK, Harness J, Paskett ED. Effectiveness of a nurse-managed, lay-led tobacco cessation intervention among Ohio Appalachian women. Cancer Epidemiol Biomarkers Prev. 2009;18:3451–8. doi: 10.1158/1055-9965.EPI-09-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.State of Ohio, Department of Insurance. Department of Job and Family Services, Department of Health, and Department of Mental Health. Ohio family health survey regional county demographic tables, 2008. Available at: http://grc.osu.edu/ofhs/datadownloads/index.cfm. Accessed: 16 July 2012. [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 34.Storr C, Reboussin B, Anthony J. The Fagerstrom test for nicotine dependence: a comparison of standard scoring and latent class analysis approaches. Drug Alcohol Depend. 2005;80:241–50. doi: 10.1016/j.drugalcdep.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 37.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edn. Hoboken, NJ: John Wiley and Sons, Inc.; 2000. [Google Scholar]
- 38.Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34:212–5. doi: 10.1016/j.amepre.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Kalman D, Morissette S, George T. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addiction. 2005;14:106–23. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benowitz N. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinberger A, George T, McKee S. Differences in smoking expectancies in smokers with and without a history of major depression. Addict Behav. 2011;36:434–7. doi: 10.1016/j.addbeh.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leach L, Christensen H. A systematic review of telephone-based interventions for mental disorders. J Telemed Telecare. 2006;12:122–9. doi: 10.1258/135763306776738558. [DOI] [PubMed] [Google Scholar]
- 43.Spek V, Cuijpers P, Nykicek I, et al. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med. 2007;37:319–28. doi: 10.1017/S0033291706008944. [DOI] [PubMed] [Google Scholar]
- 44.Farrer L, Christensen H, Griffiths KM, Mackinnon A. Internet-based CBT for depression with and without telephone tracking in a national helpline: randomised controlled trial. PLoS One. 2011;6:e28099. doi: 10.1371/journal.pone.0028099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel V, Weiss HA, Chowdhary N, et al. Lay health worker led intervention for depressive and anxiety disorders in India: impact on clinical and disability outcomes over 12 months. Br J Psychiatry. 2011;199:459–66. doi: 10.1192/bjp.bp.111.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkel C, Mauricio A, Schoenfelder E, Sandler IN. Putting the pieces together: an integrated model of program implementation. Prev Sci. 2011;12:23–33. doi: 10.1007/s11121-010-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell J, Mays MZ, Yuan NP, Muramoto ML. Who are health influencers? characterizing a sample of tobacco cessation interveners. Am J Health Behav. 2007;31:181–92. doi: 10.5555/ajhb.2007.31.2.181. [DOI] [PubMed] [Google Scholar]