Abstract
We investigated oral fluid (OF) as an alternative to sampling of rashes for varicella zoster virus (VZV) genotyping and further characterized VZV clade prevalence in the United Kingdom and Europe. VZV was detected in up to 91% of OF specimens. Paired OF and vesicle fluid samples contained identical VZV clades. While clades 1 and 3 were the most prevalent across the United Kingdom and Europe, in Western Europe, clade 5 viruses were circulating. Viruses from the same outbreak belonged to different clades, but no clade was associated with a severe-disease phenotype. OF is suitable and convenient for large-scale molecular epidemiological studies of VZV.
Keywords: Varicella-zoster virus, Clade, Oral fluid, Single nucleotide polymorphism, Vesicle fluid
Varicella zoster virus (VZV) causes varicella (chicken pox) and zoster (shingles). In temperate climates, varicella is a childhood disease that occurs during annual epidemics in winter and spring [1]. In tropical countries, however, infection is more common among adults, in whom disease is associated with increased morbidity and mortality [1].
VZV genotyping methods have identified 5 confirmed clades (clades 1–5) and 4 provisional clades (clades VI–IX) [2, 3]. Clades 1 and 3 predominate in Europe and in countries where people of European ancestry predominate, clade 2 is found in Japan and surrounding countries, and clades 4 and 5 are found in Asia and Africa. Clades VII–IX have been rarely reported, but small numbers of clade VI viruses have been isolated Western Europe, the United States, and Australia [2]. Recently, non-European clades of VZV have been spreading in European countries, particularly in regions to which there has been emigration from Asia and Africa [4]. Clade 2 viruses, including those that are pOka-like, have been identified in the United States [5]. Given the high vaccine coverage in the United States (>90%), one might expect that these clades and much of the disease due to VZV that now occurs in the United States are likely associated with imported cases of varicella.
Cocirculation of clades provides an ideal opportunity for coinfection, reinfection, and, ultimately, recombination, all of which have been described for VZV [6, 7]. Reinfection of people vaccinated with the Oka strain varicella vaccine occurs during breakthrough infection with wild-type virus. The possibility that recombination between vaccine and wild-type virus can occur necessitates the importance of surveillance programs that monitor circulating viral genotypes in communities before and after introduction of the vaccine. Because varicella is mainly a childhood infection and the majority of Oka strain vaccine recipients are children, such surveillance strategies need to be acceptable to parents. In this study, we examined the suitability of oral fluid (OF) for VZV genotyping and used genotyping data to examine the molecular epidemiology of VZV across the United Kingdom and Europe.
METHODS
Study Design
A total of 406 children presenting with varicella were recruited prospectively in the United Kingdom and Europe, and OF and/or vesicle fluid (VF) was collected. In the United Kingdom, 90 patients were recruited by 4 centers (2 in London and 1 each in Bristol and Glasgow). In Europe, 316 patients were recruited from 17 centers in 13 countries (Portugal, Greece, Spain, Czech Republic, Poland, Albania, Estonia, Italy, Germany, Sweden, Bulgaria, Austria, and France). Ethical approval and parental consent was obtained from all centers and for all patients, respectively. Data on ethnicity, duration of rash at sampling, severity of rash, secondary infections, rare complications, and hospitalization status were collected by questionnaire. For the United Kingdom study, postcode data, which specify the precise locations where each patient resides, was available for children recruited in East London.
Sample Processing and Genotyping
VF was collected in viral transport medium. OF was collected using Oracol swabs (Malvern Medical Developments), in accordance with the manufacturers’ instructions. DNA was extracted from all samples using a QIAamp DNA Mini Kit (Qiagen), according to the manufacturer's instructions. Genotyping was performed using pyrosequencing assays or direct sequencing to characterize single-nucleotide polymorphisms (SNPs) in open reading frames (ORFs) 1, 7, 21, 22, 50, 54, and 68 as previously described [6, 8].
Statistical Analysis
The Fisher exact test was used to compare the percentage of OF and VF samples positive for VZV, to compare differences in percentage positivity of each sample type over time, and to evaluate the relationship between each clade and ethnicity, hospitalization status, number of lesions, and occurrence of secondary infection.
RESULTS
Detection Rates in OF and VF Samples
Overall, 375 VF and 345 OF samples were received. VZV DNA was detected significantly more frequently in VF samples than in OF samples (97% vs 83%; P = .05). The frequency of VZV DNA detection in OF was significantly higher during days 1 and 2 after rash development (88.5% and 91% of samples, respectively), compared with days 3–8 (range, 89.2%–60%; P = .001). Compared with OF, detection in VF remained high on all days (range, 92.3%–96.9%; P = .05). Paired OF and VF samples were obtained from 313 of 407 patients (66 of 90 from the United Kingdom and 247 of 317 from Europe), and VZV was detected in 221 of 313 specimen pairs (70.6%). All VZV-positive paired OF and VF samples contained identical VZV clades. For all other patients (92 of 313), either VF, OF, or both specimens were VZV negative. Most of these children had specimens collected after day 5 of rash onset.
Geographical Distribution of Clades
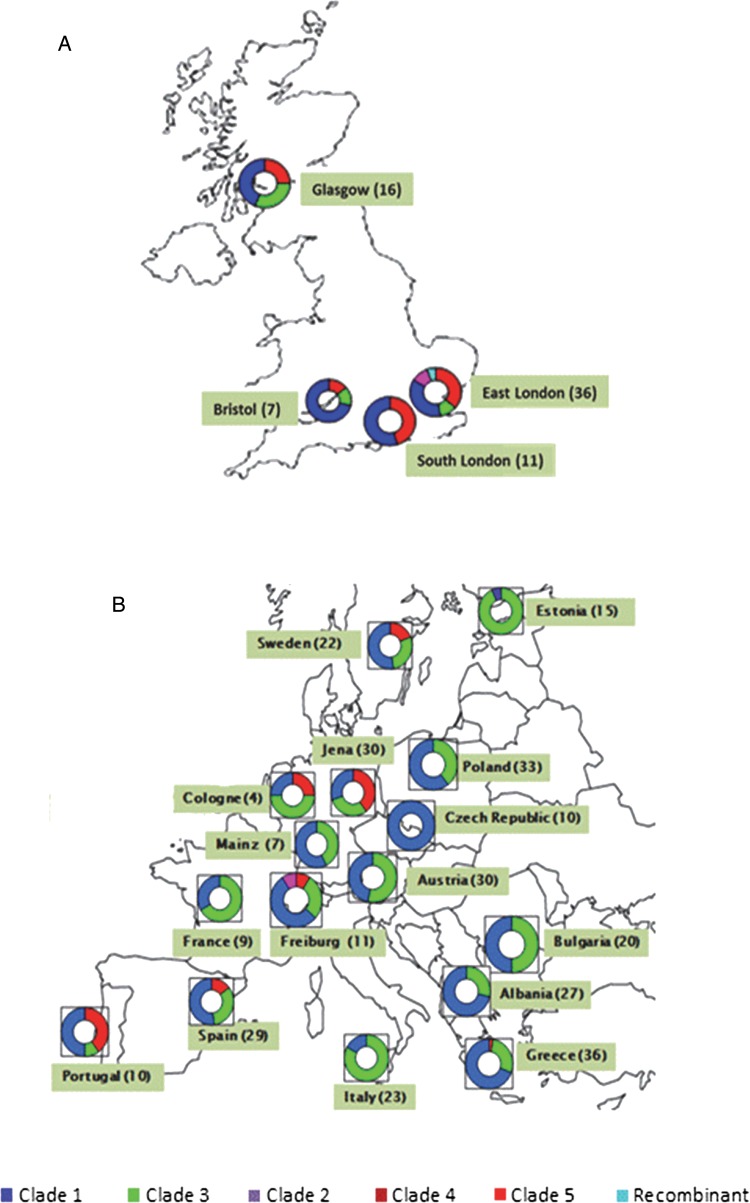
From all 4 UK centers, viruses belonged predominantly to clade 1, ranging from 30% of viruses (in East London) to 75% of viruses (in Bristol) (Figure 1A). Clade 5 was present in all centers, and clade 3 was present in all centers except South London (Figure 1A). In East London, clades 1, 3, 4, and 5 were circulating, with clades 4 and 5 together representing the majority (52.8% of viruses). Two potential clade 5/3 recombinant viruses were also identified. In eastern parts of the continent, clades 1 and 3 were predominant, with increased proportions of clade 5 in the western part of the continent (Figure 1B).
Figure 1.
Distribution of varicella zoster virus clades in the United Kingdom (A) and continental Europe (B). Numbers in parenthesis indicate the number of patients recruited from that particular city/country.
Characterization of Clades in Varicella Outbreaks
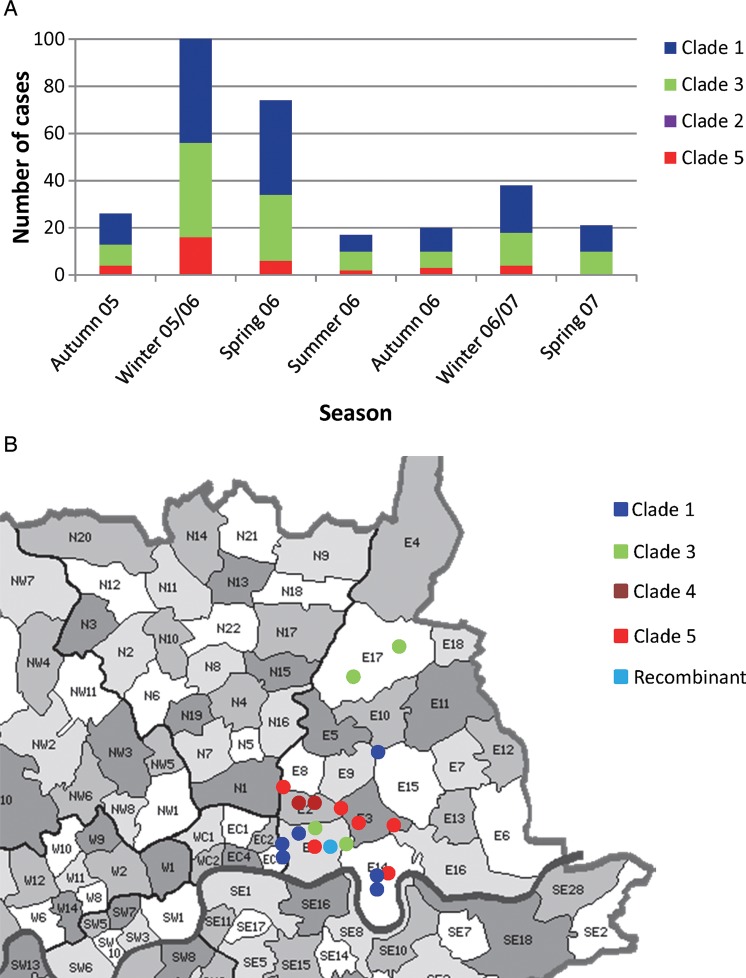
Multiple VZV clades were prevalent throughout each year of recruitment, with no seasonal differences in clade distribution (Figure 2A). Different clades were found cocirculating within and between outbreaks (Figure 2A). When we plotted viral genotype against postcode data for cases of varicella occurring within a 1-month period, we found colocalization of different clades despite having collected samples only days apart (Figure 2B).
Figure 2.
A, Seasonal distribution of varicella zoster virus (VZV) clades in Europe. B, Circulation of multiple VZV clades during varicella outbreaks in areas of East London. Alphanumeric codes denote different postcodes.
Clade Distribution in Relation to Different Ethnic Groups and Disease Severity
Nonwhite patients were significantly more likely to be infected with clade 5, compared with white patients, in both the United Kingdom and Europe (P < .01 and P < .03, respectively). No difference in clade distribution was seen between hospital inpatients and outpatients, those with >300 lesions, or those who developed secondary infections. Two geographically and temporally related clusters of rare complications were identified. Two patients with arthritis presented within 2 days to the same clinic, while 3 patients with VZV encephalitis were admitted to the same hospital over a 10-day period. Of the arthritis cases, one was caused by a clade 1 virus, and the second was caused by a clade 3 virus. Two encephalitis cases were caused by clade 3 viruses, and 1 was caused by a clade 1 virus.
DISCUSSION
To our knowledge, we are the first to demonstrate the suitability of OF for VZV genotyping. Sampling of OF is rapid and less invasive, making it more acceptable than obtaining samples from blood or vesicles. We found that the frequency of VZV detection in OF was highest during the first 2 days of illness, and despite a significant decrease in frequency, we were still able to obtain typable viral DNA up to day 8. In a previous study [9], the concordance of VZV detection in OF specimens compared with lesion samples was 100% but decreased to 30% by day 14. The latter observation is in line with our detection of VZV in 60% of OF specimens on day 5. The lower sensitivity of VZV detection in OF on days 1 and 2 in our study (88.5% and 91%, respectively) might reflect differences in sample size (19 specimens in the study by Leung et al [9] vs 247 in our study) or differences in OF collection methods.
We showed that the same clade was present in OF and VF, allowing us to use the data to analyze clade prevalence across the United Kingdom and Europe. Our study confirms that clades 1 and 3 are indigenous European strains, as postulated by others from opportunistically collected samples [2, 4]. Western Europe had a higher percentage of non-European clades as compared to Eastern Europe, where only clades 1 and 3 were found circulating. The lack of clade 5 viruses in these countries supports the observations of others [2] that viruses of this clade tend to be in countries where there is either little history of immigration from Africa and Asia, in which clade 4 and 5 viruses predominate, or where immigration is mainly from neighboring Eastern European countries. The finding in this study, as in others [7], that clade 5 viruses were significantly more prevalent among nonwhite children than white children with varicella may reflect a genetic susceptibility to infection with particular clades or, simply, may reflect population mixing patterns, with susceptible children more likely to be exposed in their community, household, or day care to others of the same ethnicity and their indigenous viruses.
By genotyping viruses using SNPs scattered across the genome, we were able to look for recombination. Two novel viruses could not be attributed to any clade. Both had the clade 5 SNP profile in all ORFs examined except ORF 54 at position 95 300, where they carried the allele characteristic of non–clade 5 viruses. While we provisionally class these viruses as recombinants, they might also represent clade 5 variants, which have been reported in other European countries [10]. Recombination has occurred in the evolutionary history of currently circulating VZV clades [11] and may contribute to the emergence of new freely circulating clades, something that will only become apparent as more genotyping is performed. A major concern, however, is that vaccine–wild-type recombinants may emerge. These could complicate molecular surveillance of VZV clades and identification of vaccine-associated adverse events.
Our survey provided 2 insights of particular clinical interest. First, multiple virus clades were recovered from 2 clusters of VZV-related complications, one of 2 cases of arthritis and a second of 3 cases of encephalitis. This makes it unlikely that transmission of a virulent variant occurred in the clusters and more likely that the severe clinical phenotypes observed occurred serendipitously or because of shared host genetic factors.
Our second insight relates to the finding that, within a single outbreak of varicella occurring in a circumscribed geographical region, many different clades were circulating (Figure 2B). Cocirculation of multiple VZV clades within a single outbreak and of different clades infecting individuals who live close to one another suggest that epidemics occurring in one locality do not result from successive transmission of a single virus within the susceptible population in a community but from multiple separate introductions of virus from different contacts. Since we observed cocirculation of multiple VZV clades in the low-varicella-incidence months at similar proportions to months with higher incidence (Figure 2A), it is likely, as predicted by modeling data from the 1950s [12], that in unvaccinated communities, multiple interepidemic cases of varicella seed the new epidemics when environmental conditions and herd immunity allow. However, our findings cannot exclude the possibility that, even in the presence of circulating varicella, virus from cases of zoster contributes to the diversity of strains in outbreaks. Varicella caused by transmission of VZV from zoster is well documented [13, 14], even within an established outbreak [14]. Although VZV transmission via saliva has not been described, the presence of viable virus in saliva during asymptomatic reactivation has been reported [15]. Because immunization with the Oka strain vaccine affords high individual protection, the opportunity for spread is reduced, and the risk to susceptible individuals is decreased. However, breakthrough infections may arise both from imported cases and from reactivated virus within naturally infected individuals in the community, so maintaining herd immunity is important.
In summary, OF has proven to be a suitable source of virus for large-scale monitoring of local and global changes in VZV clade prevalence. Ethnic predisposition to infection by certain clades was observed and probably reflects population mixing patterns. We report for the first time cocirculation of multiple VZV strains during temporarily and geographically localized chickenpox outbreaks. We also demonstrated that rare clusters of varicella-related complications were unlikely to be attributable to transmission of VZV strains with increased virulence.
Notes
Acknowledgments. We thank the following people and their coworkers, who provided samples and participated in coordination of this study: Paul Heath, St Georges Hospital, South London, United Kingdom; Ewa Majda-Stanilawska, Medical University Hospital, Lodz, Poland; Peter Fritsch, University Hospital of Graz, Graz, Austria; Georgine Kuli-Lito, University Hospital, Tirana, Albania; Adam Finn, Bristol Royal Children's Hospital, Bristol, United Kingdom; Lucina Titone, Ospedale dei Bambini, ARNAS, Palmermo, Palmero, Italy; Vera Pellantova, Faculty Hospital, Hradec Kralove, Czech Republic; Katrin Steul, Johannes Guteberg University Hospital, Mainz, Germany; Julie Keller, University Hospital of Koln, Cologne, Germany; and Magda Campins-Marti, Hospital Universitario Vall D'Hebron, Barcelona, Spain. We acknowledge the help of Mary Leedham Green and Fiona Scott with data processing.
Financial support. This work was supported by a European Society for Infectious Disease fellowship (to N. S.) and by the Wellcome Trust (grant 081703/Z/06/Z). M. Q. was funded by an investigator-led grant from Sanofi Pasteur MSD. J. B. receives funding from the University College London (UCL)–UCL Hospitals Comprehensive Biomedical Research Centre of the National Institute for Health Research. We thank the Medical Research Centre for Molecular Medical Virology for infrastructure support.
Potential conflicts of interest. J. B. receives an educational grant from Sanofi Pasteur MSD for the European Varicella Zoster Virus Identification Program. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lee BW. Review of varicella zoster seroepidemiology in India and South East Asia. Trop Med Int Health. 1998;3:886–90. doi: 10.1046/j.1365-3156.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Chanasit J, Sauerbrei A. Evolution and world-wide distribution of varicella-zoster virus clades. Infect Genet Evol. 2011;11:1–10. doi: 10.1016/j.meegid.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Zell R, Taudien S, Pfaff F, Wutzler P, Platzer M, Sauerbrei A. Sequencing of 21 varicella-zoster virus genomes reveals two novel genotypes and evidence of recombination. J Virol. 2012;86:1608–22. doi: 10.1128/JVI.06233-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett-Muir W, Nichols R, Breuer J. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J Virol. 2002;76:1971–9. doi: 10.1128/JVI.76.4.1971-1979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–23. doi: 10.1093/infdis/jiq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinlivan M, Sengupta N, Breuer J. A case of varicella caused by co-infection with two different genotypes of varicella-zoster virus. J Clin Virol. 2009;44:66–9. doi: 10.1016/j.jcv.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Quinlivan M, Hawrami K, Barrett-Muir W, et al. The molecular epidemiology of varicella-zoster virus: evidence for geographic segregation. J Infect Dis. 2002;186:888–94. doi: 10.1086/344228. [DOI] [PubMed] [Google Scholar]
- 8.Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349–58. doi: 10.1128/JVI.78.15.8349-8358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23–32. doi: 10.1086/653113. [DOI] [PubMed] [Google Scholar]
- 10.Sauerbrei A, Stefanski J, Philipps A, Krumbholz A, Zell R, Wutzler P. Monitoring prevalence of varicella-zoster virus clades in Germany. Med Microbiol Immunol. 2011;200:99–107. doi: 10.1007/s00430-010-0178-6. [DOI] [PubMed] [Google Scholar]
- 11.McGeoch DJ. Lineages of varicella-zoster virus. J Gen Virol. 2009;90:963–9. doi: 10.1099/vir.0.007658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seiler HE. A study of herpes zoster particularly in its relationship to chickenpox. The Journal of hygiene. 1949;47:253–62. doi: 10.1017/s002217240001456x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunell PA, Argaw T. Chickenpox attributable to a vaccine virus contracted from a vaccinee with zoster. Pediatrics. 2000;106:E28. doi: 10.1542/peds.106.2.e28. [DOI] [PubMed] [Google Scholar]
- 14.Nichols RA, Averbeck KT, Poulsen AG, et al. Household size is critical to varicella-zoster virus transmission in the tropics despite lower viral infectivity. Epidemics. 2011;3:12–8. doi: 10.1016/j.epidem.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–22. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]