Abstract
Background. Given the high infant measles mortality rate, there is interest in whether a measles immunization regimen beginning at <12 months of age provides lasting immunity.
Methods. Measles-specific immune responses were evaluated in 70 children aged 5–10 years after primary measles vaccine administered at 6, 9, or 12 months.
Results. At 5–10 years of age, the stimulation index for measles T-cell proliferation was 11.4 (SE, 1.3), 10.9 (SE, 1.5), and 14.4 (SE 2.1) when the first measles dose was given at 6, 9, or 12 months, respectively. Neutralizing antibody concentration (geometric mean titer [GMT]) in those immunized at 6 months of age was 125 mIU/mL (95% confidence interval [CI], 42–377) in the presence of passive antibodies (PAs) and 335 mIU/mL (95% CI, 211–531) in those without PAs; in those immunized at 9 months, GMTs were 186 mIU/mL (95% CI, 103–335) and 1080 mIU/mL (95% CI, 642–1827) in the presence and absence of PAs, respectively. The GMT was 707 mIU/mL (95% CI, 456–1095) when vaccine was administered at 12 months (P ≤ .04).
Conclusions. Measles-specific T-cell responses were sustained at 5–10 years of age regardless of age at time of primary measles immunization. Neutralizing antibody concentrations were lower in cohorts given the first vaccine dose at 6 months of age and in the presence of PAs; however, responses could be boosted by subsequent doses. Starting measles vaccination at <12 months of age may be beneficial during measles outbreaks or in endemic areas.
Keywords: Measles immunization, measles-specific B-cell immunity, measles-specific T-cell immunity
Measles remains the leading cause of vaccine-preventable childhood mortality globally despite substantial declines through immunization programs [1]. In 2008, 164 000 measles-related deaths occurred [2], with the highest fatality rates seen during the first year of life [3]. Although disease burden is greatest in developing nations, the changing epidemiology of measles in developed countries is a concern [2–12]. The United States was declared free from endemic measles transmission in 2000; nevertheless, cases continue to be imported [5, 6, 11, 12]. The year 2008 saw the highest measles incidence since 1998 (131 cases), and 21% of cases were in children younger than the recommended age for primary vaccination, with these children representing 26% of measles hospitalizations [4]. Of the 222 cases of measles in the United States in 2011, 90% of the cases were imported, and 14% of the cases occurred in infants aged <12 months [5]. Control of measles in the United States is complicated by a shift to an earlier loss of transplacentally acquired measles antibodies in infants [8–10]. In the United States, approximately 40% of infants aged 6 months lack passive antibodies (PAs) against measles [7, 8, 12] and remain vulnerable to infection until they are immunized.
Previously, high immunization coverage and persistent PAs in the United States afforded herd immunity to infants younger than the recommended age for vaccination [13], but measles caused by imported cases show that the current level of herd immunity in the United States is now insufficient for the protection of exposed infants. Recent outbreaks show a shift to increased measles incidence in children aged <12 months who lack protection by PAs [4, 5, 14]. Thus, giving the first measles vaccine dose to younger infants might benefit those aged 6–12 months in the United States, where measles has been reintroduced, and those aged <12 months in the developing world, where mortality is high in infants [15].
Measles immunization of infants aged as young as 3 months has shown partial success [16–18], with failures in younger infants attributed to interference from passively acquired antibodies and to limitations of the developing immune system [7, 17, 19]. An early primary measles immunization strategy was effective in a measles outbreak in the United States as well as in countries where measles is endemic [20, 21]. Yet, information about how well measles-specific immunity persists after early primary immunization is limited. To evaluate the longevity of both humoral and cellular immunity to measles after early immunization, we studied children aged 5–10 years, comparing those who had received their primary measles immunization at 6 or 9 months of age with those vaccinated at 12 months of age.
METHODS
Study Populations
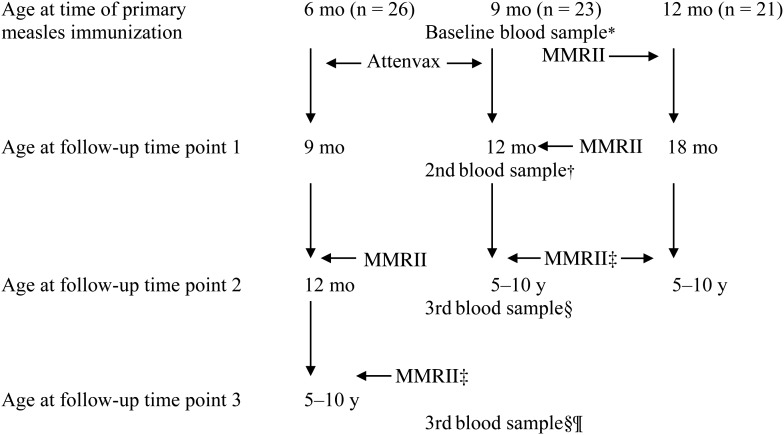
Subjects included 70 healthy children who had received their primary measles vaccine dose at 6 (n = 26), 9 (n = 23), or 12 (n = 21) months of age in an earlier study [7, 19]. Those immunized at 6 or 9 months of age received a second measles dose at 12 months of age [22], and all participants received a measles vaccine dose at 5 years of age (Figure 1). Children with chronic illness were excluded.
Figure 1.
Study design representing the timing of initial and subsequent measles vaccine doses and immune evaluations for each cohort. *Blood samples were collected on all infants, as previously reported [13], and used to determine passive antibody concentrations prior to primary measles immunization. †Blood samples were collected on all infants, as previously reported [13]. ‡Measles, mumps, and rubella virus vaccine live (MMRII) given at 5 years. §Blood samples were collected at random time points from children initially immunized at 9 (n = 23) or 12 (n = 21) months of age and who were now aged 5–10 years. ¶Blood samples were collected at random time points from children initially immunized at 6 months of age (n = 26) and who were now aged 5–10 years. Abbreviation: MMRII, measles, mumps, and rubella virus vaccine live.
A single blood sample was collected from each child when he/she was aged 5–10 years (Figure 1). Data were analyzed for the whole group and for subgroups of subjects who were aged 5–6 years (1–2 years from last measles immunization) and 7–10 years (3–5 years from last measles immunization) to differentiate peak responses to vaccine from steady state immunity, respectively.
Participants were recruited through the Palo Alto Medical Foundation, Palo Alto, California. Written consent was obtained. The Stanford University Committee for the Protection of Human Subjects and the Institutional Review Board of Palo Alto Medical Foundation approved the study. No cases of measles were identified in our area during the 10-year follow-up period.
Vaccines
Infants aged 6 and 9 months received measles virus vaccine live (Attenuvax) followed by measles, mumps, and rubella virus vaccine live at 12 months and 5 years. Infants aged 12 months received measles, mumps, and rubella virus vaccine live with a second dose at 5 years (all vaccines, Merck & Co).
Plaque Reduction Neutralization
Sera were stored at −80°C and tested for measles neutralizing antibody using a modified plaque reduction neutralization (PRN) assay [23]. In brief, serum samples were tested in parallel with the World Health Organization Measles Reference Serum II (66/202, obtained from the National Institute for Biological Standards and Control). In this assay, a titer of 1:8 corresponded to 8 mIU/mL of measles neutralizing antibody; <1:4 mIU/mL was considered negative. Measles antibody concentrations of ≥120 mIU/mL indicated measles seroprotection [24].
Immunoglobulin G Antibody Avidity
Measles virus–specific immunoglobulin G (IgG) antibody avidity was determined by enzyme immunoassay, as previously described [25]. In brief, sera samples were stored at −80°C. Flat-bottom, 96-well Maxisorp plates (Nalge Nunc Intl) coated with 1 μg/well of Edmonston measles virus–infected Vero cell lysate (Advanced Biotechnologies, Inc) were incubated with sera diluted 1:100. Avidity was determined by the dissociation of measles virus–specific IgG bond with the addition of the chaotropic agent ammonium thiocynate, NH4SCN (Sigma) and detected by alkaline phosphatase–conjugated rabbit antihuman IgG (H&L; Accurate Chemical & Scientific Corp). Substrate p-nitrophenyl phosphate (Sigma) was added to plates and read at absorbance 405 nm endpoint on microplate reader (Spectramax 190; Softmax Pro 4.0, Molecular Devices). The avidity index (AI) was determined as the concentration of NH4SCN that caused 50% reduction of measles virus–specific IgG binding. Optical density readings <0.3 in absence of NH4SCN and <30% dissociation were considered negative.
T-Cell Proliferation Assay
Peripheral blood mononuclear cells were separated from whole blood by Ficoll-Hypaque gradient and added to 96-well microtiter plates at 3.0 × 105 cells/well in Roswell Park Memorial Institute 1640 medium (Mediatech), as described previously [7, 19]. Measles antigen was prepared from lysates of Vero cells inoculated with Attenuvax measles vaccine (Merck & Co). Vero cell lysates made in parallel served as control antigen.
T-cell proliferation was measured by 3[H]-thymidine uptake after incubation of peripheral blood mononuclear cells with dilutions of 1:16 and 1:32 antigen and Vero cell control (optimal dilutions determined previously [19]) in duplicate wells for 5 days. The stimulation index (SI) is the ratio of mean counts per minute in antigen wells divided by the mean counts per minute in corresponding Vero cell control wells; the highest SI was used for statistical analysis. An SI ≥3.0 was considered positive [7, 19]. Phytohemagglutinin (0.1 mg/mL; Difco) served as the positive control and phosphate-buffered solution served as the negative control for each assay and were processed under the same conditions as the antigen and Vero cell control.
Statistics
Antibody titers are reported as geometric mean concentrations (GMCs) with 95% confidence intervals (CIs), and avidity is reported as AI. T-cell proliferation is reported as SI ± standard error (SE). Responses were compared by Student paired or unpaired t test and Fisher exact test. P < .05 was considered statistically significant.
RESULTS
Cellular Immunity
The mean SI (±SE) in 5–10 year old children was 11.4 (1.3) and 10.9 (1.5) in children given primary vaccination at 6 or 9 months of age, respectively (Table 1). These responses were comparable to the mean response of 14.4 (2.1) in children vaccinated at 12 months and 5 years of age.
Table 1.
Measles Immunity in Children Aged 5–10 Years After an Early Vaccine Regimen
| Age at Time of Primary Measles Immunizationa | No. of Measles Vaccine Doses | Interval Between Last Vaccine Dose and Blood Draw, Mean in Years (range) | GMC, mIU/mL (95% CI)b | Avidity Index, Meanc | Stimulation Index (±SE)d |
|---|---|---|---|---|---|
| 6 mo (n = 26) | 3 | 2.3 (1–5) | 199 (110–361) | 1.4 | 11.4 (1.3) |
| 9 mo (n = 23) | 3 | 2.8 (1–6) | 419 (254–690) | 1.7 | 10.9 (1.5) |
| 12 mo (n = 21) | 2 | 2.8 (1–6) | 823 (544–1244) | 2.2 | 14.4 (2.1) |
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; SE, standard error.
a First measles dose given as Attenuvax at 6 or 9 months of age or measles, mumps, and rubella virus vaccine live (MMRII) at 12 months of age; second dose given as MMRII at 12 months of age to infants given Attenuvax at 6 or 9 months of age or at 5 years of age to infants administered MMRII at 12 months of age; third dose given as MMRII at 5 years of age to children given Attenuvax at 6 or 9 months of age and MMRII at 12 months of age.
b Measured using plaque reduction neutralization test.
c Measured using enzyme immunoassay avidity assay.
d Measured using lymphoproliferation assay.
The presence of PAs at the time of primary measles immunization had no effect on the mean SI (Tables 2 and 3). No differences in T-cell responses were found in a subgroup analysis of children aged 5–6 years compared with children aged 7–10 years (boost responses vs steady state; Table 3).
Table 2.
Effects of Passive Antibodies and Age of Primary Measles Immunization on Persistence of Measles Immunity in Children Aged 5–10 Years
| Age at Time of Primary Measles Immunizationa | GMC, mIU/mL (95% CI)b | Avidity Index, Meanc | Stimulation Index (±SE)d |
|---|---|---|---|
| 6 mo with PAse | 125 (42–377) (n = 13) | 1.1 (n = 13) | 11.9 (2.6) (n = 10) |
| 6 mo without PAs | 335 (211–531) (n = 13) | 1.7 (n = 12) | 10.9 (1.4) (n = 13) |
| 9 mo with PAse | 186 (103–335) (n = 12) | 1.4 (n = 11) | 10.4 (2.5) (n = 11) |
| 9 mo without PAs | 1080 (642–1827) (n = 11) | 2.3 (n = 9) | 10.8 (1.7) (n = 7) |
| 12 mo without PAs | 707 (456–1095) (n = 21) | 2.2 (n = 20) | 14.0 (2.7) (n = 19) |
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; PA, passive antibody; SE, standard error.
a First measles dose given as Attenuvax at 6 or 9 months of age or measles, mumps, and rubella virus vaccine live (MMRII) at 12 months of age; second dose given as MMRII at 12 months of age to infants given Attenuvax at 6 or 9 months of age or at 5 years of age to infants administered MMRII at 12 months; third dose given as MMRII at 5 years of age to children given Attenuvax at 6 or 9 months of age and MMRII at 12 months of age.
b Measured using plaque reduction neutralization test.
c Measured using enzyme immunoassay avidity assay.
d Measured using lymphoproliferation assay.
e Passive antibodies to measles.
Table 3.
Effects of Passive Antibodies and Age of Primary Measles Immunization on Persistence of Measles Immunity in Children Aged 5–6 Years and 7–10 Years
| Age at Time of Primary Measles Immunizationa | GMC, mIU/mL (95% CI)b |
Avidity Index, Meanc |
Stimulation Index (±SE)d |
|||
|---|---|---|---|---|---|---|
| 5–6 y | 7–10 y | 5–6 y | 7–10 y | 5–6 y | 7–10 y | |
| 6 mo with PAse | 329 (263–412) (n = 4) | 81 (18–376) (n = 9) | 1.6 (n = 4) | 0.9 (n = 9) | 11.3 (8.0) (n = 2) | 12.0 (2.9) (n = 8) |
| 6 mo without PAs | 297 (149–588) (n = 5) | 361 (189–690) (n = 8) | 1.6 (n = 5) | 1.8 (n = 7) | 12.2 (2.0) (n = 5) | 10.1 (2.0) (n = 8) |
| 9 mo with PAse | NAf | 175 (93–331) (n = 11) | NAf | 1.3 (n = 11) | NAf | 10.8 (2.7) (n = 10) |
| 9 mo without PAs | 1922 (1007–3691) (n = 6) | 541 (456–644) (n = 5) | 2.6 (n = 6) | 1.8 (n = 3) | 11.9 (4.1) (n = 3) | 10.1 (1.0) (n = 4) |
| 12 mo without PAs | 1139 (580–2222) (n = 6) | 584 (342–998) (n = 15) | 2.4 (n = 4) | 2.1 (n = 13) | 12.6 (3.7) (n = 5) | 14.5 (3.0) (n = 13) |
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; NA, not applicable; PA, passive antibody; SE, standard error.
a First measles dose given as Attenuvax at 6 or 9 months of age or measles, mumps, and rubella virus vaccine live (MMRII) at 12 months of age; second dose given as MMRII at 12 months of age to infants given Attenuvax at 6 or 9 months of age or at 5 years of age to infants administered MMRII at 12 months; third dose given as MMRII at 5 years of age to children given Attenuvax at 6 or 9 months of age and MMRII at 12 months of age.
b Measured using plaque reduction neutralization test.
c Measured using enzyme immunoassay avidity assay.
d Measured using lymphoproliferation assay.
e Passive antibodies to measles.
f Not done because of too few infant samples.
Humoral Immunity
Both PA and younger age, but not sex, at the time of initial measles immunization were associated with lower measles antibody titers at 5–10 years of age after 3 measles vaccine doses (Tables 1–3). As can be seen from the tables, the GMCs in children who were initially immunized at 6 months of age, with or without PAs, or at 9 months of age in the presence of PAs were significantly lower than those immunized at 9 months of age without PAs or at 12 months of age (6 months of age with PAs and without PAs and 9 months of age with PAs vs 12 and 9 months of age without PAs; P ≤ .04).
GMCs in children aged 5–6 years, who were 1–2 years from last measles immunization, were higher for most groups than GMCs in children aged 7–10 years, who were 3–5 years from last measles immunization (Table 3), which suggests that responses were boosted after a measles dose administered at 5 years of age. However, steady-state antibody titers resulted in lower GMCs among children initially immunized in the presence of PAs when tested at 7–10 years of age when compared with subjects from the same vaccine cohort immunized in the absence of PAs (6 and 9 months of age with PAs vs 12 months of age without PAs, and 9 months of age with PAs vs without PAs; P ≤ .05).
When the quality of measles IgG antibody response was evaluated, the mean AI was lower in children aged 5–10 years initially immunized in the presence of PAs at 6 and 9 months of age and in the absence of PAs at 6 months of age (Tables 1–3; 6 months of age with PAs and without PAs and 9 months of age with PAs vs 12 and 9 months of age without PAs; P ≤ .01).
Both PAs and age affected the mean AI evaluated at 5–6 years of age for subjects given their primary measles immunization at 6 months of age (Tables 2 and 3; 6 months of age with PAs and without PAs vs 9 and 12 months of age without PAs; P < .01); comparisons could not be made for infants aged 9 months with PAs because there were too few subjects. This difference persisted through 7–10 years of age but only for those initially immunized at 6 months of age in the presence of PAs and not in the absence of PAs (with PAs vs without PAs for all comparisons, P < .01). In contrast, the mean AIs were comparable at 7–10 years of age for all children initially immunized at 6 or 9 months of age in the absence of PAs.
Protective Immunity
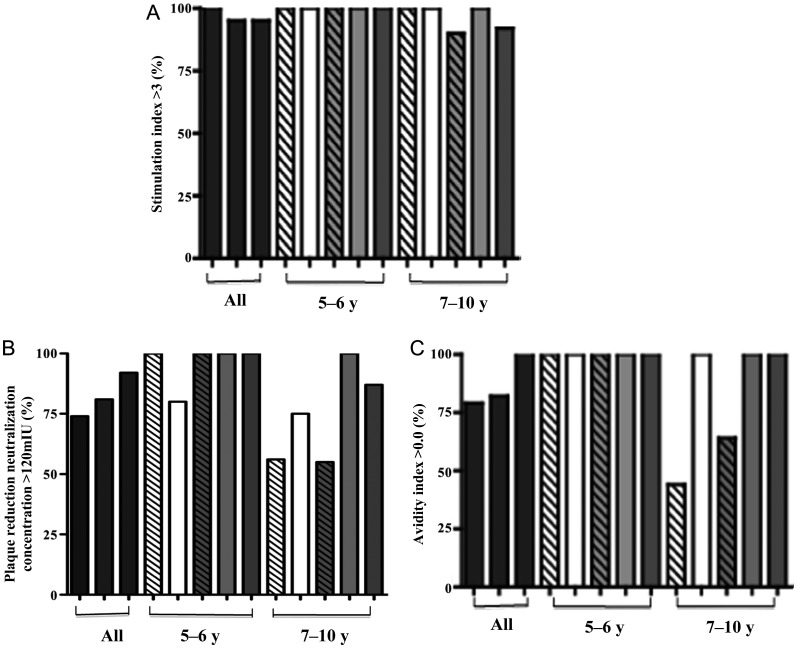
Only 1 of 70 children (1%) lacked both humoral and cellular immunity to measles at 5–10 years of age. Two children had a negative measles-specific T-cell immune response (SI < 3) (Figure 2A) [7, 19]. PAs and age at first immunization had no effect on measles T-cell responses at 5–10 years of age (Figure 2A; P > .5 for all comparisons). Of the 2 children with SI < 3, 1 was originally immunized at 12 months and had evidence of humoral immunity with a PRN titer ≥120 mIU/mL, whereas the other child was originally immunized at 9 months in the presence of PAs and had a PRN titer <120 mIU/mL. This was the only child in the study who lacked immunity to measles immunization at follow-up (Figure 2).
Figure 2.
Protective measles immunity in children aged 5–10 years after an early vaccine. Graphs show the percentage of infants with stimulation index ≥3 (A), the percentage of infants with plaque reduction neutralization concentration ≥120 mU/mL (B), and the percentage of detectable avidity index (C) after 3 measles doses in children aged 5–10 years who were initially immunized against measles at 6 months of age in the presence of passive antibodies (white striped bars) or the absence of passive antibodies (white bars) or at 9 months in the presence of passive antibodies (light gray striped bars) or the absence of passive antibodies (light gray bars), and after 2 measles doses in children immunized initially at 12 months of age in the absence of passive antibodies (dark gray bars). Immune responses are reported in all children aged 5–10 years (left bracket), and cohorts of children aged 5–6 years (middle bracket) or 7–10 years (right bracket).
Based on a PRN titer of ≥120 mIU/mL [24], the percentage of children aged 5–10 years with serologic evidence of protection against measles was equivalent in all cohorts. PRN titers were ≥120 mIU/mL in 74%, 81%, and 92% of children after primary measles immunization at 6, 9, and 12 months, respectively (Figure 2B). When the data were analyzed by subgroups to look at time from last measles immunization, fewer children had PRNs >120 mIU/mL if they had received their primary measles immunization in the presence of PAs and were aged 7–10 years (considered steady state), but not at 5–6 years when titers were boosted (Figure 2B; 6 and 9 months of age with PAs vs 12 months of age without PAs, P ≤ .04). The percentage of children with PRNs ≥120 mIU/mL was similar for children in all subgroups (those analyzed at 5-6 years of age and those analyzed at 7-10 years of age), who received their first vaccine dose in the absence of PAs.
All children aged 5–6 years had a positive avidity antibody response representing responses in close timing to last dose of measles immunization administered at 5 years of age. However, at 7–10 years of age, when responses that were 3–5 years from last measles dose were measured, 56% of children aged 7–10 years who were initially immunized in presence of PAs at 6 months of age and 36% of children aged 7–10 years who were initially immunized in presence of PAs at 9 months of age had a negative avidity antibody response, which was significant for the children receiving primary immunization at 6 months of age (6 months with PAs and without PAs for those aged 5–6 years vs those aged 7–10 years, P = .04; Figure 2C).
DISCUSSION
Despite substantial progress toward measles control, the latest documented global mortality rate in 2008 is 164 000 persons, with children aged <5 years disproportionately affected [2]. Effective control of measles necessitates a 2-dose immunization strategy [26] and, with recent epidemiologic changes, may require an early 2-dose vaccine schedule. Measles outbreaks in countries with high measles vaccine coverage have shown a shift in measles incidence to children aged <12 months [4, 5, 27, 28]. Further, the number of susceptible infants aged <12 months is expected to expand in highly vaccinated populations because the majority of women of childbearing age have vaccine-induced immunity to measles [8, 9, 29], with recent studies showing 99% of infants born to vaccinated mothers lack detectable antibodies to measles by 6 months of age [30]. Historically protection of infants aged 6–12 months was provided by a combination of PAs and herd immunity; however, this barrier has been disrupted by global importation of measles [4, 5, 11, 12] and, paradoxically, by the success of the measles vaccine program as vaccine-induced maternal immunity wanes earlier in infants as compared with infection-induced immunity [31].
In this study, we describe measles humoral and cellular responses in children aged 5–10 years who were given their primary measles immunization at 6 or 9 months of age. To our knowledge, this is the first report to offer these data. Longitudinal evaluations have previously described either humoral or T-cell immunity after an early dose of measles vaccine or immediately after an early 2-dose vaccine strategy [21, 32, 33].
Our study showed that T-cell responses to measles vaccine are robust and persisted up to 10 years of age, even when the first vaccine dose was given at 6 months of age. Importantly, these responses are not affected by PAs or an age-dependent maturation of the developing immune system. We have shown previously that the mean SI seen after the first dose of measles vaccine does not increase substantially after subsequent doses [19]. In the current study, T-cell responses did not wane, and the number of children with SI ≥3 was 97% when measured at 5–10 years of age. Thus, once established, T-cell immunity against measles is sustained and is not boosted upon antigen re-exposure [7, 19].
That measles T-cell responses were unaffected by the presence of PAs or age-related maturational deficits and that these responses persisted despite primary measles immunization before 12 months of age are critical to assessing the role that early measles immunization might have in global measles control. Markers for protection from measles have not been fully characterized. Humoral immunity is clearly important, and a high PRN antibody concentration is correlated with protection [24], but evidence suggests that cell-mediated immunity is essential for viral clearance, recovery from acute disease, and for persistence of long-term immunity [34–36]. In addition, T-cell responses are needed to initiate and amplify the humoral responses [35]. In contrast, the absence of detectable antibody concentrations does not appear to reflect susceptibility to infection if T-cell immunity is present, as has been shown in individuals who suffer from B-cell immune defects [37]. Protection from measles infection has been shown in the absence of detectable antibody titers in seronegative individuals, and measles viral clearance is not impacted in macaques who lack B-cell but have T-cell immunity after measles challenge [34, 38, 39]. In contrast, individuals that lack T-cell immunity have protracted measles disease and increased disease severity [36, 40].
Our observations demonstrate that measles-specific humoral immunity follows a different pattern than T-cell responses to measles. When infants received their first dose of vaccine at 6 months of age, humoral responses measured at 5–10 years of age were diminished compared with the responses of those vaccinated at 12 months of age. These differences were observed in the cohort that had PAs when first immunized and in those who did not, suggesting that age-dependent differences of the immune system at the time of primary measles immunization may be a factor in the latter cohort. By 9 months of age, maturational deficiencies were no longer apparent, but PA effects persisted. When GMCs were compared among the cohorts of children aged 5–6 years and children aged 7–10 years, concentrations were higher in those aged 5–6 years, which represents a boosted response after recent immunization. This pattern suggests that a boost in serum antibody titers occurs after measles immunization administered at 5 years of age but is not sustained. In the cohort tested at 7–10 years of age, which represented a steady state, the antibody levels were lower among children who had received their primary measles immunization at 6 months of age, regardless of presence of PAs, and at 9 months of age in the presence of PAs.
High avidity antibody responses followed a pattern similar to that seen with neutralizing antibodies. All children had a measurable AI when evaluated within 2 years of their measles vaccine administered at 5 years of age. Interestingly, these responses were lost by 2–5 years after the third measles vaccine dose in more children who were originally immunized in the presence of PAs when compared with those originally immunized in the absence of PAs.
Taken together, these results show the long-term pattern of measles immunity after an early primary immunization regimen. Measles T-cell responses are elicited by the initial vaccine dose regardless of PAs and persist but are not enhanced after subsequent doses. In contrast, despite the rise in antibody concentrations seen after measles vaccine doses given at 12 months [19] and 5 years of age, steady-state humoral responses were not sustained at the same level in children who had low neutralizing antibody concentrations after primary measles immunization associated with the presence of PAs or immunologic immaturity. Although the effects of PAs on long-term humoral immunity are well established, these results suggest a more global B-cell limitation.
The generation and maintenance of memory B cells is not fully understood, but it is clear that long-lived memory B cells appear to be dependent on the induction of plasmablasts at the time of initial antigen exposure [41]. Little is known about the factors necessary for establishing an adequate initial memory B-cell compartment, yet it appears to be of importance for subsequent B-cell responses upon antigen re-exposures. Thus, both the quantity and quality of the anamnestic antibody response are primarily driven by the quantity and quality of the initial memory B-cell compartment [42]. This study suggests that this initial B-cell response is diminished in some measles vaccine recipients immunized in the presence of PAs or at 6 months of age. Nevertheless, lower antibody concentrations after the initial vaccine dose were not indicative of a completely fixed state, as evidenced by boosting of both antibody concentrations and avidity after a measles immunization of these children at 12 months [19] and 5 years of age.
Information has shown that CD4+ T-cell help is crucial for the induction phase of B-cell memory but is not required to maintain long-lived plasma cells [43], and therefore the kinetics of memory T and B cells need not be concordant, as is consistent with the findings in this study and others [44]. Research evaluating PA interference to vaccines favors the hypothesis that passively acquired antibodies directly mask specific B-cell determinants, thus preventing antigen binding and recognition by infant B cells [45, 46]. T-cell immunity is spared because the binding of the passively acquired antibody to vaccine antigen results in antibody–antigen complexes that are processed by antigen presenting cells for T-cell presentation [47]. This allows for adequate T-cell but limited B-cell responses after the first antigen exposure and would allow for T-cell priming of B-cell responses to subsequent antigen exposures, as seen in this and other vaccine studies [48]. Further studies are needed to determine what age-dependent immunologic factors interfere with the production of high neutralizing antibody concentrations and high avidity antibodies in some children immunized at 6 months of age in the absence of PAs.
The clinical efficacy of early measles immunization has been shown in epidemics in the United States and in areas of endemic measles disease [16, 17, 49] but could not be tested in the small cohort of children in this study who were not exposed to measles. In addition to the small sample size in this study, an additional limitation of our study was the requirement that all children receive the routine measles vaccine schedule; thus our early primary measles immunization recipients received 3 measles doses and the control group received only 2 doses. Despite these challenges, our data suggest that it is unlikely that the children in this study who received an early primary measles dose would be susceptible to measles infection given the strong anamnestic humoral responses elicited by repeat measles immunizations and the persistent T-cell responses present in virtually all children in this study. In highly endemic countries that have utilized an early 2-dose measles strategy, vaccinated children were spared measles mortality and severe disease [49]. This must, however, be studied further, especially given a recent Canadian measles outbreak that showed increased cases in children immunized with their primary dose at 12 months of age compared with 15 months of age [50]. Importantly, this study indicates that humoral immunity is not inhibited permanently by early immunization, as has been suggested [32], and can be boosted to high concentrations [19]. Given the high measles mortality rate in infants aged <12 months and the growing population of infants susceptible to measles at younger ages in the developed world, early measles immunization may be the only effective strategy for protection against measles during this vulnerable period and should be studied further.
Notes
Acknowledgments. We thank the families, physicians, and nursing and laboratory personnel at the Palo Alto Medical Foundation for their participation in this study. We thank Drs Suzanne Epstein, Douglas Pratt, and Joao Pedras-Vasconcelos for their critical review of this work.
Financial support. This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI37127).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Global measles mortality, 2000–2008. MMWR Morb Mortal Wkly Rep. 2009;58:1321–6. [PubMed] [Google Scholar]
- 3.Zarocostas J. Mortality from measles fell by 91% in Africa from 2000 to 2006. BMJ. 2007;335:1173. doi: 10.1136/bmj.39419.393275.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: measles—United States, January–July 2008. MMWR Morb Mortal Wkly Rep. 2008;57:893–6. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Measles imported by returning US travelers aged 6–23 months, 2001–2011. MMWR Morb Mortal Wkly Rep. 2011;60:397–400. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Measles—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:253–7. [PubMed] [Google Scholar]
- 7.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280:527–32. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 8.Maldonado YA, Lawrence EC, DeHovitz R, Hartzell H, Albrecht P. Early loss of passive measles antibody in infants of mothers with vaccine-induced immunity. Pediatrics. 1995;96:447–50. [PubMed] [Google Scholar]
- 9.Markowitz LE, Albrecht P, Rhodes P, et al. Changing levels of measles antibody titers in women and children in the United States: impact on response to vaccination. Kaiser Permanente measles vaccine trial team. Pediatrics. 1996;97:53–8. [PubMed] [Google Scholar]
- 10.Papania M, Baughman AL, Lee S, et al. Increased susceptibility to measles in infants in the United States. Pediatrics. 1999;104:e59. doi: 10.1542/peds.104.5.e59. [DOI] [PubMed] [Google Scholar]
- 11.Papania MJ, Seward JF, Redd SB, Lievano F, Harpaz R, Wharton ME. Epidemiology of measles in the United States, 1997–2001. J Infect Dis. 2004;189(suppl 1):S61–8. doi: 10.1086/381557. [DOI] [PubMed] [Google Scholar]
- 12.Yip FY, Papania MJ, Redd SB. Measles outbreak epidemiology in the United States, 1993–2001. J Infect Dis. 2004;189(suppl 1):S54–60. doi: 10.1086/379377. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Recommended childhood immunization schedule—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:31–3. [PubMed] [Google Scholar]
- 14.Gindler J, Tinker S, Markowitz L, Atkinson W, Dales L, Papania MJ. Acute measles mortality in the United States, 1987–2002. J Infect Dis. 2004;189(suppl 1):S69–77. doi: 10.1086/378565. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Measles vaccine: WHO position paper. Wkly Epidemiol Rec. 2009;35:349–60. [PubMed] [Google Scholar]
- 16.Johnson CE, Nalin DR, Chui LW, Whitwell J, Marusyk RG, Kumar ML. Measles vaccine immunogenicity in 6- versus 15-month-old infants born to mothers in the measles vaccine era. Pediatrics. 1994;93:939–44. [PubMed] [Google Scholar]
- 17.Kumar ML, Johnson CE, Chui LW, Whitwell JK, Staehle B, Nalin D. Immune response to measles vaccine in 6-month-old infants of measles seronegative mothers. Vaccine. 1998;16:2047–51. doi: 10.1016/s0264-410x(98)00083-8. [published erratum appears in Vaccine 1999;17:2206] [DOI] [PubMed] [Google Scholar]
- 18.Markowitz LE, Sepulveda J, Diaz-Ortega JL, et al. Immunization of six-month-old infants with different doses of Edmonston-Zagreb and Schwarz measles vaccines. N Engl J Med. 1990;322:580–7. doi: 10.1056/NEJM199003013220903. [published erratum appears in N Engl J Med 1990;322:863] [DOI] [PubMed] [Google Scholar]
- 19.Gans HA, Yasukawa LL, Alderson A, et al. Humoral and cell-mediated immune responses to an early 2-dose measles vaccination regimen in the United States. J Infect Dis. 2004;190:83–90. doi: 10.1086/421032. [DOI] [PubMed] [Google Scholar]
- 20.Aaby P, Andersen M, Sodemann M, Jakobsen M, Gomes J, Fernandes M. Reduced childhood mortality after standard measles vaccination at 4–8 months compared with 9–11 months of age. BMJ. 1993;307:1308–11. doi: 10.1136/bmj.307.6915.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchins SS, Dezayas A, Le Blond K, et al. Evaluation of an early two-dose measles vaccination schedule. Am J Epidemiol. 2001;154:1064–71. doi: 10.1093/aje/154.11.1064. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Pediatrics. Red book: 2012 report of the Committee on Infectious Diseases. Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics. 2012 [Google Scholar]
- 23.Albrecht P, Herrmann K, Burns GR. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–60. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 25.Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J Infect Dis. 2007;196:1339–45. doi: 10.1086/522519. [DOI] [PubMed] [Google Scholar]
- 26.Gustafson TL, Lievens AW, Brunell PA, Moellenberg RG, Buttery CM, Sehulster LM. Measles outbreak in a fully immunized secondary-school population. N Engl J Med. 1987;316:771–4. doi: 10.1056/NEJM198703263161303. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson WL, Hadler SC, Redd SB, Orenstein WA. Measles surveillance—United States, 1991. MMWR CDC Surveill Summ. 1992;41:1–12. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Measles—United States, 1992. MMWR Morb Mortal Wkly Rep. 1993;42:378–81. [PubMed] [Google Scholar]
- 29.Lennon JL, Black FL. Maternally derived measles immunity in era of vaccine-protected mothers. J Pediatr. 1986;108:671–6. doi: 10.1016/s0022-3476(86)81039-3. [DOI] [PubMed] [Google Scholar]
- 30.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 31.Carson MM, Spady DW, Albrecht P, et al. Measles vaccination of infants in a well-vaccinated population. Pediatr Infect Dis J. 1995;14:17–22. doi: 10.1097/00006454-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Linnemann CC, Jr, Dine MS, Roselle GA, Askey PA. Measles immunity after revaccination: results in children vaccinated before 10 months of age. Pediatrics. 1982;69:332–5. [PubMed] [Google Scholar]
- 33.Pabst HF, Spady DW, Carson MM, Krezolek MP, Barreto L, Wittes RC. Cell-mediated and antibody immune responses to AIK-C and Connaught monovalent measles vaccine given to 6 month old infants. Vaccine. 1999;17:1910–8. doi: 10.1016/s0264-410x(98)00472-1. [DOI] [PubMed] [Google Scholar]
- 34.Permar SR, Klumpp SA, Mansfield KG, et al. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. J Infect Dis. 2004;190:998–1005. doi: 10.1086/422846. [DOI] [PubMed] [Google Scholar]
- 35.van Els CA, Nanan R. T cell responses in acute measles. Viral Immunol. 2002;15:435–50. doi: 10.1089/088282402760312322. [DOI] [PubMed] [Google Scholar]
- 36.Jaye A, Magnusen AF, Whittle HC. Human leukocyte antigen class I- and class II-restricted cytotoxic T lymphocyte responses to measles antigens in immune adults. J Infect Dis. 1998;177:1282–9. doi: 10.1086/515271. [DOI] [PubMed] [Google Scholar]
- 37.Good R, Zak S. Disturbacnes in gamma globulin synthesis as “experiments of nature.”. Pediatrics. 1956;18:109–49. [PubMed] [Google Scholar]
- 38.Ruckdeschel J, Graziano K, Mardiney MJ. Additional evidence that the cell-associated immune system is the primary host defense against measles (rubeola) Cell Immunol. 1975;17:11–8. doi: 10.1016/s0008-8749(75)80002-5. [DOI] [PubMed] [Google Scholar]
- 39.Permar SR, Klumpp SA, Mansfield KG, et al. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol. 2003;77:4396–400. doi: 10.1128/JVI.77.7.4396-4400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA. 1992;267:1237–41. [PubMed] [Google Scholar]
- 41.Odendahl M, Mei H, Hoyer BF, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–21. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 42.Durandy A, Revy P, Fischer A. Human models of inherited immunoglobulin class switch recombination and somatic hypermutation defects (hyper-IgM syndromes) Adv Immunol. 2004;82:295–330. doi: 10.1016/S0065-2776(04)82007-8. [DOI] [PubMed] [Google Scholar]
- 43.Vieira P, Rajewsky K. Persistence of memory B cells in mice deprived of T cell help. Int Immunol. 1990;2:487–94. doi: 10.1093/intimm/2.6.487. [DOI] [PubMed] [Google Scholar]
- 44.Njie-Jobe J, Nyamweya S, Miles DJ, et al. Immunological impact of an additional early measles vaccine in Gambian children: responses to a boost at 3 years. Vaccine. 2012;30:2543–50. doi: 10.1016/j.vaccine.2012.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jelonek MT, Maskrey JL, Steimer KS, Potts BJ, Higgins KW, Keller MA. Maternal monoclonal antibody to the V3 loop alters specificity of the response to a human immunodeficiency virus vaccine. J Infect Dis. 1996;174:866–9. doi: 10.1093/infdis/174.4.866. [DOI] [PubMed] [Google Scholar]
- 46.Panpitpat C, Thisyakorn U, Chotpitayasunondh T, et al. Elevated levels of maternal anti-tetanus toxin antibodies do not suppress the immune response to a Haemophilus influenzae type B polyribosylphosphate-tetanus toxoid conjugate vaccine. Bull World Health Organ. 2000;78:364–71. [PMC free article] [PubMed] [Google Scholar]
- 47.Siegrist C, Barrios C, Martinex X, et al. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol. 1998;28:4138–48. doi: 10.1002/(SICI)1521-4141(199812)28:12<4138::AID-IMMU4138>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 48.Weeratna RD, Brazolot Millan CL, McCluskie MJ, Siegrist CA, Davis HL. Priming of immune responses to hepatitis B surface antigen in young mice immunized in the presence of maternally derived antibodies. FEMS Immunol Med Microbiol. 2001;30:241–7. doi: 10.1111/j.1574-695X.2001.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 49.Aaby P, Martins CL, Garly ML, Rodrigues A, Benn CS, Whittle H. The optimal age of measles immunisation in low-income countries: a secondary analysis of the assumptions underlying the current policy. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000761. pii:e000761. doi:10.1136/bmjopen-2011-000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Serres G, Boulianne N, Defay F, et al. Higher risk of measles when the first dose of a 2-dose schedule of measles vaccine is given at 12–14 months versus 15 months of age. Clin Infect Dis. 2012;55:394–402. doi: 10.1093/cid/cis439. [DOI] [PubMed] [Google Scholar]