Abstract
Background. The effect of nonthymidine nucleoside reverse-transcriptase inhibitors (NRTIs) on fat mitochondrial DNA (mtDNA) content and function is unclear.
Methods. A5202 randomized antiretroviral therapy–naive human immunodeficiency virus–infected subjects to abacavir-lamivudine (ABC/3TC) versus tenofovir DF–emtricitabine (TDF/FTC) with efavirenz (EFV) or atazanavir-ritonavir (ATV/r). A5224s, substudy of A5202, enrolled 269 subjects with fat measurements by dual-energy x-ray absorptiometry and computed tomography. A subset of subjects underwent fat biopsies at baseline and week 96 for mtDNA content (real-time polymerase chain reaction) and oxidative phosphorylation nicotinamide adenine dinucleotide (reduced) dehydrogenase (complex I) and cytochrome c oxidase (complex IV) activity levels (immunoassays). Intent-to-treat analyses were performed using analysis of variance and paired t tests.
Results. Fifty-six subjects (87% male; median age, 39 years) were included; their median body mass index, CD4 cell count, and fat mtDNA level were 26 kg/m2, 227 cells/μL, and 1197 copies/cell, respectively. Fat mtDNA content decreased within the ABC/3TC and TDF/FTC groups (combining EFV and ATV/r arms; median change, −341 [interquartile range, −848 to 190; P = .03] and −400 [−661 to −221; P < .001] copies/cell, respectively), but these changes did not differ significantly between the 2 groups (P = .57). Complex I and IV activity decreased significantly in the TDF/FTC group (median change, −12.45 [interquartile range, −24.70 to 2.90; P = .003] and −8.25 [−13.90 to −1.30; P < .001], optical density × 103/µg, respectively) but not the ABC/3TC group. Differences between the ABC/3TC and TDF/FTC groups were significant for complex I (P = .03).
Conclusions. ABC/3TC and TDF/FTC significantly and similarly decreased fat mtDNA content, but only TDF/FTC decreased complex I and complex IV activity levels.
Clinical Trials Registration. NCT00118898.
Keywords: lipoatrophy, lipodystrophy, metabolic disease, mitochondrial dysfunction, mitochondrial toxicity, oxidative phosphorylation
Patients with human immunodeficiency virus type 1 (HIV-1) infection receiving thymidine nucleoside reverse-transcriptase inhibitors (NRTIs) experience a high rate of metabolic abnormalities, including lipoatrophy. Depletion of adipose tissue mitochondrial DNA (mtDNA) and impairment of the oxidative phosphorylation system are associated with lipoatrophy induced by thymidine NRTI–containing regimens [1]. Mitochondrial oxidative phosphorylation enzymes nicotinamide adenine dinucleotide (reduced; NADH) dehydrogenase (complex I) and cytochrome c oxidase (complex IV) contain polypeptides of mtDNA-encoded subunits and so are affected by mtDNA depletion.
In the current era of nonthymidine NRTI–containing regimens, lipoatrophy incidence has significantly decreased but has not been completely prevented [2]. In addition, subjects who have established lipoatrophy while taking thymidine NRTI–containing regimens experience only a slow and incomplete resolution of lipoatrophy after switching to nonthymidine NRTI–based therapy [3–5], putting into question the mitochondrial toxicity–free characteristics of nonthymidine NRTIs. Prospective randomized studies have also yielded conflicting findings about the independent effects on mitochondria of other antiretroviral therapy (ART) classes, such as nonnucleoside analogue reverse-transcriptase inhibitor (NNRTI) or protease inhibitor (PI) therapy. Because of the remaining uncertainty about the effect of nonthymidine NRTI–containing regimens on mitochondrial toxicity and the potential effect of the concomitant PI or NNRTI therapy, we sought to compare changes in mitochondrial indices in the context of a randomized trial.
METHODS
AIDS Clinical Trials Group (ACTG) A5224s was a metabolic substudy of A5202 in which ART-naive subjects ≥16 years old with HIV-1 RNA levels >1000 copies/mL were randomized in a double-blinded fashion to coformulated tenofovir disoproxil fumarate–emtricitabine (TDF/FTC) or abacavir-lamivudine (ABC/3TC), along with open-labeled efavirenz (EFV) or atazanavir-ritonavir (ATV/r) [2, 6]. Randomization was stratified by screening HIV-1 RNA level (<100 000 vs ≥100 000 copies/mL). A preplanned mitochondrial substudy of A5224s included all subjects at 13 participating sites who agreed to undergo fat biopsies at entry into A5224s and again at week 96. This mitochondrial substudy was designed with the primary objective of comparing the effects of 96 weeks of ABC/3TC versus TDF/FTC on mtDNA levels; secondary objectives were to compare changes in mitochondrial function between ABC/3TC and TDF/FTC and changes in mtDNA levels and mitochondrial function between EFV and ATV/r.
The A5224s exclusion criteria were untreated hypogonadism or thyroid disease, Cushing syndrome, diabetes mellitus, and the use of growth hormone, anabolic steroids, or glucocorticoids. Any subject entering A5202 and its substudy A5224s who met the fat mitochondrial substudy criteria was eligible to enroll (A5202 randomization was stratified by intention to participate in A5224s and in the mitochondrial substudy). The duration of the study was 96 weeks. Each subject signed a written informed consent, which was approved by each participating site's local institutional review board.
A5224s evaluations included whole body dual-energy x-ray absorptiometry to measure limb and central fat and a single-section noncontrast computed tomographic scan of the abdomen at the L4–L5 level to measure central subcutaneous and visceral fat at baseline and week 96. Technicians were instructed to use the same machine on the same subject throughout the study and all scans were standardized at the participating sites and centrally read (at Tufts University) by personnel blinded to treatment assignment. Mitochondrial substudy evaluations included an excisional fat biopsy from the lower abdomen. These biopsies were performed with local anesthesia by an experienced physician.
MtDNA Quantitation
MtDNA content in adipose tissue was measured by quantitative real-time polymerase chain reaction, as described elsewhere [7]. Briefly, DNA was extracted from fat frozen in RNAlater using a Qiagen DNA kit (Qiagen). Standardization of real-time polymerase chain reaction was performed using LightCycler FastStart DNA Master SYBR Green I with the Roche LightCycler instrument (Roche). A dilution series of the control plasmid containing the 90–base pair mtDNA NADH dehydrogenase, subunit 2 and the 98–base pair Fas ligand gene was prepared to set up the standard. Each sample and standard was assayed in duplicate, and the results were analyzed with version 4.0 LightCycler software.
Oxidative Phosphorylation Protein and Enzyme Activity Immunoassays
Enzyme levels of oxidative phosphorylation NADH dehydrogenase (complex I) and cytochrome c oxidase (complex IV) were determined in the Molecular Medicine and Infectious Diseases Laboratory in the Department of Cell and Molecular Biology, as described elsewhere [8]. An approximately 64-mm2 piece of flash-frozen fat was homogenized with a Qiagen TissueRuptor (Qiagen) for 30 seconds in 0.5 mL of ice-cold extraction buffer (1.5% lauryl maltoside, 25 mmol/L HEPES [pH 7.4], 100 mmol/L sodium chloride, and a PI cocktail [PI-78410; Thermo Fisher Scientific]). Samples were mixed gently and kept on ice for 20 minutes, and then spun in a microcentrifuge at 18 000 relative centrifugal force and at 4°C for 20 minutes to remove insoluble cell debris. The supernatant, an extract of detergent-solubilized cellular proteins (10 μg), was then assayed with the oxidative phosphorylation immunoassays (MS130 and MS430; MitoSciences). The sample protein concentration was determined with BCA assay (Thermo Fisher Scientific). Equal amounts of total cell protein were assayed using an amount previously established with control samples to generate signals within the linear range of the assay. Therefore, the resulting signal was directly proportional to the amount of oxidative phosphorylation enzyme activity in the sample. The signal was quantified by densitometric scanning with a Hamamatsu ICA-1000 reader (Hamamatsu). Laboratory personnel were blinded to patients' characteristics.
Statistical Analysis
The primary objective was to compare, between pooled, randomized NRTI components (ABC/3TC vs TDF/FTC with third drugs combined), changes from baseline to week 96 in fat mtDNA levels. Other objectives were considered secondary. All analyses were performed using intent-to-treat principles, which are based on randomized treatment assignment and used all available data with modifications to randomized treatment and missing values ignored. Comparisons used a factorial analysis approach in which, after assessment for treatment effect modification by the other component, the NRTI effect was assessed by combining EFV and ATV/r arms, and vice versa by combining NRTIs for the PI/NNRTI comparison. Spearman rank correlation coefficients were used to describe the relationship between 2 continuous variables. P values <.05 were considered statistically significant, and nominal values are reported without adjustment for multiple comparisons. Analyses were performed using SAS software, version 9.2 (SAS Institute).
The comparison of ABC/3TC and TDF/FTC with EFV and ATV/r combined (factorial analysis) was performed at each time point, because there was no significant evidence that the NRTI effect differed at 96 weeks according to the NNRTI or PI component (all P ≥ .20). Similarly, EFV and ATV/r were compared with ABC/3TC and TDF/FTC combined, because there was no significant evidence that the third drug effect differed at 96 weeks according to the NRTI components (all P ≥ .17). The sample size represents all available subjects from ACTG sites participating in A5224s biopsy substudy who agreed to undergo the fat biopsy procedures.
RESULTS
Subject Characteristics
As detailed elsewhere [2, 6], 269 subjects were randomized to 1 of the 4 regimens and included in the A5224s analysis (see Appendix for A5224s sites). Of these 269 subjects, 56 (21%) were enrolled in the mitochondrial substudy from 13 ACTG sites in the United States and Puerto Rico. Among these 56, 13 were randomized to EFV plus TDF/FTC, 15 to EFV plus ABC/3TC, 15 to ATV/r plus TDF/FTC, and 13 to ATV/r plus ABC/3TC. Baseline characteristics are summarized in Table 1. Overall, 87% of the subjects were male, and 39% were white non-Hispanics. At baseline, the median age was 39 years; the median CD4 cell count, 227 cells/µL; and the median HIV-1 RNA level, 4.73 log10 copies/mL. Baseline characteristics were balanced across arms and were similar between the 56 subjects included in this mitochondrial substudy and the A5224s subjects not included (data not shown).
Table 1.
Baseline Characteristics of Study Subjects by Randomized Arms
| Characteristic | EFV + TDF/FTC (n = 13) | EFV + ABC/3TC (n = 15) | ATV/r + TDF/FTC (n = 15) | ATV/r + ABC/3TC (n = 13) | P |
|---|---|---|---|---|---|
| Male sex, No. (%) | 12 (92) | 13 (87) | 12 (80) | 12 (92) | .82 |
| Age, y | 39 (36–44) | 42 (31–47) | 38 (32–43) | 39 (36–47) | .86 |
| Race/ethnicity, No. (%) | |||||
| White Non-Hispanic | 7 (54) | 4 (27) | 5 (33) | 6 (46) | .43 |
| Black Non-Hispanic | 2 (15) | 3 (20) | 2 (13) | 5 (38) | |
| Hispanic (regardless of race) | 4 (31) | 8 (53) | 5 (33) | 2 (15) | |
| BMI, kg/m2 | 29.0 (25.9–31.1) | 25.5 (21.4–28.9) | 25.3 (22.0–29.3) | 26.1 (23.8–29.9) | .41 |
| CD4 cell count, cells/μL | 226 (97–301) | 241 (120–363) | 193 (45–312) | 211 (53–311) | .93 |
| Log HIV-1 RNA, copies/mL | 4.8 (4.4–5.2) | 4.9 (4.1–5.2) | 4.6 (4.2–5.3) | 4.7 (4.3–4.7) | .78 |
| Limb fat, g | 8290 (7697–14 992) | 7841 (5212–10 477) | 7000 (5044–17 167) | 6544 (6186–1140) | .60 |
| Visceral fat, cm2 | 95.0 (82.8–146.8) | 102 (71–132) | 87.9 (56.9–129.0) | 86.8 (75.7–103.1) | .35 |
| Fat mtDNA, copies/cell | 1312 (920–1609) | 1250 (998–1886) | 1150 (721–1423) | 1125 (1049–1588) | .48 |
| Complex I activity, OD × 103/µg | 45.4 (36.7–52.1) | 39.3 (25.1–47.5) | 48.7 (33.0–58.3) | 45.2 (36.3–46.2) | .51 |
| Complex IV activity, OD × 103/µg | 27.2 (22.2–32.1) | 21.5 (16.9–30.6) | 32.1 (20.3–39.1) | 26.5 (25.4–31.8) | .48 |
Unless otherwise specified, data represent median value (interquartile range).
Abbreviations: ABC/3TC, abacavir-lamivudine; ATV/r, atazanavir-ritonavir; BMI, body mass index; complex I, nicotinamide adenine dinucleotide (reduced) dehydrogenase; complex IV, cytochrome c oxidase; EFV, efavirenz; HIV-1, human immunodeficiency virus type 1; mtDNA, mitochondrial DNA levels; OD, optical density; TDF/FTC, tenofovir DF–emtricitabine.
Overall, a total of 17 subjects did not undergo a 96-week fat biopsy. No substudy discontinuations were related to fat biopsies or adverse events from study drugs; 1 subject died of disseminated metastatic cervical cancer, 10 were unable to adhere to the study schedule, 4 were lost to follow-up, and 2 were from ACTG sites that were defunded. The baseline characteristics of these 17 subjects who prematurely discontinued the study were similar to those of the 39 subjects who underwent biopsy at week 96 (data not shown).
Among subjects with 96-week biopsies, none switched their NRTI components during the study. Two modified their randomized NNRTI or PI components (both taking EFV plus ABC/3TC); the first switched to nevirapine plus ABC/3TC secondary to a rash at week 2 and the other switched to fosamprenavir-ritonavir plus ABC/3TC at week 72 because of depression. At week 96, only 2 subjects had HIV-1 RNA levels >50 copies/mL (11 611 and 15 041 copies/mL). One of the 2 subjects with detectable HIV-1 RNA was receiving EFV plus TDF/FTC, the other ATV/r plus ABC/3TC.
Changes in Fat mtDNA Content
Changes by NRTI Components
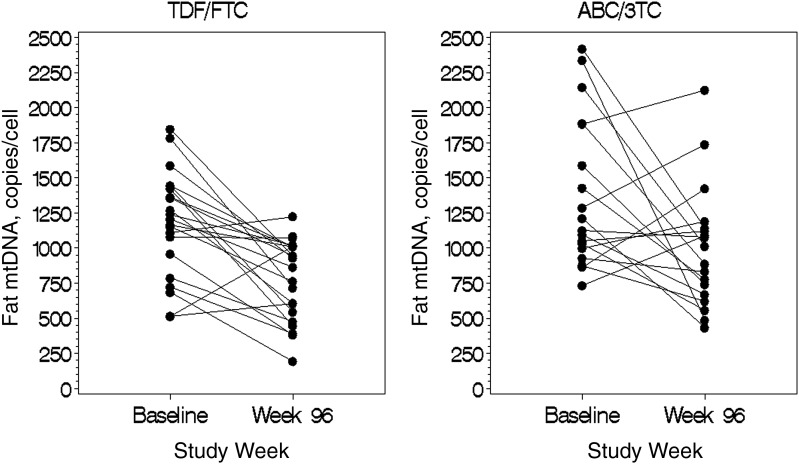
As shown in Table 2 and Figure 1, when third drugs were combined, there was a significant decrease in fat mtDNA at week 96 compared with baseline in subjects randomized to either ABC/3TC (median change, −341 copies/cell; interquartile range [IQR] −848 to 190; P = .03) or TDF/FTC (−400 copies/cell; −661 to −221; P < .001). However, the decrease did not differ between ABC/3TC and TDF/FTC groups (P = .57).
Table 2.
Changes Between Baseline and Week 96 in Human Immunodeficiency Virus Disease, Metabolic, and Mitochondrial Indices in Patients Receiving Abacavir-Lamivudine Versus Tenofovir DF–Emtricitabine (n = 39)
| Index | ABC/3TC | P (Within ABC/3TC) | TDF/FTC | P (Within TDF/FTC) | P (Between Groups) |
|---|---|---|---|---|---|
| CD4 cell count, cells/μL | 220 (142–307) | <.001 | 238 (116–357) | <.001 | .82 |
| Log10 HIV-1 RNA, copies/mL | −3.8 (−4.1 to −3.1) | <.001 | −3.8 (−4.6 to −3.2) | <.001 | .47 |
| BMI, kg/m2 | 1.2 (0.3–2.0) | .045 | 2.0 (0.2–4.9) | .02 | .12 |
| Limb fat, g | 906 (−349 to 1900) | .03 | 1479 (353–3032) | .008 | .30 |
| Visceral fat, cm2 | 14.2 (−7.5 to 30.7) | .06 | 16.1 (−4.6 to 33.6) | .21 | .93 |
| Fat mtDNA, copies/cell | −341 (−848 to 190) | .03 | −400 (−661 to −221) | <.001 | .57 |
| Complex I activity, OD × 103/µg | 2.6 (−4.4 to 11.1) | .67 | −12.45 (−24.7 to 2.9) | .003 | .03 |
| Complex IV activity, OD × 103/µg | −3.5 (−8.9 to 1.7) | .09 | −8.25 (−13.9 to −1.3) | <.001 | .13 |
The third drugs were combined in this comparison. Values represent median change (interquartile range).
Abbreviations: ABC/3TC, abacavir-lamivudine; BMI, body mass index; complex I, nicotinamide adenine dinucleotide (reduced) dehydrogenase; complex IV, cytochrome c oxidase; HIV-1, human immunodeficiency virus type 1; mtDNA, mitochondrial DNA levels; OD, optical density; TDF/FTC, tenofovir DF–emtricitabine.
Figure 1.
Changes in fat tissue mitochondrial DNA levels in abacavir-lamivudine and tenofovir DF–emtricitabine arms (third drugs combined) for all subjects with available paired samples (n = 39). Abbreviations: ABC/3TC, abacavir-lamivudine; mtDNA, mitochondrial DNA; TDF/FTC, tenofovir DF–emtricitabine.
Changes by NNRTI or PI Components
At week 96, when NRTIs were combined, there was a significant decrease in fat mtDNA compared with baseline within the EFV-containing (median change, −434 copies/cell; IQR, −1000 to 90; P = .006) and ATV/r-containing (−351 copies/cell; −576 to −49; P = .001) arms, without a difference between EFV and ATV/r (P = .45; Table 3). Removing the 2 subjects with ART switches during the study did not change the results. Furthermore, there was no evidence of an interaction between the NRTI or NNRTI/PI components and the HIV-1 RNA stratum.
Table 3.
Changes Between Baseline and Week 96 in Human Immunodeficiency Virus Disease, Metabolic, and Mitochondrial Indices in Patients Receiving Efavirenz Versus Atazanavir-Ritonavir (n = 39)
| Index | EFV | P (Within EFV) | ATV/r | P (Within ATV/r) | P (Between Groups) |
|---|---|---|---|---|---|
| CD4 count, cells/μL | 144 (108–292) | <.001 | 250 (210–352) | <.001 | .22 |
| Log10 HIV-1 RNA, copies/mL | −3.8 (−4.1 to −3.1) | <.001 | −3.7 (−4.3 to −3.1) | <.001 | .85 |
| BMI, kg/m2 | 1.1 (−0.3 to 2.2) | .25 | 1.6 (0.3–4.4) | <.001 | .24 |
| Limb fat, g | 812 (−349 to 1503) | .14 | 1807 (391–2982) | <.001 | .053 |
| Visceral fat, cm2 | 11.5 (−17.6 to 30.9) | .48 | 18.9 (−0.2 to 33.6) | .01 | .40 |
| Fat mtDNA, copies/cell | −434 (−1000 to 90) | .006 | −351 (−576 to −49) | .001 | .45 |
| Complex I activity, OD × 103/µg | −0.85 (−20.2 to 8.8) | .15 | −2.4 (−15.9 to 2.9) | .09 | .99 |
| Complex IV activity, OD × 103/µg | −10.0 (−13.8 to −1.3) | .005 | −3.7 (−7.3 to 0.2) | .01 | .39 |
The nucleoside reverse-transcriptase inhibitors were combined in this comparison. Values represent median change (interquartile range).
Abbreviations: ATV/r, atazanavir-ritonavir; BMI, body mass index; complex I, nicotinamide adenine dinucleotide (reduced) dehydrogenase; complex IV, cytochrome c oxidase; EFV, efavirenz; mtDNA, mitochondrial DNA levels; HIV-1, human immunodeficiency virus type 1; OD, optical density.
Changes in Oxidative Phosphorylation Activity
Changes by NRTI Components
Despite the decrease in fat mtDNA copies/cell, no significant changes in complex I and complex IV activity levels were seen in ABC/3TC arms (median change, 2.6 [IQR, −4.4 to 11.1; P = .67] and −3.5 [−8.9 to 1.7; P = .09] optical density [OD] × 103/µg, respectively). In contrast, both complex I and complex IV activity levels decreased significantly in the TDF/FTC-containing arms (−12.45 [−24.7 to 2.9; P = .003] and −8.25 [−13.9 to −1.3; P < .001] OD × 103/µg). The difference in the changes between groups was significant only for complex I activity (P = .03).
Changes by NNRTI or PI Components
Complex I activity did not significantly change in either EFV- or ATV/r-containing arms (median change, −0.85 [IQR, −20.2 to 8.8; P = .15] and −2.4 [−15.9 to 2.9; P = .09] OD × 103/µg, respectively). Complex IV activity, however, significantly decreased in both EFV and ATV/r arms (−10.0 [−13.8 to −1.3; P = .05] and −3.7 [−7.3 to 0.2; P = .01] OD × 103/µg), but the fall was similar in the 2 groups (P = .39). Again, removing the 2 subjects with ART switches during the study did not change the results, and for both complex I and complex IV, there was no evidence of an interaction between the NRTI or NNRTI/PI components and the HIV-1 RNA stratum (P ≥ .44).
Correlations of the Changes in Mitochondrial Indices
For the entire group, regardless of treatment assignment, the changes in fat mtDNA content did not correlate with changes in limb fat or trunk fat measured with dual-energy x-ray absorptiometry or with total, visceral, or subcutaneous abdominal fat measured with computed tomography. Similarly, the changes in oxidative phosphorylation complex I and complex IV activity levels did not correlate with any of these fat measurements (P ≥ .13). Moreover, the changes in all 3 mitochondrial indices did not correlate with changes in baseline or changes in CD4 cell counts or HIV-1 RNA levels (all P ≥ .21).
We then considered separately the patients randomized to TDF/FTC or ABC/3TC therapy. In the subgroup randomized to ABC/3TC-containing regimens, no correlation was found between any of the mitochondrial indices and body composition measures, HIV-1 RNA level, or CD4 cell count. However, in subjects receiving TDF/FTC, inverse correlations were found between changes in complex I and complex IV activity levels and changes in trunk fat (complex I, r = −0.42 and P = .04; complex IV, r = −0.41 and P = .06), total abdominal fat (r = −0.53 and P = .01; r = −0.50 and P = .02), visceral abdominal fat (r = −0.52 and P = .02; r = −0.43; P = .05), and subcutaneous abdominal fat (r = −0.56 and P = .01; r = −0.49; P = .01).
DISCUSSION
This report, for the first time, details changes in mtDNA and mitochondrial function among subjects randomized to 1 of 4 commonly used nonthymidine NRTI–containing initial ART regimens. We found that ART initiation with both ABC/3TC- and TDF/FTC-containing regimens results in a significant decrease in fat mtDNA levels. In addition, in subjects treated with TDF/FTC (but not ABC/3TC), there was evidence of mitochondrial respiratory chain dysfunction, as assessed by decreases in both complex I and complex IV activity levels. In this same group (TDF/FTC treated), mitochondrial alterations were correlated with gains in subcutaneous and visceral abdominal fat. We also found in EFV- and ATV/r-containing arms significant but similar declines in fat mtDNA and in oxidative phosphorylation complex IV activity levels.
To date, no data are available on longitudinal fat mitochondrial changes in ART-naive subjects. This current study did not enroll an ART-naive control group who underwent serial biopsies without starting ART. Therefore, we cannot firmly conclude that the observed declines in mitochondrial indices are due to the effect of ART (and not HIV itself). However, we believe that these were unlikely to be due to HIV-1 infection alone. Indeed untreated HIV-1 infection has been linked in most studies but not all [9], to declines in mtDNA levels and in oxidative phosphorylation enzymes in peripheral blood mononuclear cells [10–12] or in adipose tissue [13]. Miura et al have also reported that mtDNA levels in HIV-1–infected individuals were inversely correlated with HIV-1 RNA levels [14]. Therefore, treating HIV-1 infection and suppressing the virus is expected to improve mitochondrial indices and not worsen them, as seen in our study.
The lack of correlation between the decline in mitochondrial indices and changes in fat content in the overall group should not necessarily lessen concerns about potential mitochondrial toxicity of nonthymidine NRTIs. Our study was relatively short, and it is conceivable that nonthymidine NRTI–induced mitochondrial toxicity is mild compared with that seen with thymidine NRTIs, resulting in slower development of clinical phenotypes such as lipoatrophy. It is also possible that the mitochondrial toxicity seen in our study could become problematic in subjects receiving other medications known to affect mitochondrial function, such as metformin [15], ethambutol [16], or linezolid [17], or in those exposed to excessive alcohol, a known mitochondrial toxin.
Moreover, in the TDF/FTC-treated group (regardless of the ATV/r vs EFV randomization), we found consistent inverse correlations between the activity levels of oxidative phosphorylation complex I and complex IV and several measures of fat content, at both visceral and subcutaneous levels, suggesting that lower mitochondrial function is associated with generalized fat gain in this TDF/FTC-treated group. Similar associations between complex I and complex IV activity levels and limb and trunk fat were recently reported from another study of TDF-based regimens [18]. Although the pathogenesis of lipohypertrophy is poorly understood, the role of mitochondrial toxicity has not been ruled out. Indeed, we and others have found significant mitochondrial abnormalities in dorsocervical adipose tissue, even from HIV-infected persons with lipoatrophy [19–20].
In this mitochondrial substudy of A5224s, the body fat composition results are overall consistent with the main A5224s results [2]. Limb fat and visceral fat increased significantly in the ATV/r arms but not in the EFV arms. In ACTG A5142, a randomized study in which patients received NRTIs of choice, including thymidine NRTIs in many, EFV was associated with higher rates of lipoatrophy than the PI lopinavir-ritonavir [21]. This finding suggested that EFV may have been associated with greater mitochondrial toxicity than lopinavir-ritonavir. This concept was further corroborated by a study showing that EFV inhibited mitochondrial function, including complex I activity, in human hepatic cells in vitro [22]. However, in our study, we found similar decreases in all mitochondrial indices with EFV- and ATV/r-containing arms.
Our study has some limitations, including small sample size and a significant number of premature study discontinuations before week 96. We recorded mitochondrial indices only at 2 time points and not beyond 96 weeks. Consequently, we could not exclude early improvements followed by worsening of these measures over time. All of these limitations are frequently encountered in studies requiring invasive tissue sampling, and it is not realistic to obtain a sufficient quantity of fat tissue at closely spaced time intervals in a substantial number of subjects.
In conclusion, we have shown significant perturbation in mitochondrial indices after 96 weeks of nonthymidine NRTI–containing regimens which were assigned randomly. In the TDF/FTC group, changes in oxidative phosphorylation complex I and complex IV activity levels consistently were inversely correlated with changes in several objective measures of body fat, including in both subcutaneous and visceral compartments. Ongoing investigations will clarify the impact of these regimens on fat apoptosis and oxidative stress. Given the anticipated use of life-long ART and the central role of NRTIs in current regimens, larger and longer studies are needed to better characterize the mitochondrial and body composition effects of nonthymidine NRTI–containing regimens. Finally, studies investigating other potential consequences of mitochondrial abnormalities, such as frailty and premature aging, are much needed.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health, US Department of Health and Human Services (grants AI065348 to G. A. M., AI068636, AI068634, AI38855, and AI069434 to A. C. C., and MD000173, GM103341, and MD007601 to M. G.). Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
Potential conflicts of interest. G. A. M. has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Abbott, Tibotec, and Gilead Sciences; has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, Abbott, Merck, and Gilead Sciences; and is currently serving as the Data and Safety Monitoring Board chair for a Pfizer-sponsored study. E. S. D. serves as a consultant for Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, and ViiV and receives research grant support from Abbott Laboratories, Merck, Gilead, ViiV, and Pfizer. A. C. C. has received research grants from Boehringer-Ingelheim, Gilead Sciences, Merck, Schering-Plough, and Tibotec-Virco; served as a Data and Safety Monitoring Board member for a Merck-sponsored study; and owns stock in Abbott Laboratories, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. J. L. S. has served as a speaker and consultant for Gilead, Merck, and Bristol-Myers Squibb and has received research grants from Merck and Gilead. C. J. F. receives research support from ViiV, Pfizer, and Janssen and serves as a speaker for Janssen. P. E. S. serves as a consultant for Abbott, Bristol-Myers Squibb, Gilead, GSK, Merck, Janssen, and ViiV and receives grant support from Gilead, Merck, and Tibotec. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix for A5224s
Sadia Shaik, MD, and Ruben Lopez, MD, Harbor-UCLA Medical Center (site 603); CTU grant AI0694241 and UCLA CTSI grant UL1TR000124.
Susan L. Koletar, MD, and Diane Gochnour, RN, Ohio State University Medical Center (site 2301); CTU grant AI069474.
Geyoul Kim, RN, and Mark Rodriguez, RN, Washington University (site 2101); CTU grant U01AI069495, GCRC grant UL1 RR024992.
Elizabeth Lindsey, RN, and Tamara James, BS, Alabama Therapeutics CRS (site 5801); CTU grant U01 AI069452.
Ann C. Collier, MD, and Jeffrey Schouten, MD, JD, University of Washington (site 1401); CTU grant AI069434, UL1 RR025014.
Jorge L. Santana Bagur, MD, and Santiago Marrero, MD, Puerto Rico-AIDS Clinical Trials Unit (site 5401); CTU grant 5 U0I AI069415–03.
Jenifer Baer, RN, BSN, and Carl J Fichtenbaum, MD, University of Cincinnati (site 2401); CTU grant AI069513.
Patricia Walton, BSN, RN, and Barbara Philpotts BSN, RN, Case Western Reserve (site 2501); CTU grant AI69501.
Princy Kumar, MD, and Joseph Timpone, MD, Georgetown University (site 1008); CTU grant, ACTG grant 5U01AI069494.
Donna Pittard, RN, BSN, and David Currin, RN, University of North Carolina (site 3201); CTU grant 5-U01 AI069423-03, UNC CFAR grant P30 AI050410(-11), UNC CTRC grant UL 1RR 025747.
Julie Hoffman, RN, and Edward Seefried, RN, San Diego Medical Center UC (site 701); CTU grant AI69432.
Susan Swindells, MBBS, and Frances Van Meter, APRN, University of Nebraska (site 1505); CTU grant AI 27661.
Deborah McMahon, MD, and Barbara Rutecki, MSN, MPH, CRNP, University of Pittsburgh (site 1001); CTU grant 1 U01 AI069494-01.
Michael P. Dube, MD, and Martha Greenwald, RN, MSN, Indiana University (site 2601); CTU grant 5U01AI025859, GCRC M01 RR00750.
Ilene Wiggins, RN, and Eric Zimmerman, RN, Johns Hopkins University (site 201); CTU grant AI27668, CTSA grant UL1 RR025005.
Judith. Aberg, MD, and Margarita Vasquez, RN, New York University/NYC HHC at Bellevue Hospital Center (site 401); CTU grant AI27665, new grant No. AI069532.
Martin McCarter and M. Graham Ray, RN, MSN, Colorado AIDS Clinical Trials Unit, (site 6101); CTU grant AI69450, RR025780.
Mamta Jain, MD–PI, and Tianna Petersen, MS, University of Texas Southwestern Medical Center (site 3751); CTU grant 3U01AI046376-05S4.
Emily Stumm, BS, and Ian Frank MD, University of Pennsylvania, Philadelphia (site 6201); CTU grant P30-AI0450008-11, CFAR grant UO1-AI069467-04.
Mary Albrecht, MD, and Neah Kim, NP, Beth Israel Deaconess (Partners/Harvard) CRS (site 103); CTU grant U01 AI069472-04.
Paul Edward Sax, MD, and Joanne Delaney, RN, Brigham and Women's Hospital (site 107) CTU grant UOI AI 069472.
Christine Hurley, RN, and Roberto Corales, DO, AIDS Care (site 1108); CTU grant U01AI069511-02 (as of 12 February 2008), GCRC UL1 RR 024160.
Keith Henry, MD, and Bette Bordenave, RN, Hennepin County Medical Center (site 1502); CTU grant N01 AI72626.
Wendy Armstrong, MD, and Ericka R. Patrick, RN, MSN, CCRC, Emory University HIV/AIDS Clinical Trials Unit (site 5802); CTU grant UO1Al69418-01/CFAR grant P30Al050409.
Jane Reid, RNC, MS, and Mary Adams, RN, MPh, University of Rochester (site 1101); CTU grant U01AI069511-02 (as of 12 February 2008), GCRC UL1 RR 024160.
Gene D. Morse, PharmD, FCCP, BCPS, SUNY, Buffalo, Erie County Medical Center (site 1102); CTU grant AI27658.
Michael P. Dube, MD, and Martha Greenwald, RN, MSN, Wishard Memorial Hospital, Indiana University (site 2603); CTU grant 5U01AI025859, GCRC grant M01 RR00750.
Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN, Rush University Medical Center (site 2702); CTU grant U01 AI069471.
Nancy Hanks, RN, and Debra Ogata-Arakaki, RN, University of Hawaii at Manoa, Leahi Hospital (site 5201); CTU grant AI34853.
Ardis Moe, MD, and Maria Palmer PA-C, UCLA Medical Center (site 601); CTU grant 1U01AI069424-01.
References
- 1.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004;4:111–8. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53:185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McComsey GA, Ward DJ, Hessenthaler SM, et al. Improvement in lipoatrophy associated with highly active antiretroviral therapy in human immunodeficiency virus-infected patients switched from stavudine to abacavir or zidovudine: the results of the TARHEEL study. Clin Infect Dis. 2004;38:263–70. doi: 10.1086/380790. [DOI] [PubMed] [Google Scholar]
- 4.Martin A, Smith DE, Carr A, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS. 2004;18:1029–36. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 5.Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20:2043–50. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 6.McComsey G, Kitch D, Daar E, et al. Bone mineral density and fractures in antiretroviral-naïve subjects randomized to abacavir/lamivudine or tenofovir disoproxil fumarate /emtricitabine along with efavirenz or atazanavir/ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McComsey GA, Libutti DE, O'Riordan M, et al. Mitochondrial RNA and DNA alterations in HIV lipoatrophy are linked to antiretroviral therapy and not to HIV infection. Antivir Ther. 2008;13:715–22. [PMC free article] [PubMed] [Google Scholar]
- 8.Brogly SB, DiMauro S, Van Dyke RB, et al. Short communication: transplacental nucleoside analogue exposure and mitochondrial parameters in HIV-uninfected children. AIDS Res Hum Retroviruses. 2011;27:777–83. doi: 10.1089/aid.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morse CG, Voss JG, Rakocevic G, et al. HIV infection and antiretroviral therapy have divergent effects on mitochondria in adipose tissue. J Infect Dis. 2012;205:1778–87. doi: 10.1093/infdis/jis101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladha JS, Tripathy MK, Mitra D. Mitochondrial complex I activity is impaired during HIV-1-induced T-cell apoptosis. Cell Death Differ. 2005;12:1417–28. doi: 10.1038/sj.cdd.4401668. [DOI] [PubMed] [Google Scholar]
- 11.Miro O, Lopez S, Martinez E, et al. Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clin Infect Dis. 2004;39:710–6. doi: 10.1086/423176. [DOI] [PubMed] [Google Scholar]
- 12.Tripathy MK, Mitra D. Differential modulation of mitochondrial OXPHOS system during HIV-1 induced T-cell apoptosis: up regulation of complex-IV subunit COX-II and its possible implications. Apoptosis. 2010;15:28–40. doi: 10.1007/s10495-009-0408-9. [DOI] [PubMed] [Google Scholar]
- 13.Garrabou G, Lopez S, Moren C, et al. Mitochondrial damage in adipose tissue of untreated HIV-infected patients. AIDS. 2011;25:165–70. doi: 10.1097/QAD.0b013e3283423219. [DOI] [PubMed] [Google Scholar]
- 14.Miura T, Goto M, Hosoya N, et al. Depletion of mitochondrial DNA in HIV-1-infected patients and its amelioration by antiretroviral therapy. J Med Virol. 2003;70:497–505. doi: 10.1002/jmv.10423. [DOI] [PubMed] [Google Scholar]
- 15.Detaille D, Guigas B, Chauvin C, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–87. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 16.Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int. 2002;40:573–84. doi: 10.1016/s0197-0186(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 17.De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42:1111–7. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 18.Gerschenson M, Shikuma CM, Ananworanich J, et al., editors. Seattle, WA: 2012. Baseline PBMC mitochondrial enzymes can predict fat changes at 24 or 72 weeks in Thai subjects initiated on HAART. 19th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 19.Sevastianova K, Sutinen J, Greco D, et al. Comparison of dorsocervical with abdominal subcutaneous adipose tissue in patients with and without antiretroviral therapy-associated lipodystrophy. Diabetes. 2011;60:1894–900. doi: 10.2337/db11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, Libutti D, Chow D, Lee F, Gerschenson M, McComsey G, editors. Mexico City, Mexico: 2008. Dorsocervical fat of HIV+ lipoatrophic patients and mitochondrial function. 17th International AIDS Conference; 3–8 August. [Google Scholar]
- 21.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–18. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blas-Garcia A, Apostolova N, Ballesteros D, et al. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology. 2010;52:115–25. doi: 10.1002/hep.23647. [DOI] [PubMed] [Google Scholar]