Abstract
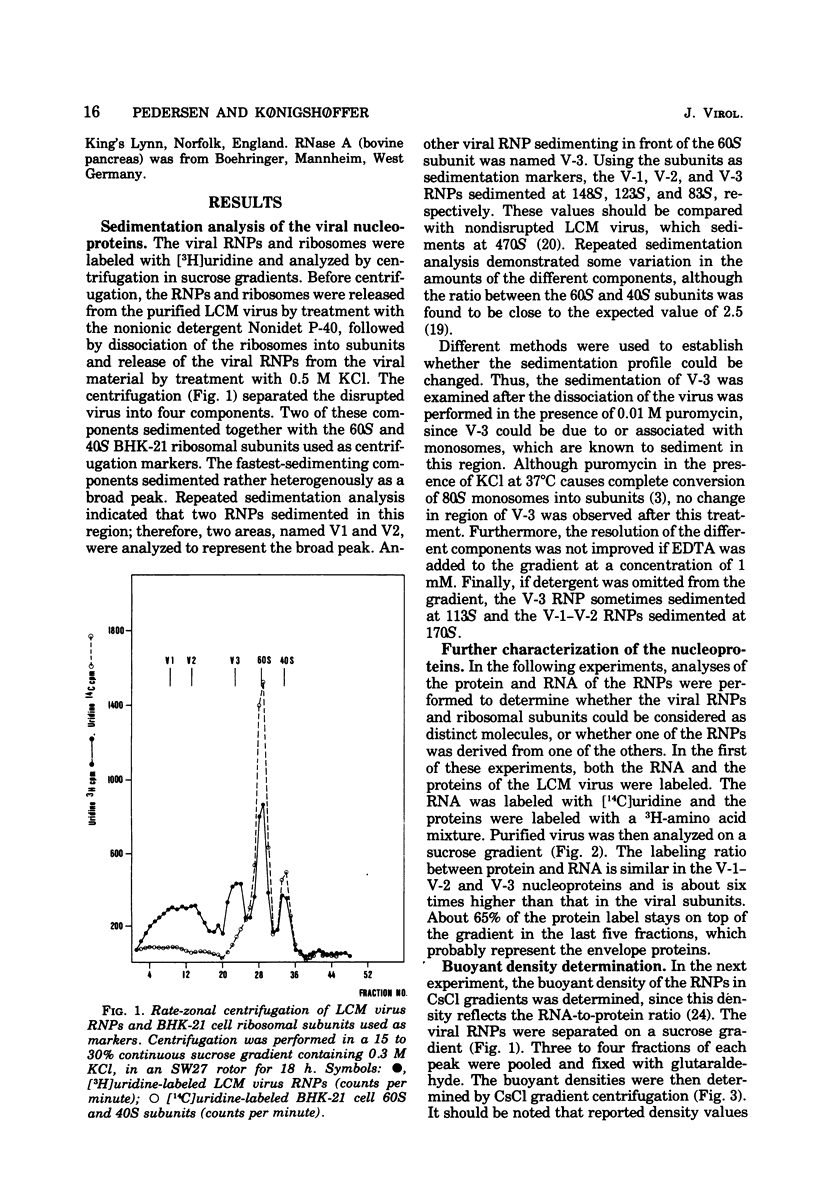
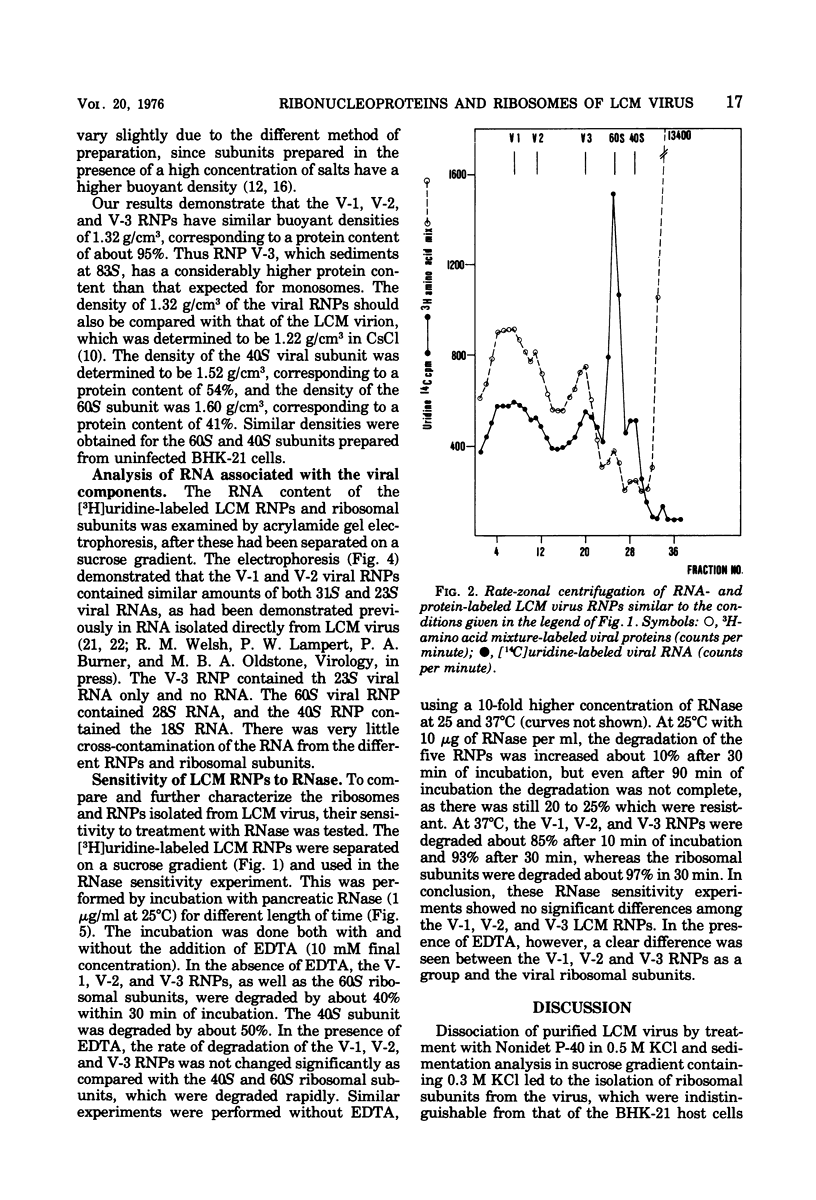
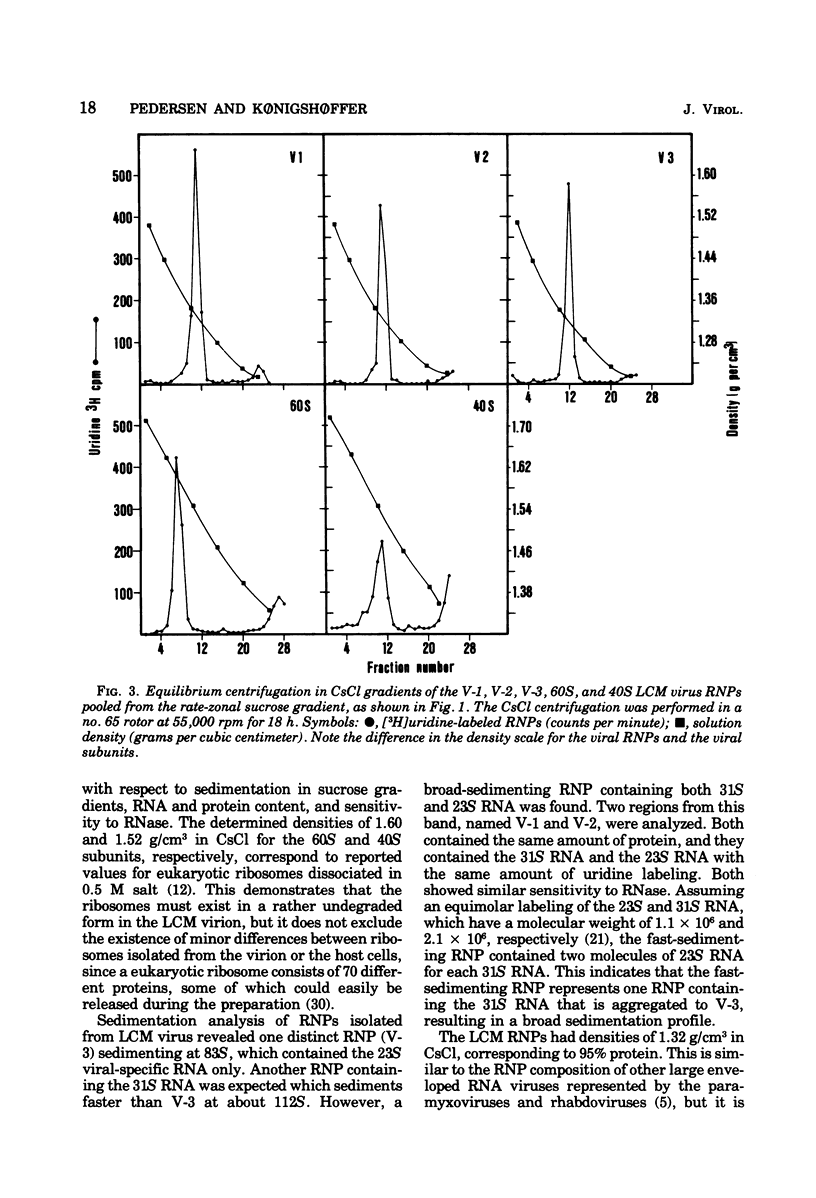
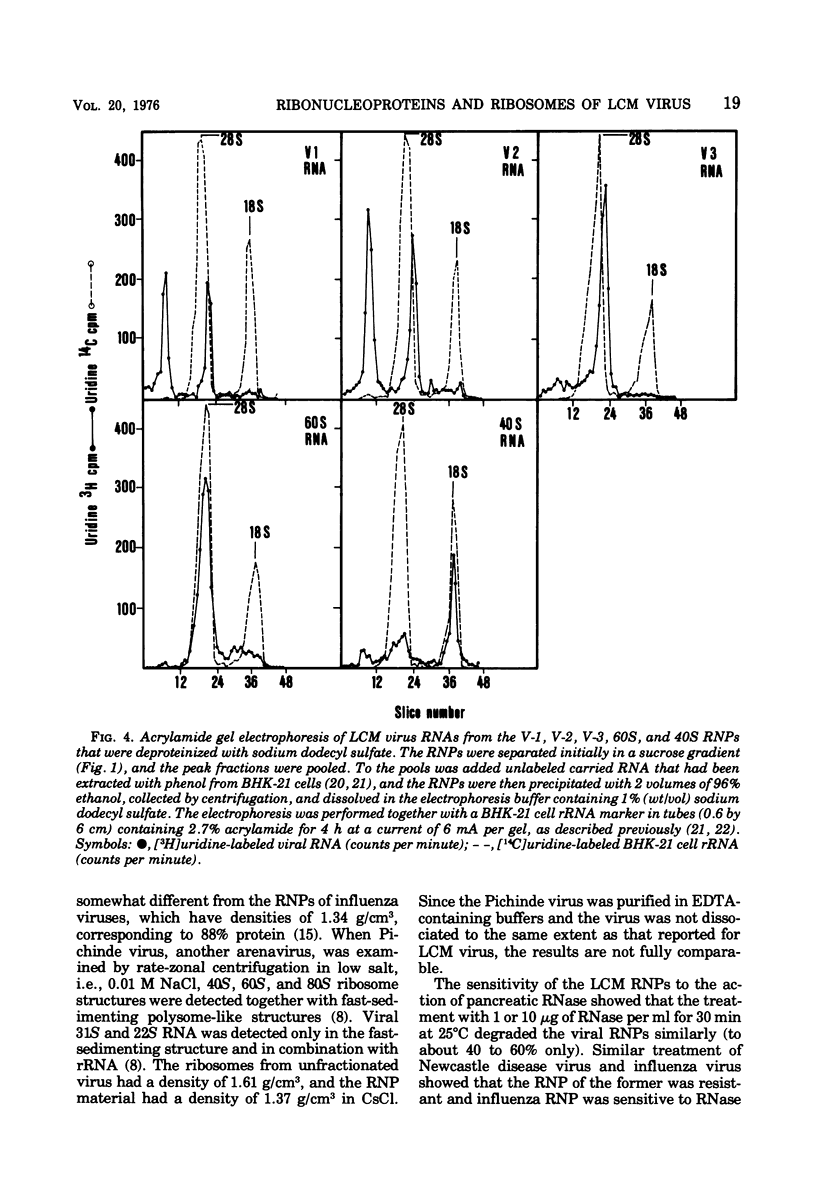
Disruption of purified lymphocytic choriomeningitis (LCM) virus with Nonidet P-40 in 0.5 M KCl followed by sucrose gradient centrifugation in 0.3 M KCl led to the isolation of two viral nucleoproteins (RNPs) as well as 40S and 60S ribosomal subunits. The largest viral RNP sedimented heterogenously at 123S to 148S and was associated with 23S and 31S viral RNA. The other viral RNP sedimented at 83S and was associated with 23S viral RNA. The buoyant density in CsCl was determined to be 1.32 g/cm3 for the viral RNP. Densities of 1.52 and 1.60 g/cm3 were determined for the 40S and 60S subunits, similar to those of the BHK-21 cells subunits dissociated by 0.5 M KCl. The viral RNPs were partly sensitive to RNase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. Optimal conditions for counting of precipitated 3H-RNA on glass-fiber filters. Anal Biochem. 1970 Sep;37(1):178–182. doi: 10.1016/0003-2697(70)90275-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Carter M. F., Biswal N., Rawls W. E. Characterization of nucleic acid of pichinde virus. J Virol. 1973 Jan;11(1):61–68. doi: 10.1128/jvi.11.1.61-68.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Farber F. E., Rawls W. E. Isolation of ribosome-like sturctures from Pichinde virus. J Gen Virol. 1975 Jan;26(1):21–31. doi: 10.1099/0022-1317-26-1-21. [DOI] [PubMed] [Google Scholar]
- Gschwender H. H., Rutter G., Popescu M. Use of iodinated organic compounds for the density gradient centrifugation of viruses. Arch Virol. 1975;49(4):359–364. doi: 10.1007/BF01318245. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Balitmore D. Initiation of polyribosome formation in poliovirus-infected HeLa cells. J Mol Biol. 1970 Feb 14;47(3):275–291. doi: 10.1016/0022-2836(70)90302-5. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Darlington R. W. Isolation and properties of Newcastle disease virus nucleocapsid. J Virol. 1968 Mar;2(3):248–255. doi: 10.1128/jvi.2.3.248-255.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Webster R. G. Some properties of influenza virus nucleocapsids. J Virol. 1969 Sep;4(3):219–225. doi: 10.1128/jvi.4.3.219-225.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Kumar A., Subramanian A. R. Ribosome assembly in HeLa cells: labeling pattern of ribosomal proteins by two-dimensional resolution. J Mol Biol. 1975 May 25;94(3):409–423. doi: 10.1016/0022-2836(75)90211-9. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Wool I. G. Formation of active hybrids from subunits of muscle ribosomes from normal and diabetic rats. Proc Natl Acad Sci U S A. 1968 Jun;60(2):569–574. doi: 10.1073/pnas.60.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R. Density gradient centrifugation studies on lymphocytic choriomeningitis virus and on viral ribonucleic acid. J Virol. 1970 Oct;6(4):414–420. doi: 10.1128/jvi.6.4.414-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Different classes of ribonucleic acid isolated from lymphocytic choriomeningitis virus. J Virol. 1973 Mar;11(3):416–423. doi: 10.1128/jvi.11.3.416-423.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Lymphocytic choriomeningitis virus RNAs. Nat New Biol. 1971 Nov 24;234(47):112–114. doi: 10.1038/newbio234112a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- Pfau C. J., Bergold G. H., Casals J., Johnson K. M., Murphy F. A., Pedersen I. R., Rawls W. E., Rowe W. P., Webb P. A., Weissenbacher M. C. Arenaviruses. Intervirology. 1974;4(4):207–214. doi: 10.1159/000149964. [DOI] [PubMed] [Google Scholar]
- Pfau C. J. Biochemical and biophysical properties of the arenaviruses. Prog Med Virol. 1974;18(0):64–80. [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. I. Experiments on the conditions of conjugation. Immunology. 1970 Jun;18(6):865–873. [PMC free article] [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. II. A reproducible method. Immunology. 1970 Jun;18(6):875–881. [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Traut R. R. Recent advances in the preparation of mammalian ribosomes and analysis of their protein composition. Methods Cell Biol. 1973;7:67–103. doi: 10.1016/s0091-679x(08)61772-0. [DOI] [PubMed] [Google Scholar]


