Abstract
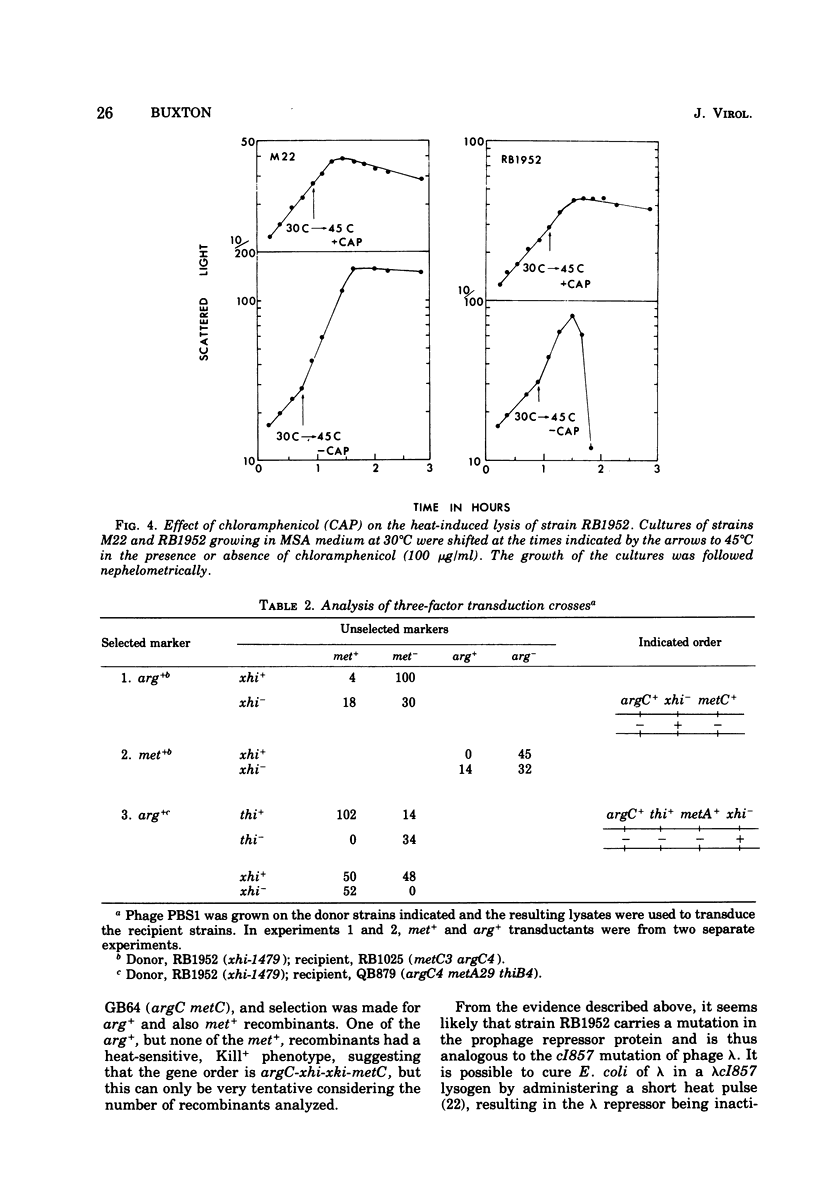
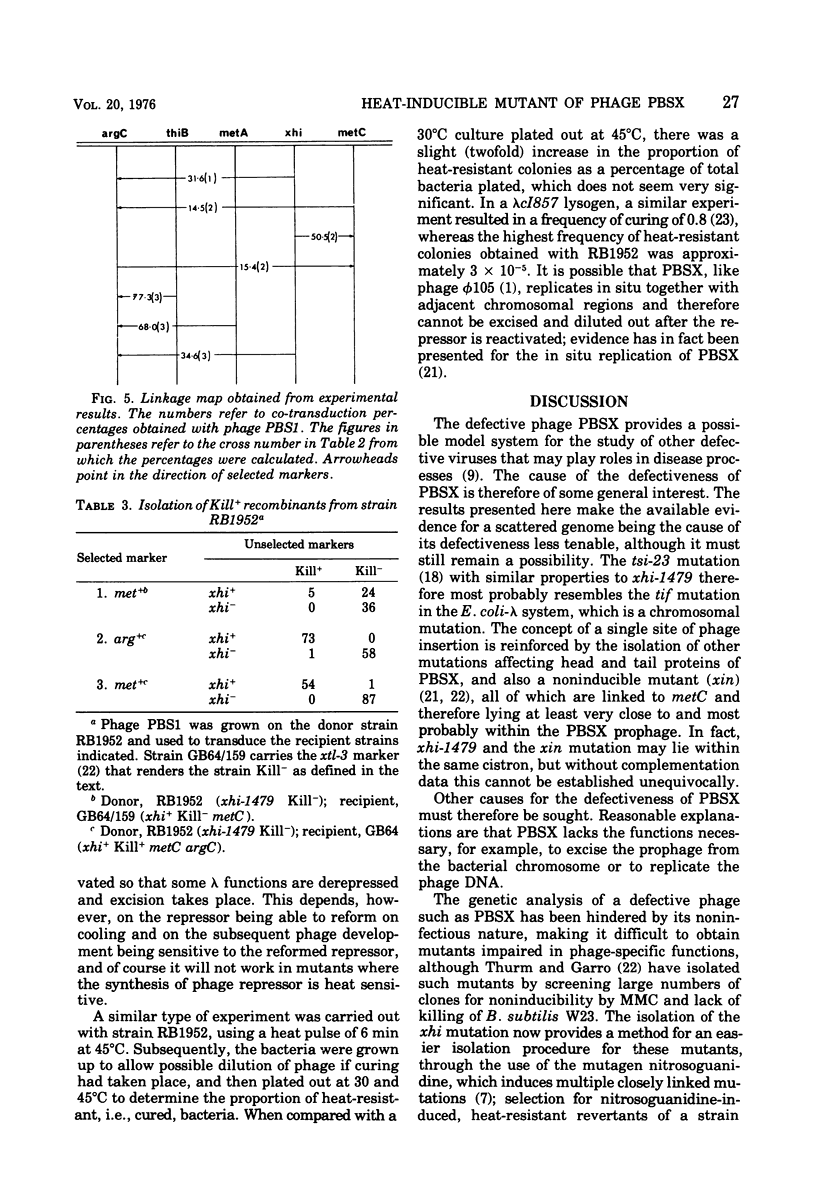
A mutant of Bacillus subtilis 168 has been isolated in which the defective phage PBSX was heat inducible, whereas another phage, phi105, was not so induced. A culture of the mutant grown at 30 degrees C, when shifted to 45 degrees C, began to lyse after 45 min; cell viability began to decrease after 10 min. Heat-induced lysis of the mutant was prevented by chloramphenicol. DNA, RNA, protein, and peptidoglycan synthesis were normal at the nonpermissive temperature up to the time of lysis. The site of xhi-1479 mutation causing this phenotype was linked (50%) in phage PBS1-mediated transduction to the host marker metC and to another PBSX marker xtl and was thus thought to map within the PBSX prophage. The order of markers was argC-thiB-metA-xhi-metC. The xhi mutation was thus distinct from another mutation, tsi-23, causing a similar heat inducibility of PBSX (Siegel and Marmur, 1969), which was unlinked to the metC marker. tsi-23 is therefore thought to be a host mutation, and the available evidence for a scattered phage genome being the cause of the defective nature of PBSX is thus less tenable. It was shown that the mutant, besides carrying the xhi mutation, also carried another closely linked mutation, xki-1479, which caused the PBSX produced to have no killing activity on the sensitive strain W23. The xki mutation was separated from xhi by recombination.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentrout R. W., Rutberg L. Heat induction of prophage phi 105 in Bacillus subtilis: replication of the bacterial and bacteriophage genomes. J Virol. 1971 Oct;8(4):455–468. doi: 10.1128/jvi.8.4.455-468.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Rutberg L. Restriction and modification in Bacillus subtilis. Induction of a modifying activity in Bacillus subtilis 168. Mol Gen Genet. 1974;133(2):175–177. doi: 10.1007/BF00264838. [DOI] [PubMed] [Google Scholar]
- Garro A. J., Leffert H., Marmur J. Genetic mapping of a defective bacteriophage on the chromosome of Bacillus subtilis 168. J Virol. 1970 Sep;6(3):340–343. doi: 10.1128/jvi.6.3.340-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Bryan T. Productive infection of Bacillus subtilis 168, with bacteriophage SP-10, dependent upon inducing treatments. J Virol. 1968 Aug;2(8):805–812. doi: 10.21236/ad0686354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Cerdá-Olmedo E. Distribution of mutations induced by ethyl methanesulfonate and ultraviolet radiation in the Escherichia coli chromosome. Mutat Res. 1975 Jul;29(1):145–147. doi: 10.1016/0027-5107(75)90028-7. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Karamata D., Gross J. D. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol Gen Genet. 1970;108(3):277–287. doi: 10.1007/BF00283358. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb M. Studies of heat-inducible lambda bacteriophage. I. Order of genetic sites and properties of mutant prophages. J Mol Biol. 1966 Mar;16(1):149–163. doi: 10.1016/s0022-2836(66)80269-3. [DOI] [PubMed] [Google Scholar]
- Lindegren G., Courtis W. S., Shult E. E. Linked genes arising among multiple mutants in EMS-treated Sacchararomyces. Can J Genet Cytol. 1968 Jun;10(2):470–472. doi: 10.1139/g68-063. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Buxton R. S. Magnesium and anion requirements of rodB mutants of Bacillus subtilis. J Bacteriol. 1976 Feb;125(2):556–564. doi: 10.1128/jb.125.2.556-564.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Sargent M. G. Synchronous cultures of Bacillus subtilis obtained by filtration with glass fiber filters. J Bacteriol. 1973 Nov;116(2):736–740. doi: 10.1128/jb.116.2.736-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Marmur J. Temperature-sensitive induction of bacteriophage in Bacillus subtilis 168. J Virol. 1969 Nov;4(5):610–618. doi: 10.1128/jvi.4.5.610-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. INCORPORATION OF BACTERIOPHAGE GENOME BY SPORES OF BACILLUS SUBTILIS. J Bacteriol. 1964 Jun;87:1499–1502. doi: 10.1128/jb.87.6.1499-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm P., Garro A. J. Bacteriophage-specific protein synthesis during induction of the defective Bacillus subtilis bacteriophage PBSX. J Virol. 1975 Jul;16(1):179–183. doi: 10.1128/jvi.16.1.179-183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm P., Garro A. J. Isolation and characterization of prophage mutants of the defective Bacillus subtilis bacteriophage PBSX. J Virol. 1975 Jul;16(1):184–191. doi: 10.1128/jvi.16.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg R. A., Gallant J. A. Dual function of the lambda prophage repressor. J Mol Biol. 1967 May 14;25(3):537–544. doi: 10.1016/0022-2836(67)90204-5. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


