Abstract
Tissue factor pathway inhibitor (TFPI) is a factor Xa dependent inhibitor of tissue factor initiated blood coagulation. In recent years several alternatively spliced forms of TFPI have been identified. These alternatively spliced forms have different C-terminal regions and have different mechanisms for association with cell surfaces. They are differentially expressed in human and mouse tissues and may have distinct physiological functions.
Keywords: Tissue factor pathway inhibitor, alternative splicing, factor VIIa, factor Xa, Review
2. INTRODUCTION
Tissue factor pathway inhibitor (TFPI) is an anticoagulant protein found primarily on the surface of endothelium1, but also within platelets2, 3 and circulating in plasma4. TFPI directly inhibits the tissue factor-factor VIIa (TF-fVIIa) catalytic complex that initiates blood coagulation. The in vivo importance of TFPI as an inhibitor of TF-fVIIa activity has been demonstrated through of studies of mice lacking TF, fVIIa and TFPI. TFPI null mice die during embryogenesis from consumptive coagulopathy5. This embryonic lethal phenotype is rescued by breeding TFPI null mice into mice with low amounts of TF6 or into mice lacking fVIIa7, thereby demonstrating that TFPI directly counterbalances TF-fVIIa activity. The initial structural studies of TFPI described a protein containing an acidic N-terminal region, three Kunitz-type serine protease inhibitor domains and a highly basic C-terminal region.8 Functional studies identified the second Kunitz domain as a direct inhibitor of factor Xa (fXa) and the first Kunitz domain as a fXa dependent inhibitor of TF-fVIIa9. An inhibitory function for the third Kunitz domain has not yet been identified. Since its initial characterization, alternatively spliced forms of TFPI have been identified that are differentially expressed during mouse development and may have distinct physiological functions10–13.
3. ALTERNATIVELY SPLICED FORMS OF TFPI
TFPI is produced in four alternatively spliced isoforms14 (Figures 1–4). Each isoform has the acidic N-terminal region and the first two Kunitz domains that are responsible for TFPI anticoagulant activity. However, they differ in the domain structure of their C-terminal regions and their mechanism of association with cell surfaces.
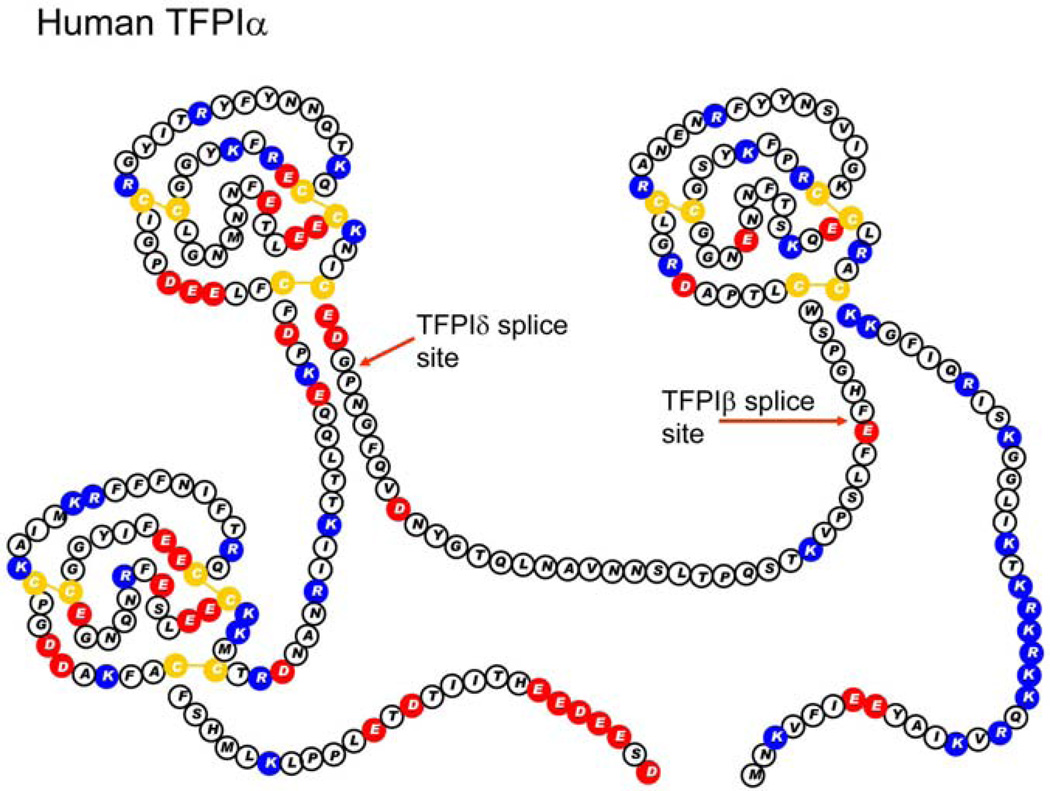
Figure 1.
The amino acid sequence and Kunitz domain structure of human TFPI alpha. Red circles indicate acidic amino acids. Blue circles indicate basic amino acids. Yellow circles are cysteine residues.
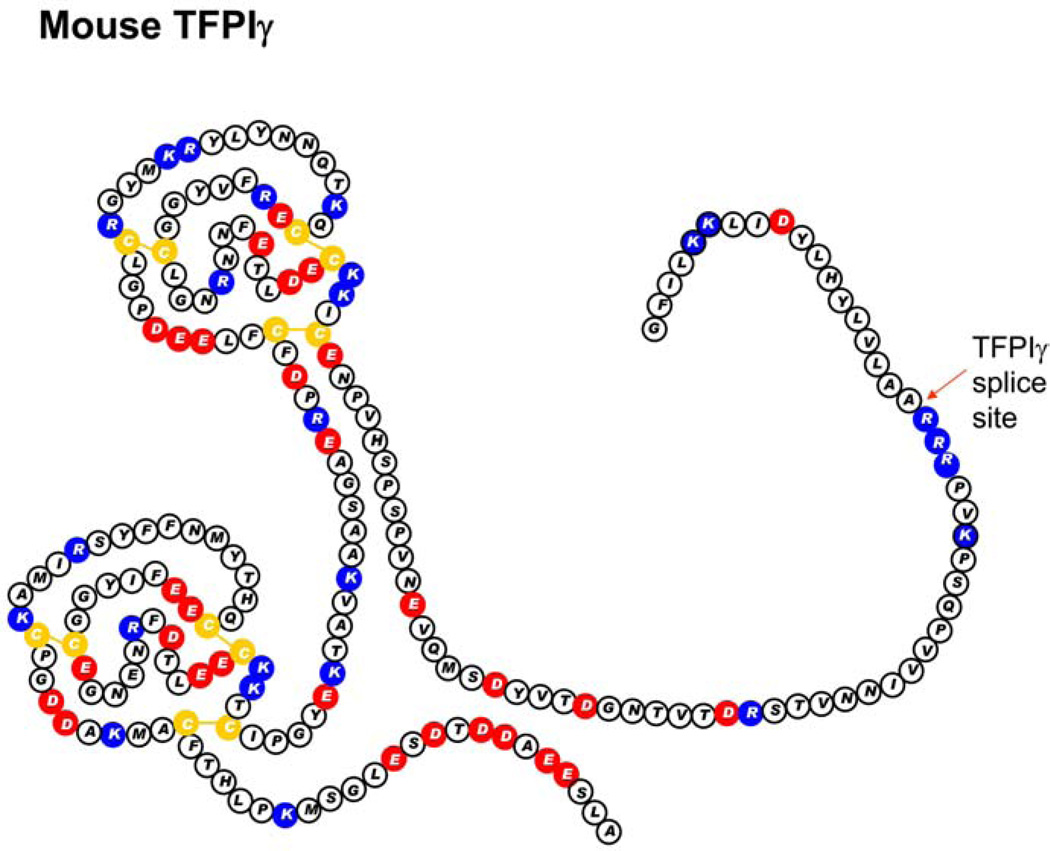
Figure 4.
The amino acid sequence and Kunitz domain structure of mouse TFPI delta. Red circles indicate acidic amino acids. Blue circles indicate basic amino acids. Yellow circles are cysteine residues.
TFPIalpha is the full-length form of TFPI. It has all three Kunitz domains and a basic amino acid rich C-terminal region. The C-terminal region of human TFPIalpha is more basic than mouse TFPIalpha, with human having 14 basic amino acids, while mice have only nine. The third Kunitz domain and/or the basic C-terminal region may be important in mediating binding of TFPIalpha to endothelium15. TFPIalpha associates with the endothelium surface in two ways. About 90% is indirectly bound through an, as yet, unident4ified GPI-anchored protein16, 17. The association of TFPIalpha with endothelium through a GPI-anchored protein localizes TFPI to caveolae, where it may interact with caveolin-1 which increases its surface expression and anticoagulant activity18. The remaining 10% of TFPIalpha is nonspecifically bound to cell surface glycosaminoglycans17. In humans, the glycosaminoglycan bound TFPI alpha is released into plasma following heparin infusion causing the plasma TFPI concentration to increase 2- to 4-fold19, 20.
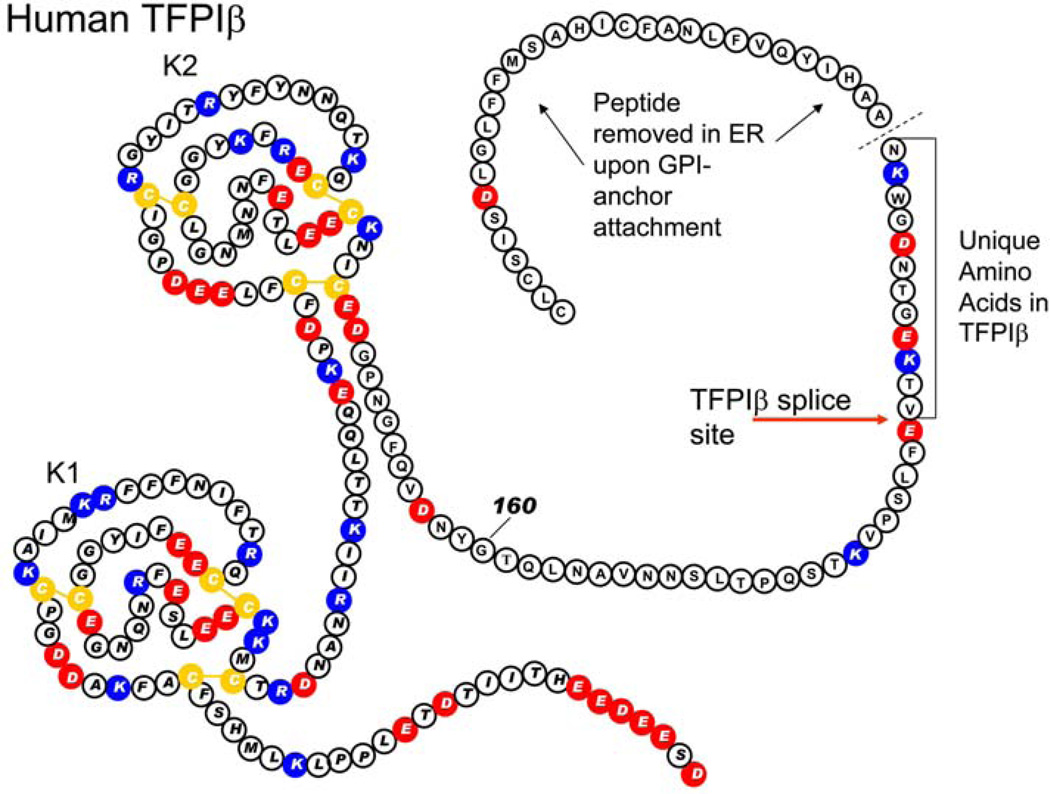
TFPIbeta is a C-terminally truncated form of TFPI10. It has the first two Kunitz domains. Alternative splicing occurs just prior to the third Kunitz domain and produces a sequence encoding a GPI-anchor attachment site and, consequently, TFPIbeta directly associates with endothelium10, 11. After processing of the C-terminal region to attach the GPI-anchor, human TFPIbeta protein has 12 amino acids not present in TFPIbeta, while mouse TFPIbeta has eight. Thus far, attempts to make high affinity antibodies that recognize the unique C-terminal region of TFPIbeta have not been successful making it difficult to directly identify TFPIbeta in tissues.
TFPIgamma is a C-terminally truncated form of TFPI found only in mice12. It has the first two Kunitz domains. Alternatively splicing occurs at the same 5’ splice acceptor site as TFPIbeta, just prior to the third Kunitz domain, but the 3’ splice acceptor site is 276 nucleotides past the TFPIbeta stop codon within the TFPIbeta 3’ untranslated region12. This produces a protein sequence with 18 amino acids not present in TFPIalpha or TFPIbeta. As with TFPIbeta, attempts to make high affinity antibodies that recognize the unique C-terminal region of TFPIgamma have not been successful making it difficult to directly identify TFPIgamma in tissues. The C-terminal region of TFPIgamma does not encode a predicted GPI-anchor attachment sequence and following transfection into CHO cells, it is processed as a secreted protein12.
TFPIdelta is a C-terminally truncated form of TFPI. Sequences are present within the NCBI GenBank database, but no other information about this isoform has been published to date. Alternative splicing occurs immediately following the second Kunitz domain and a sequence encoding a new C-terminal region of 12 amino acids is present.
4. EVOLUTION OF ALTERNATIVELY SPLICED ISOFORMS OF TFPI
TFPIalpha is well conserved from man to zebra fish who last shared a common ancestor 430 million years ago. The third Kunitz domain has maintained a similar high degree of sequence identity as the first and second Kunitz domains suggesting it has an important biological function13. In contrast, TFPIbeta-specific sequence has been identified in humans, other primates, mice, and rats13. This is not surprising because TFPIbeta sequence conservation between man and mouse is fairly poor (43%) with the exception of the 7 amino acid region proximal to the predicted GPI-anchor modification site. Analysis of zebrafish genomic sequence between the exons encoding K2 and K3 revealed no potential alternative exon that might encode the TFPIbeta specific or the TFPIgamma specific sequence. This suggests that TFPIbeta represents a ‘recent’ evolutionary adaptation whereas TFPIalpha existed prior to the divergence of the boney fish over 430 million years ago.
5. TISSUE EXPRESSION OF ALTERNATIVELY SPLICED FORMS OF TFPI
Examination of the expression of TFPIalpha and TFPIbeta mRNA in human and mouse tissues and endothelial cell lines using real time PCR revealed that each tissue has more message for TFPIalpha than TFPIbeta. This ranges from 4- to 50-fold with message for TFPIalpha on average 10-fold more abundant than message for TFPIbeta11, 12. At the level of protein production, it appears that TFPIalpha is the major TFPI isoform produced in humans. TFPIalpha protein has been identified in human platelets3 and placenta17. As mentioned, human plasma contains TFPIalpha and the heparin-releasable form of TFPI is TFPIalpha20. TFPI beta protein has not been identified in humans. The lack of a high affinity antibody that recognizes TFPIbeta but not TFPIalpha makes it difficult to differentiate TFPIbeta from partially degraded forms of TFPIalpha. Further studies are needed to definitively define the alternatively spliced isoforms of TFPI produced in different human vascular beds.
In mice, the placenta and embryo produce both TFPIalpha and TFPIbeta protein with placenta producing much more TFPIalpha than TFPIbeta and embryo producing approximately equal amounts of each13. However, as mice mature, the production of TFPIalpha decreases and adult mice produce predominantly TFPIbeta protein in all major vascular beds13. Mouse plasma contains essentially only TFPIbeta and heparin infusion has only minimal effect on the plasma TFPI concentration13. This finding is consistent with the structure of TFPIbeta, which lacks the basic C-terminal region responsible for association of TFPIalpha with glycosaminoglycans in human vasculature. Interestingly, it appears that mouse platelets make only TFPIalpha (Maroney SA et al., ATVB 2011 In Press). Thus, adult mice produce TFPIbeta in all tissues expect for platelets that make TFPIalpha. TFPIgamma and TFPIdelta protein have thus far not been identified in vivo12. Since TFPIalpha message is more abundant than TFPIbeta message in adult mouse tissues, control of TFPI protein production in mice occurs during mRNA translation.
6. PERSPECTIVE: POTENTIAL FOR DIFFERENTIAL FUNCTION OF ALTERNATIVELY SPLICED ISOFORMS OF TFPI
There is accumulating evidence that alternative splicing of TFPI produces physiologically relevant changes in TFPI activity. While all TFPI isoforms have the first two Kunitz domains and are capable of inhibiting TF-fVIIa and fXa catalytic activity, it is unclear whether they have relative equal inhibitory activity in vivo or whether they differentially inhibit TF procoagulant and pro-inflammatory activities. The evolutionary conservation of TFPIalpha and its conserved production by mouse embryo, placenta and platelets suggests that the third Kunitz domain and basic C-terminal region may have specific functions during vascular development and/or wound healing not performed by the other isoforms. The third Kunitz domain and C-terminal region of soluble TFPIalpha directly interact with fXa21, perhaps partially explaining the enhanced anticoagulant activity of TFPIalpha compared to truncated forms of TFPI in solution phase assays22–24. The anticoagulant activity of TFPIalpha is enhanced through interactions between the third Kunitz domain and protein S that produces enhanced inhibition of fXa by the second Kunitz domain25, 26, but not of TF-fVIIa by the first Kunitz domain in solution phase assays27. Further studies are needed to determine how protein S may enhance the activity of surface associated TFPIalpha. This enhancing effect of protein S on inhibition of fXa was not observed in mouse plasma, a finding that is consistent with TFPIbeta as the major alternatively spliced form of TFPI in adult mice.
Several studies using in vitro and in vivo assays have reported physiological activities of peptides corresponding to the basic C-terminal region of TFPI that occur independent of TFPI anticoagulant activity further demonstrating that TFPIalpha may have unique functions not performed by the other isoforms. These include studies demonstrating that the TFPIalpha C-terminal peptide inhibits endothelial cell proliferation in vitro as well as primary and metastatic tumor growth in vivo28. The C-terminal peptide has also been shown to have complement dependent antibacterial activity29, 30. It is important to note that these studies have been performed using soluble forms of TFPI. Yet, most TFPI within the body is associated with cell surfaces which can greatly alter its activity. Further studies of the biological activity of the different alternatively spliced isoforms associated with cell surfaces are needed to understand their physiological functions.
Figure 2.
The amino acid sequence and Kunitz domain structure of human TFPI beta. Red circles indicate acidic amino acids. Blue circles indicate basic amino acids. Yellow circles are cysteine residues.
Figure 3.
The amino acid sequence and Kunitz domain structure of human TFPI gamma. Red circles indicate acidic amino acids. Blue circles indicate basic amino acids. Yellow circles are cysteine residues.
REFERENCES
- 1.Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured Normal Human Hepatocytes do not Synthesize Lipoprotein-Associated Coagulation Inhibitor: Evidence that Endothelium is the Principal Site of Its Synthesis. PNAS. 1990;87:8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- 3.Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Purification and characterization of the lipoprotein-associated coagulation inhibitor from human plasma. J Biol Chem. 1989;264:18832–71883. [PubMed] [Google Scholar]
- 5.Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 6.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105:2777–2782. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 7.Chan JC, Carmeliet P, Moons L, Rosen ED, Huang ZF, Broze GJ, Jr, Collen D, Castellino FJ. Factor VII deficiency rescues the intrauterine lethality in mice associated with a tissue factor pathway inhibitor deficit. J Clin Invest. 1999;103:475–482. doi: 10.1172/JCI5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wun TC, Kretzmer KK, Girard TJ, Miletich JP, Broze GJ., Jr Cloning and characterization of a cDNA coding for the lipoprotein- associated coagulation inhibitor shows that it consists of three tandem Kunitz-type inhibitory domains. J Biol Chem. 1988;263:6001–6004. [PubMed] [Google Scholar]
- 9.Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 10.Chang JY, Monroe DM, Oliver JA, Roberts HR. TFPIbeta, a second product from the mouse tissue factor pathway inhibitor TFPI) gene. Thromb Haemost. 1999;81:45–49. [PubMed] [Google Scholar]
- 11.Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108:623–627. doi: 10.1161/01.CIR.0000078642.45127.7B. 2003. [DOI] [PubMed] [Google Scholar]
- 12.Maroney SA, Ferrel JP, Collins ML, Mast AE. TFPIgamma is an active alternatively spliced form of TFPI present in mice but not in humans. J Thromb Haemost. 2008;6:1344–1351. doi: 10.1111/j.1538-7836.2008.03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maroney SA, Ferrel JP, Pan S, White TA, Simari RD, McVey JH, Mast AE. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7:1106–1113. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroney SA, Ellery PE, Mast AE. Alternatively spliced isoforms of tissue factor pathway inhibitor. Thromb Res. 2010;125(Suppl 1):S52–S56. doi: 10.1016/j.thromres.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piro O, Broze GJ., Jr Role for the Kunitz-3 Domain of Tissue Factor Pathway Inhibitor-{alpha} in Cell Surface Binding. Circulation. 2004;110:3567–3572. doi: 10.1161/01.CIR.0000148778.76917.89. [DOI] [PubMed] [Google Scholar]
- 16.Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mast AE, Acharya N, Malecha MJ, Hall CL, Dietzen DJ. Characterization of the association of tissue factor pathway inhibitor with human placenta. Arterioscler Thromb Vasc Biol. 2002;22:2099–2104. doi: 10.1161/01.atv.0000042456.84190.f0. [DOI] [PubMed] [Google Scholar]
- 18.Lupu C, Hu X, Lupu F. Caveolin-1 Enhances Tissue Factor Pathway Inhibitor Exposure and Function on the Cell Surface. J Biol Chem. 2005;280:22308–22317. doi: 10.1074/jbc.M503333200. [DOI] [PubMed] [Google Scholar]
- 19.Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor EPI) Thromb Res. 1988;50:803–813. doi: 10.1016/0049-3848(88)90340-4. [DOI] [PubMed] [Google Scholar]
- 20.Novotny WF, Palmier M, Wun TC, Broze GJ, Jr, Miletich JP. Purification and properties of heparin-releasable lipoprotein- associated coagulation inhibitor. Blood. 1991;78:394–400. [PubMed] [Google Scholar]
- 21.Cunningham AC, Hasty KA, Enghild JJ, Mast AE. Structural and functional characterization of tissue factor pathway inhibitor following degradation by matrix metalloproteinase-8. Biochem J. 2002;367:451–458. doi: 10.1042/BJ20020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wesselschmidt R, Likert K, Girard T, Wun TC, Broze GJ., Jr Tissue factor pathway inhibitor: the carboxy-terminus is required for optimal inhibition of factor Xa. Blood. 1992;79:2004–2010. [PubMed] [Google Scholar]
- 23.Lockett JM, Mast AE. Contribution of regions distal to glycine-160 to the anticoagulant activity of tissue factor pathway inhibitor. Biochemistry. 2002;41:4989–4997. doi: 10.1021/bi016058n. [DOI] [PubMed] [Google Scholar]
- 24.Hamamoto T, Yamamoto M, Nordfang O, Petersen JG, Foster DC, Kisiel W. Inhibitory properties of full-length and truncated recombinant tissue factor pathway inhibitor TFPI). Evidence that the third Kunitz-type domain of TFPI is not essential for the inhibition of factor VIIa- tissue factor complexes on cell surfaces. J Biol Chem. 1993;268:8704–8710. [PubMed] [Google Scholar]
- 25.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci U S A. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndonwi M, Tuley EA, Broze GJ., Jr The Kunitz-3 domain of TFPI-alpha is required for protein S-dependent enhancement of factor Xa inhibition. Blood. 2010;116:1344–1351. doi: 10.1182/blood-2009-10-246686. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ndonwi M, Broze G., Jr Protein S enhances the tissue factor pathway inhibitor inhibition of factor Xa but not its inhibition of factor VIIa-tissue factor. J Thromb Haemost. 2008;6:1044–1046. doi: 10.1111/j.1538-7836.2008.02980.x. [DOI] [PubMed] [Google Scholar]
- 28.Hembrough TA, Ruiz JF, Swerdlow BM, Swartz GM, Hammers HJ, Zhang L, Plum SM, Williams MS, Strickland DK, Pribluda VS. Identification and characterization of a very low density lipoprotein receptor-binding peptide from tissue factor pathway inhibitor that has antitumor and antiangiogenic activity. Blood. 2004;103:374–3380. doi: 10.1182/blood-2003-07-2234. [DOI] [PubMed] [Google Scholar]
- 29.Schirm S, Liu X, Jennings LL, Jedrzejewski P, Dai Y, Hardy S. Fragmented tissue factor pathway inhibitor TFPI) and TFPI C-terminal peptides eliminate serum-resistant Escherichia coli from blood cultures. J Infect Dis. 2009;199:1807–1815. doi: 10.1086/599097. [DOI] [PubMed] [Google Scholar]
- 30.Papareddy P, Kalle M, Kasetty G, Morgelin M, Rydengard V, Albiger B, Lundqvist K, Malmsten M, Schmidtchen A. C-terminal peptides of tissue factor pathway inhibitor are novel host defense molecules. J Biol Chem. 2010;285:28387–28398. doi: 10.1074/jbc.M110.127019. [DOI] [PMC free article] [PubMed] [Google Scholar]