Abstract
Alzheimer's disease (AD) is the most common progressive age-related dementia in the elderly and the fourth major cause of disability and mortality in that population. The disease is pathologically characterized by deposition of β-amyloid plaques neurofibrillary tangles in the brain. Current strategies for the treatment of AD are symptomatic only. As such, they are less than efficacious in terms of significantly slowing or halting the underlying pathophysiological progression of the disease. Modulation by cell therapy may be new promising disease-modifying therapy. Recently, we showed reduction in amyloid-β (Aβ) levels/β-amyloid plaques and associated astrocytosis following low-dose infusions of mononuclear human umbilical cord blood cells (HUCBCs). Our current study extended our previous findings by examining cognition via (1) the rotarod test, (2) a 2-day version of the radial-arm water maze test, and (3) a subsequent observation in an open pool platform test to characterize the effects of monthly peripheral HUCBC infusion (1×106 cells/μL) into the transgenic PSAPP mouse model of cerebral amyloidosis (bearing mutant human APP and presenilin-1 transgenes) from 6 to 12 months of age. We show that HUCBC therapy correlates with decreased (1) cognitive impairment, (2) Aβ levels/β-amyloid plaques, (3) amyloidogenic APP processing, and (4) reactive microgliosis after a treatment of 6 or 10 months. As such, this report lays the groundwork for an HUCBC therapy as potentially novel alternative to oppose AD at the disease-modifying level.
Introduction
Alzheimer's disease (ad) is the most common progressive age-related dementia, and is pathologically characterized by the deposition of amyloid-β peptide (Aβ) as amyloid plaques in the brain parenchyma and neurofibrillary tangles (NFTs) within neurons. As a result of the atrophy that occurs in both cortical and subcortical regions, patients suffer cognitive and emotional dysregulation leading eventually to an inability to perform acts of daily living independently and safely. In fact, AD has emerged as a national and international pandemic. According to the World Alzheimer Report 2010, dementia patients account for 35.6 million in worldwide, and are expected to increase to 65.7 million by 2030 and 115.4 million by 2050. Currently, the number of AD patients is around 1% of the world's gross domestic product. Therefore, it is becoming increasingly evident that a more effective treatment or prophylaxes are needed in the near future. This is because Aβ plaques are potent activators of both microglia and astrocytes—central nervous system (CNS) resident immuno-competent cells that respond to cerebral amyloidosis by chronic, pro-inflammatory activation, also known as “inflammaging” (see review [1]). While it was once thought that activation of microglia and astrocytes in AD brains was an epiphenomenon and not a pathoetiological contributor to AD, more recent studies implicate this Aβ-mediated inflammatory cascade as an etiological perpetrator of AD. For example, therapeutic strategies aimed at manipulating this inflammatory cascade, including Aβ immunization, non-steroidal anti-inflammatory drugs, and modulation of microglial activation, are all able to reduce AD-like pathology and improve cognitive impairments in AD transgenic mouse models [2] and, in some cases, reduce AD pathology in humans [3].
While it is true that no model fully recapitulates AD, transgenic animal models pose novel insights into the pathophysiology of Aβ toxicity. This is especially so with regards to the effects of various Aβ species and the probable pathogenic role of Aβ oligomers [4]. In the PSAPP mouse model of cerebral amyloidosis (bearing mutant human APPsw and presenilin-1 transgenes), there are large numbers of compact Aβ plaques in the hippocampus and cerebral cortex. These mice demonstrate greatly accelerated β-amyloid deposition compared with Tg APPsw mice that is apparent as early as 16 weeks of age [5]. Concurrently, they show increased levels of both Aβ1–40 and Aβ1–42 in their parenchyma and a reduced performance of spatial working memory in the period preceding overt Aβ deposition [5]. Such findings support a critical role of Aβ1–42 in the pathogenesis of AD and suggest a neurotoxic effect of soluble forms of Aβ as well [6].
Human umbilical cord blood cells (HUCBCs) have a unique immunomodulatory potential. Therapeutic benefits derived from HUCBC treatment have been suggested to arise from modulation of peripheral inflammatory processes, which in turn affects inflammation in the brain parenchyma, and the mobilization of adult stem cells from the bone marrow (BM) [7–11]. Indeed, in the animal model of stroke, HUCBCs have been shown to promote a strong anti-inflammatory T helper 2 (Th2) response [7], as opposed to the deleterious proinflammatory T helper cell type 1 (Th1) response. Interestingly, this observation was seen in conjunction with reduced infarct volume and very importantly with rescue of neurological deficits [7,12–14].
HUCBC transplantation has been therapeutically beneficial [1] in other neuroinflammatory conditions as well, including Parkinson's disease and amyotrophic lateral sclerosis. At single high dose, HUCBC treatment into an AD preclinical model was even linked to an extension of lifespan [15]. Moreover, HUCBC infusion mediated recovery after brain injury within days [9,16], suggesting that in this scenario, transplanted cells do not infiltrate the site of brain injury and mediate reinnervation.
As mentioned earlier, another potential mechanism by which HUCBCs may confer therapeutic benefit is via modulation and mobilization of endogenous adult stem cell populations from BM that are believed to mature into microglia upon CNS migration [10,11]. Recently, BM-derived cells, especially monocytes/macrophages (MO/MØ), have been shown to be able to cross the blood–brain barrier and differentiate into functional microglia [17–21].
HUCBCs may promote reductions in amyloidosis through several other indirect mechanisms as well. For example, they produce a number of neurotrophic factors, including nerve growth factor (NGF), colony stimulating factor-1 (CSF-1), thrombopoietin, and anti-inflammatory cytokine, interleukin (IL)-11 [22,23]. Conversely, HUCBCs downregulate proinflammatory cytokines, including tumor necrosis factor-α and IL-1β [7]. Thus, therapeutic effects of HUCBC infusion in PSAPP mice might result from this beneficial immune modulation in the periphery.
Recently, we showed reduction in Aβ levels/β-amyloid pathology and associated astrocytosis following low-dose infusions of HUCBCs. In that study [24], we initiated treatment at 6 months of age where appreciable quantities of Aβ deposition can be seen. This correlates to early stage of AD. We then treated groups for 6 or 10 months. Mice were then euthanized at 16 months of age, reflecting late-stage AD. Treatments encompassed short- and long-term immunotherapeutic strategies, yielding significant reductions in Aβ levels/β-amyloid pathology [24].
Since multiple cognitive domains are affected by AD, our current study used a comprehensive battery of locomotor ability and cognitive function tests to characterize the effects of HUCBCs infused throughout most of adult life (from 6 to 12 months of age) into PSAPP mice as an immunotherapeutic strategy for AD. There were 2 prevailing questions driving this current study: (1) how could HUCBCs' effect on manipulating the immune system impact AD? and (2) can HUCBCs influence locomotor ability and cognitive function? Therefore, throughout the course of 10 months, during a period that correlates with adulthood then old age, PSAPP mice underwent a comprehensive battery of locomotor ability and cognitive function tests that were administered 1 week after each HUCBC treatment. Our data demonstrated reductions in cognitive rescue that correlated positively with reduced AD-like pathology.
Materials and Methods
Animals and cytokine level analysis
All procedures, herein, were in accordance with the animal protocol approved by the University of South Florida (USF) Institutional Animal Care and Use Committee. Treatment was initiated at 6 months of age (after appreciable Aβ deposits). Blood was collected by submandibular bleeding before and after treatment at 0, 2, 4, and 6 months to monitor plasma cytokine levels [24]. Before and after treatment, all mice were maintained on a 12-h light/12-h dark cycle at ambient temperature and humidity. The PSAPP transgenic mice and wild-type (WT) littermates were originally obtained from the Jackson Laboratory (Bar Harbor, ME) [25,26]. Given the existence of gender differences in Aβ deposition in this model, we used both males and females. All animals in this study were observed in a blinded, randomized approach.
HUCBC transplantation
The right tail vein was identified and vasodilated using warm water. About 33×106 cells/kg per mouse (n=20: 10♀/10♂) or 3.3 mL phosphate buffered saline (PBS)/kg per mouse control (n=10: 5♀/5♂) was delivered using a 27-gauge needle attached to a 1 mL syringe. This treatment was repeated biweekly for the first month and monthly for the remaining 6 and 10 months, respectively. Mice were then kept for 6 or 10 months in shared cages with ad libitum water and food. All animals survived for the entirety of the transplantation periods with no indication of aberrant cell growth or tumor formation. Mice were randomly assigned into the following 4 groups: Group 1 (n=5, PSAPP/PBS), Group 2 (n=10, PSAPP/HUCBCs), Group 3 (n=5, WT/PBS), and Group 4 (n=10, WT/HUCBCs).
Motor activity and cognitive tests
PSAPP transgenic mice and their WT littermates were divided into 1 of 4 groups and underwent a comprehensive battery of motor activity and cognitive tests administered 1 week after each HUCBC treatment to determine the therapeutic implications of low-dose administrations of HUCBCs over time. Mice were randomly assigned into the following 4 groups: Group 1 (n=5, PSAPP/PBS), Group 2 (n=10, PSAPP/HUCBCs), Group 3 (n=5, WT/PBS), and Group 4 (n=10, WT/HUCBCs).
Rotarod test
The rotarod test was performed for 2 consecutive days. The main objectives of this sensorimotor task are to exclude possibility that positive effects of any treatment in the cognitive tasks are due to differences in sensorimotor ability. The current method is designed to evaluate motor performance without a practice confound. Mice were positioned on the rod (diameter 3.6 cm) of the equipment (Rotarod 7650 accelerating model; Ugo Basile, Biological Research Apparatus, Varese, Italy) to gauge differences in balance and motor coordination. This was accomplished by measuring the balance and coordination of fore- and hind limbs. The rod on the apparatus was set at 1.0 rpm and mice were placed, 5 at a time, each in an assigned location on rod. The rod was allowed to steadily accelerate up to 40.0 rpm over a 3-min session. Mice were then removed and allowed to rest for 30 min until returning to the remaining 3 sessions of the test, yielding a total of 4 trials on day 1. This was repeated on day 2. Evaluation was made by monitoring clinging from the top of the rotating barrel, or by latency to fall. Mice training occurred in 1 trial and this was adequate to attain a performance baseline and to establish habituation. As a precautionary measure against physical damages from potential falls, a soft pad was placed under the equipment.
Radial-arm water maze test
The radial-arm water maze (RAWM) is remarkably robust and is used to examine learning and memory deficits in AD transgenic mouse. This test also optimizes sample size requirements when compared with other typical rodent memory tasks [27]. The RAWM was conducted over 2 days and consisted of triangular wedges in a water pool configured to form swim lanes that enclose a central open space [28]. According to this design, the maze can be organized to independently detect errors in working and reference memory. The mouse was dropped into a random start arm (predetermined on a score sheet) and allowed to swim until it located and climbed onto the platform (goal) over a period of 1 min. Errors accrued when the mouse failed to enter an arm for 15 s. If the mouse was unable to find platform for that time duration, it was guided to the platform. Latency to locate the platform and error numbers were recorded. Once on the platform was found, either by self-discovery or by guidance, the mouse was allowed to rest there for 15 s. Subsequently, the mouse was then removed, towel-dried, and replaced in cage with a heat lamp slightly overhead for a 30-min rest period. Another mouse was selected and the process repeated until all mice were tested. Each mouse was continued this protocol for a total of 16 trials each. On day 1, the goal alternated between being visible and hidden as the trials proceeded for each mouse, while on day 2, the goal was always hidden. The goal arms remained in the same location for both days, while the start arm changed with the randomized position recorded on the score sheets.
Visible platform in an open pool test
This test was conducted during the last day of the test battery to examine whether the animals possess the skills sufficient to complete the water maze task. In brief, it was performed in the same pool for the RAWM; however, the triangular wedges were removed and the pool was left open with a visible platform in an imagined quadrant. This platform has insignias that are visible above the water, while the platform remains slightly below the water [28]. For each trial, the mouse was dropped in the same start location, while the goal was placed in a different location per each mouse. Here, just as in the RAWM test, each mouse was tested and latency to reach the platform was recorded. The test ran for 15 trials. Mice were considered as impaired if they had not accomplished within the 20-s latency criterion for the last 3 trials of the visible platform task and were thus excluded.
Tissue preparation
After all neurocognitive testing was completed, mice were euthanized at either 6 or 10 months after transplantation with isofluorane anesthesia and then transcardially perfused with an ice-cold physiological saline. Prior to transcardial perfusion, hind limbs (for BM) and 500 μL peripheral blood were collected. Brains were rapidly isolated and the left hemispheres were frozen immediately in liquid nitrogen and stored at −80°C. For molecular analysis, the left hemispheres were sonicated in RIPA buffer (Cell Signaling Technology, Danvers, MA) and centrifuged at 14,000 rpm for 1 h at 4°C. Supernatant was transferred to a new tube for soluble Aβ analysis and the pellet was used for insoluble Aβ extraction as described previously [29]. The right hemispheres were placed in 4% paraformaldehyde in 0.1 M PBS at 4°C overnight, and then transferred to a graded series of sucrose solution (10%, 20%, and 30%, each at 4°C overnight) for cryostat sectioning. Sequential 25-μm coronal sections were cut and free-floating sections were then stored at 4°C in 24-well plates containing PBS with 100 mM sodium azide.
Immunohistochemical analysis
Immunohistochemical staining was performed using mouse monoclonal anti-human Aβ17–24 antibody (clone 4G8; Covance Research Products, Emeryville, CA) or rat anti-mouse CD45 antibody (AbD serotec, Raleigh, NC) in conjunction with the VectaStain Elite ABC kit (Vector Laboratories, Burlingame, CA) coupled with diaminobenzidine substrate. For all the staining, a set of sections without adding primary antibody were used as negative staining control. Aβ or CD45 burden was determined by quantitative image analysis. Images of five 25-μm sections (150 μm apart) through hippocampus and neocortex were captured and a threshold optical density was obtained that discriminated staining from background. Data are reported as percentage of immunolabeled area captured (positive pixels divided by total pixels captured). Quantitative image analysis was performed by a single examiner (T.M.) blinded to sample identities.
Enzyme-linked immunosorbance assay
This was performed according to our previous methods [30]. Soluble Aβ40,42 in brain homogenates was detected at 1:10 dilutions. Detergent-insoluble Aβ40,42 was detected in brain homogenates by extracting pellets in 5 M guanidine HCl buffer, followed by a 1:20 dilution in lysis buffer. Aβ40,42 was quantified in all samples using Aβ40,42 ELISA kits (Invitrogen, Grand Island, NY) in strict accordance with manufacturer's instructions. To allow for sample normalization, the BCA protein assay (Thermo Fisher Scientific, Waltham, MA) was performed to measure protein concentration from each brain homogenates assayed prior to quantification of cytokines by enzyme-linked immunosorbance assay (ELISA).
Western blot analysis
Following the sample preparation as described previously, an aliquot corresponding to 40 μg of total protein was electrophoretically separated using 10% Tris-sodium dodecyl sulfate gels or 10%–20% Tris-tricine gels (Bio-Rad, Richmond, CA) and transferred to polyvinylidene fluoride membranes (Bio-Rad). As a positive control, Aβ oligomers were prepared from synthetic human Aβ42 according to published methods [31,32]. Membranes were blocked for 1 h at room temperature in Tris-buffered saline (containing 0.1% Tween 20 with 5% nonfat dry milk) and were then incubated with the following primary antibodies: rabbit anti-APP C-terminus polyclonal antibody (pAb751/770, 1:1,000; Calbiochem, Billerica, MA), mouse monoclonal Aβ1−16 antibody (clone 6E10, 1:2,000; Covance Research Products), or mouse monoclonal β-actin antibody (1:4,000; Sigma-Aldrich, St. Louis, MO). Afterward, membranes were immunoblotted with anti-mouse (1:2,000; Cell Signaling Technology) or anti-rabbit (1:10,000; Thermo Fisher Scientific) IgG secondary antibodies conjugated with horseradish peroxidase. Proteins were detected with Super Signal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) and BIOMAX-MR Film (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis
Data are presented as mean±standard error (SE). In instances of single mean comparisons, Levene's test followed by t-test for independent samples was performed. The other statistics were calculated using one-way analysis of variance for multiple comparisons. A P value of <0.05 was considered significant. The statistical package for the social sciences release 10.0.5 (IBM, Armonk, NY) was used for all data analysis.
Results
HUCBC infusion improves motor skills and spatial working memory
Previous work in a mouse model of stroke has shown that HUCBC infusion results in significant reduction in the infarct volume as well as rescue of neurological deficits associated with decreased proinflammatory cytokine production [19]. Additionally, we previously found reduced parenchymal and vascular β-amyloid deposits in AD mouse model. Thus, here we sought to determine whether HUCBC (95%–98% mononuclear cells) infusion could impact Aβ-associated cognitive deficits in PSAPP AD mouse model. To evaluate whether HUCBC administrations could improve locomotive and cognitive capabilities of transgenic AD mice, PBS-treated and HUCBC-treated PSAPP mice and their WT littermates were assessed for rotarod balance and endurance, RAWM spatial learning, and open pool coordination activity. The rotarod test disclosed a significantly higher endurance or “riding time” for the HUCBC-treated PSAPP mice (Group 2) and their HUCBC-treated WT littermates (Group 4) versus their WT littermates (Group 3) or PSAPP mice treated with PBS (Group 4), respectively. All mice, with the exception of the PBS-treated PSAPP animals (Group 1), exhibited increased riding times. Further, PSAPP mice infused with HUCBCs over a period of 10 months showed overall greater balance and coordination abilities than their WT littermates or PSAPP mice treated with PBS.
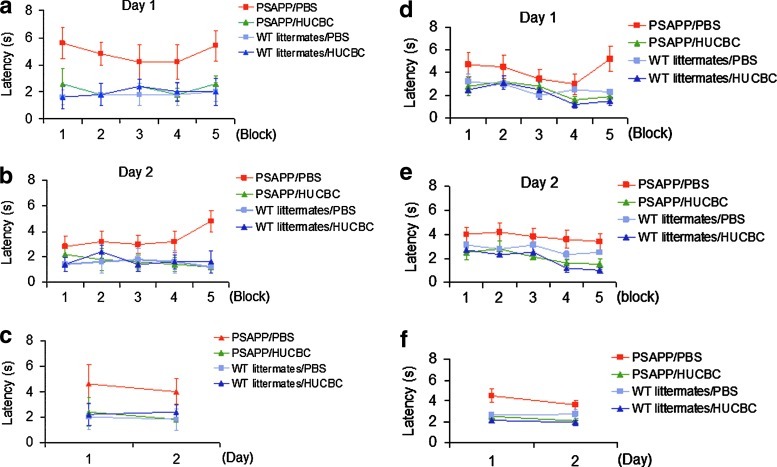
At 12 and 16 months of age, HUCBC-infused PSAPP mice exhibited a significant elevation in memory function in the RAWM—hippocampus-dependent cognitive test. Compared with control mice (WT littermates/PBS), HUCBC-treated mice displayed superior cognitive agility to locate and swim to the goal arm of the maze with less errors (shorter latency; Fig. 1a–c, 6-month treatment; Fig. 1d, e, 10-month treatment). This finding shows that Aβ-associated spatial memory cognitive impairments can be rescued by infusion of HUCBCs. The above results were further reinforced using the open pool platform test to assess whether the animals possessed the motor skills sufficient to complete the water maze tasks. Notably, at both 6- and 10-month time points, animals treated with HUCBCs were able to perform similarly to control animals (WT littermates/PBS) with less errors (shorter latency) compared with PSAPP positive controls.
FIG. 1.
HUCBC infusion reverses working memory deficits in PSAPP transgenic mice. PSAPP mice and WT littermates were treated with a monthly HUCBC injection of 33×106 cells/kg per mouse or 3.3 mL PBS/kg per mouse for 6 (a–c) or 10 months (d–f), followed by neurocognitive testing in a 2-day version of the radial-arm water maze (RAWM). In overall working memory performance, WT littermates/PBS, WT littermates/HUCBC, and PSAPP/HUCBC mice reveal significantly lower escape latencies than PSAPP/PBS mice (P<0.05). HUCBC, human umbilical cord blood cell; WT, wild-type; PBS, phosphate buffered saline. Color images available online at www.liebertpub.com/scd
Cerebral parenchymal β-amyloid plaques are reduced in AD transgenic mice peripherally infused with HUCBCs
Here, we correlated whether HUCBC infusion could impact Aβ-associated pathology in concert with observed cognitive improvements in PSAPP mice. Each group was evaluated for both soluble and insoluble Aβ40,42 cerebral levels by ELISA. When compared with PBS-treated PSAPP mice, analyses of brain homogenates from HUCBC-treated PSAPP mice at both 6- and 10-month treatment groups exhibited significant decreased levels of both soluble (Fig. 2a, b) and insoluble (Fig. 2c, d) Aβ40 and Aβ42. A t-test revealed a significant difference in the cerebral parenchyma regions between HUCBC-infused versus PBS-treated mice.
FIG. 2.
HUCBC treatment results in decreased Aβ and increased anti-amyloidogenic APP processing. Brain homogenates from both 6- and 10-month treatments were evaluated using Aβ ELISA and immunoblotting analyses. These analyses show decreased levels of both soluble (a, b) and insoluble (c, d) Aβ40 and Aβ42 in brain homogenates prepared from PSAPP/HUCBC mice in both 6- and 10-month treatment groups when compared with PSAPP/PBS controls [n=10 (5♀/5♂)]. A t-test revealed a significant difference between PBS-injected and HUCBC-infused conditions, in both soluble Aβ40,42 and insoluble Aβ40,42 [n=10 (5♀/5♂)]. Aβ species and β-CTF were analyzed in mouse brain homogenates from (e) 6-month treatment and (f) 10-month treatment group using monoclonal Aβ1–16 antibody (6E10). (g) Densitometry analysis shows the ratio of Aβ to β-actin. *P<0.05; ***P<0.001. Aβ, amyloid-β; ELISA, enzyme-linked immunosorbance assay; CTF, C-terminal fragment.
To corroborate these findings, an immunoblotting (IB) analysis for soluble Aβ levels and APP proteolytic products was performed. We conducted an analysis of mouse brain homogenates using monoclonal Aβ1–16 antibody (6E10) to analyze Aβ species and β-C-terminal fragments (β-CTFs), from both the 6- and 10-month treated groups. The 6E10 antibody specifically recognizes APP and β-CTFs. IB with this antibody showed hippocampal reductions in β-CTF and Aβ species in both HUCBC-infused PSAPP mice compared with PBS-treated mice (Fig. 2e, 6-month treatment; Fig. 2f, 10-month treatment). To substantiate that 6E10 IB accurately reflected the quantitative change in Aβ protein levels in the brain, the area of pyramidal cell layer immunoreactive to 6E10 was quantified and standardized in regard to the amount of total β-actin protein. As to the ratio of Aβ levels to β-actin (n=10: 5♀/5♂ for each group), densitometric analysis proved to be statistically significant. At both time points, Aβ species were significantly decreased (Fig. 2g). This reflects the widely accepted notion of an upregulation of Aβ40 and Aβ42 early on in AD. Further, cognitive decline robustly connects with this elevation of Aβ40 and Aβ42. Indeed, cognitive degeneration [33,34] has been associated with the long and the most toxic isoform of Aβ, Aβ42, and this isoform has also been shown to elicit a strong proinflammatory immunological response in the brain [18].
To evaluate reductions of Aβ plaques in HUCBC-infused mice brains, we performed immunohistochemistry using monoclonal Aβ17–24 antibody (4G8) staining. Mouse brain sections from both 6- and 10-month HUCBC-treated PSAPP mice were stained with 4G8 antibody at 12 and 16 months of age, respectively. It was observed that there was a distinct reduction of cerebral Aβ pathology as detected by 4G8 staining in HUCBC-infused PSAPP mice. A marked reduction in amyloid plaques was seen in the hippocampi of both 6- and 10-month HUCBC-infused PSAPP mice compared with 6- or 10-month-old PBS-infused PSAPP mice (Fig. 3a). Quantitative image analysis for the percentage of 4G8 immunoreactive plaques from hippocampal brain regions [mean±SE, n=10 (5♂/5♀ per group)] of PSAPP mice infused with HUCBCs (PSAPP/HUCBCs) and PSAPP mice peripherally infused with PBS (PSAPP/PBS) revealed substantial differences between groups (Fig. 3b). A t-test for independent samples revealed significances between groups for the 10-month, HUCBC-infused PSAPP mice while the 6-month treatment group did not reach significance. Specifically, these results suggest that, in vivo, HUCBC infusion lowers Aβ pathology in PSAPP mice after 10 months of treatment.
FIG. 3.
HUCBC infusion reduces β-amyloid pathology in PSAPP mice. (a) Mouse brain sections from both 6- and 10-month treated PSAPP mice with HUCBCs were stained with anti-Aβ17–24 antibody (4G8: brown) at 12 and 16 months of age. (b) Percentage of Aβ 4G8 antibody immunoreactive plaques from hippocampus [mean±SE; n=10 (5♀/5♂ per group)] was quantified by image analysis. A t-test for independent samples revealed significances between groups (**P<0.01). SE, standard error. Color images available online at www.liebertpub.com/scd
HUCBC infusion mitigates microgliosis in PSAPP mice
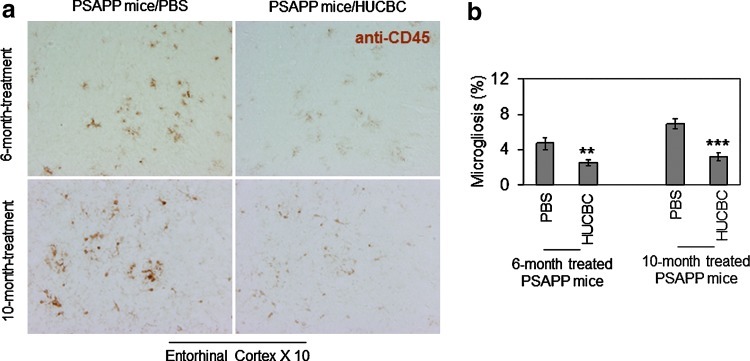
There has been much accumulated evidence that activation of microglia is a crucial event mediating inflammatory responses in AD brains [35,36]. Therefore, after Aβ pathology assessment, sections of mouse brains from 12- and 16-month-old HUCBC-treated PSAPP mice (from both the 6- and 10-month infusion regimes) were stained with monoclonal anti-CD45 antibody. HUCBC infusion in PSAPP mice resulted in marked reduction of microgliosis (Fig. 4a) as assayed by monoclonal anti-CD45 antibody immunohistochemistry. Quantitative image analysis comparison of PSAPP/HUCBC to PSAPP/PBS mice, in terms of entorhinal cortex regions that comprised CD45 positive cells per mouse [mean±SE, n=10 (5♂/5♀ per group)], showed striking significances (Fig. 4b). A t-test for independent samples confirmed that the expression of CD45 in microglia was significantly reduced in the hippocampi of both 6- and 10-month-old PSAPP/HUCBC groups compared with 6- and 10-month PSAPP/PBS groups. In vivo, these findings provide lucid evidence that indicates that microglial activation, subsequent to either a 6- or 10-month HUCBC infusion, is significantly lowered in PSAPP mice.
FIG. 4.
HUCBC infusion reduces microgliosis in PSAPP mice. (a) Mouse brain sections from both 6- and 10-month treated PSAPP mice with HUCBCs were stained with anti-CD45 antibody (brown) at 12 and 16 months of age. (b) Percentage of CD45 positive microglial cells from entorhinal cortex [mean±SE; n=10 (5♀/5♂ per group)] was quantified by image analysis. A t-test for independent samples revealed significances between groups (**P<0.01; ***P<0.001). Color images available online at www.liebertpub.com/scd
Collectively, these results suggest that multiple low-dose infusions of HUCBCs are effective in reducing Aβ deposits and in rescuing cognitive impairments. Moreover, these effects persist past the finishing point of a 10-month regimen of HUCBC treatments.
Discussion
An array of biochemical, genetic, and postmortem evidence suggests that Aβ peptides are key etiological contributors to AD pathogenesis [37]. For example, recent studies in a mouse model of stroke showed that HUCBC transplantation results in significant reduction in the infarct volume as well as rescue of neurological deficits associated with decreased production of proinflammatory cytokines [7]. We previously showed that HUCBC (95%–98% mononuclear cells) infusion could impact Aβ-associated pathology in PSAPP mice [38]. It has been shown that Aβ peptides mediate proinflammatory and neurodegenerative changes and oligomeric forms of the peptide are neurotoxic as well as synapse toxic [39]. Indeed, it is well established that brain inflammatory mechanisms mediated by reactive glia are activated in response to Aβ plaques [40–42] and these changes could negatively affect cognitive function.
For this work, starting at 6 months of age, animals were given monthly tail vein injections of either HUCBCs or PBS. After each administration, mice underwent a comprehensive battery of motor ability and cognitive tests to investigate the efficacy of multiple low-dose HUCBC administrations as an immunotherapeutic strategy for AD. At 12 and 16 months of age, after completion of neurocognitive analyses, mice were euthanized and evaluated AD-like pathology.
The 6-month-old PSAPP mice represent a very well-known period of appreciable Aβ deposits. Though Aβ is ubiquitous in most cells of the body, its formation of plaques and the pathogenic chain of events contributing to neurodegeneration and dementia currently remain elusive. Most interventions address one or more of the following therapeutic approaches: preventing oligomerization of Aβ and deposition, enhancing Aβ degradation, targeting Aβ neurotoxicity directly or indirectly, preventing or reducing Aβ production, and improving Aβ efflux from the brain into the peripheral blood plasma [43]. We chose to administer multiple low doses of HUCBCs based on our optimized method for reduction of vascular and parenchymal deposits used previously [24]. In addition, while it is likely that toxicity and other safety issues, such as tumors and immunization problems, may arise from high doses, low doses may be more efficacious for translation to humans.
In AD, inflammatory responses mediated by glial cells are upregulated by Aβ plaque deposition and this increase is believed to mediate neuronal injury [36,41,42,44–47] and finally cognitive decline [48]. CD45 is a marker of microglia in the brain. We found that HUCBCs reduced the number of these cells in the brain. As microglia are central to the inflammatory process of the disease, their observed reduction upon HUCBC infusion holds strong potential therapeutic value. Further, in regard to the anti-inflammatory potential of HUCBCs, we and others previously found expression profiles of 2 proinflammatory molecules implicated in dementia [29,49–52], CD40 and CD40L. Both are markedly increased in and around Aβ plaques in AD patients and in mouse models of the disease [53,54] and were mitigated by HUCBC infusion [24].
Indeed, we have previously suggested that transplanted HUCBCs confer their effect on reducing cerebral amyloidosis by causing the host to secrete a soluble factor or factors. This may act to reduce the CD40L–CD40 interaction on microglia which in turn promotes Aβ microglial clearance. This mechanism is supported by our previous observation of decreased brain levels of soluble form of CD40L in HUCBC-infused PSAPP mice, and by increased Aβ phagocytosis/removal by microglia cultured in the presence of HUCBC-infused PSAPP mouse sera or cultured from adult HUCBC-treated PSAPP mice, and our previous observations that microglial CD40 ligation shifts these cells away from an Aβ phagocytic phenotype and toward a proinflammatory response (see review [44,50,51]). Future studies designed to characterize specific soluble factors crucial for these therapeutic effects to identify a pharmacotherapeutic target or targets(s).
In addition, previous works indicated that a direct microglial stimulation of naive CD4+T cells is dampened by HUCBC-induced inhibition of microglial activation [8,55,56]. This in turn will also yield less of the co-stimulatory proinflammatory molecules and CD40. Together, these could prevent infiltrating T cells from being activated and thus downregulate proinflammatory secretions [8,45,57].
HUCBC studies done in vitro have shown that these cells secrete soluble factors that have salutary effects [16,58]. Cultured HUCBC supernatants, for example, stimulate survival of neural cells and peripheral blood mononuclear cells cultured under conditions designed to induce cell stress and limit protein synthesis [12]. Moreover, HUCBCs have the capacity to stimulate generation of a vast amount of cytokines and neurotrophic factors that modify inflammatory responses, including IL-11, CSF-1, NGF, and thrombopoietin [7,22,23]. It has been reported that HUCBC entry into the brain is not required to promote neuroprotection [59]. According to the report just outlined, recovery following brain injury is mediated through peripheral anti-inflammatory responses resulting in brain recovery [9]. This is in accord with our results that indicated more of a peripheral, HUCBC-mediated CNS affect, since the cells were not detected in the mouse brain for any significant amount of time.
On the other hand, it should be noted that it has been shown that after irradiation, peripheral macrophages are able to penetrate the brain and mitigate cerebral amyloidosis in AD mice, implying that hematogenously derived macrophages are efficient at phagocytosing and clearing Aβ deposits [18]. Nevertheless, earlier reports have shown that Aβ can also be phagocytosed or cleared by brain-resident microglia [58,60,61].
In the current experimental paradigm, we did not detect the presence of brain-infiltrating macrophages. Specifically, we stained for CD45 (a marker for both macrophages and microglia), and observed that in and around Aβ plaques there were process-bearing cells that morphologically resembled microglia. Further, vascular “cuffing” that would suggest the presence of infiltrating macrophages that are frequently observed in other CNS inflammatory conditions, such as experimental autoimmune encephalomyelitis [62], was not detected. Also, given the difficulties inherent to distinguishing macrophages from microglia, and the ease of peripheral macrophages to engraft into the brain, as well as changes of microglial phenotype after brain injury [63], it remains possible that peripheral macrophages contribute to decreased cerebral amyloidosis after treatment with HUCBCs.
In this report, we have demonstrated that HUCBC infusion decreases Aβ/β-amyloid pathology in the brain parenchyma, reduces brain inflammation evidenced by reduction of activated microglia, and improves cognitive impairments associated with the AD-like pathology in PSAPP mice. These HUCBC-imparted beneficial effects, which correlate with increased brain-to-blood efflux of Aβ and a shift from proinflammatory Th1 to anti-inflammatory Th2 cytokines both in the brain and in the periphery, are similar to what we observed in previous studies after Aβ immunization [64–66]. When taken together, our results provide the basis for a novel immunomodulatory strategy for AD using HUCBCs. While the exact mechanism of efficacy of multiple low-dose HUCBC infusions in AD patients is currently being elucidated, further studies investigating which HUCBC secreted factors are capable of modulating neuroinflammation, reducing AD-like pathology, and rescuing cognitive impairments will need to be explored.
Acknowledgments
This work was supported by the NIH/NIA [R01AG032432 and R42AG031586 (J.T.)]. We would like to thank Md Shahaduzzaman for helpful discussion.
Author Disclosure Statement
P.R.S. is a cofounder and J.T. is a consultant for Saneron CCEL Therapeutics, Inc., and are inventors on a patent application submitted by USF. P.R.S. was not involved in any data acquisition and analysis.
References
- 1.Giunta B. Fernandez F. Nikolic WV. Obregon D. Rrap E. Town T. Tan J. Inflammaging as a podrome to Alzheimer's disease. J Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biscaro B. Lindvall O. Tesco G. Ekdahl CT. Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer's disease. Neurodegener Dis. 2012;9:187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lue LF. Walker DG. Rogers J. Modeling microglial activation in Alzheimer's disease with human postmortem microglial cultures. Neurobiol Aging. 2001;22:945–956. doi: 10.1016/s0197-4580(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 4.Elder GA. Gama Sosa MA. De Gasperi R. Transgenic mouse models of Alzheimer's disease. Mt Sinai J Med. 2010;77:69–81. doi: 10.1002/msj.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb L. Gordon MN. McGowan E. Yu X. Benkovic S. Jantzen P. Wright K. Saad I. Mueller R, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffer EL. Figueiro M. Gattaz WF. Insights into Alzheimer disease pathogenesis from studies in transgenic animal models. Clinics (Sao Paulo) 2011;66(suppl 1):45–54. doi: 10.1590/S1807-59322011001300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vendrame M. Cassady J. Newcomb J. Butler T. Pennypacker KR. Zigova T. Sanberg CD. Sanberg PR. Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 8.Vendrame M. Gemma C. de Mesquita D. Collier L. Bickford PC. Sanberg CD. Sanberg PR. Pennypacker KR. Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 9.Sanberg PR. Willing AE. Garbuzova-Davis S. Saporta S. Liu G. Sanberg CD. Bickford PC. Klasko SK. El-Badri NS. Umbilical cord blood-derived stem cells and brain repair. Ann N Y Acad Sci. 2005;1049:67–83. doi: 10.1196/annals.1334.008. [DOI] [PubMed] [Google Scholar]
- 10.Taguchi A. Soma T. Tanaka H. Kanda T. Nishimura H. Yoshikawa H. Tsukamoto Y. Iso H. Fujimori Y, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns K. Finklestein SP. Growth factors and stem cells as treatments for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S135–S142. doi: 10.1016/s1047-9651(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 12.El-Badri NS. Hakki A. Saporta S. Liang X. Madhusodanan S. Willing AE. Sanberg CD. Sanberg PR. Cord blood mesenchymal stem cells: potential use in neurological disorders. Stem Cells Dev. 2006;15:497–506. doi: 10.1089/scd.2006.15.497. [DOI] [PubMed] [Google Scholar]
- 13.Garbuzova-Davis S. Gografe SJ. Sanberg CD. Willing AE. Saporta S. Cameron DF. Desjarlais T. Daily J. Kuzmin-Nichols N, et al. Maternal transplantation of human umbilical cord blood cells provides prenatal therapy in Sanfilippo type B mouse model. FASEB J. 2006;20:485–487. doi: 10.1096/fj.05-4684fje. [DOI] [PubMed] [Google Scholar]
- 14.Henning RJ. Burgos JD. Ondrovic L. Sanberg P. Balis J. Morgan MB. Human umbilical cord blood progenitor cells are attracted to infarcted myocardium and significantly reduce myocardial infarction size. Cell Transplant. 2006;15:647–658. doi: 10.3727/000000006783981611. [DOI] [PubMed] [Google Scholar]
- 15.Ende N. Chen R. Ende-Harris D. Human umbilical cord blood cells ameliorate Alzheimer's disease in transgenic mice. J Med. 2001;32:241–247. [PubMed] [Google Scholar]
- 16.Newman MB. Davis CD. Kuzmin-Nichols N. Sanberg PR. Human umbilical cord blood (HUCB) cells for central nervous system repair. Neurotox Res. 2003;5:355–368. doi: 10.1007/BF03033155. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor GF. Tauchi T. Ohyashiki K. Matsumiya T. Enriched levels of erythropoietin in human umbilical cord blood stimulate hematopoietic progenitor cells. J Biochem Mol Biol Biophys. 2002;6:65–70. doi: 10.1080/10258140290010214. [DOI] [PubMed] [Google Scholar]
- 18.Simard AR. Soule D. Gowing G. Julien JP. Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Hess P. Altenhöfer A. Salam Khan A. Daryab N. Sik Kim K. Hacker J. Oelschlaeger TA. A salmonella fim homologue in Citrobacter freundii mediates invasion in vitro and crossing of the blood-brain barrier in the rat pup model. Infect Immun. 2004;72:5298–5307. doi: 10.1128/IAI.72.9.5298-5307.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naert G. Rivest S. Hematopoietic CC-chemokine receptor 2 (CCR2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. Mol Med. 2012;18:297–313. doi: 10.2119/molmed.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H. Sun J. Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol. 2008;82:7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suen Y. Lee SM. Schreurs J. Knoppel E. Cairo MS. Decreased macrophage colony-stimulating factor mRNA expression from activated cord versus adult mononuclear cells: altered posttranscriptional stability. Blood. 1994;84:4269–4277. [PubMed] [Google Scholar]
- 23.McGowan E. Sanders S. Iwatsubo T. Takeuchi A. Saido T. Zehr C. Yu X. Uljon S. Wang R, et al. Amyloid phenotype characterization of transgenic mice overexpressing both mutant amyloid precursor protein and mutant presenilin 1 transgenes. Neurobiol Dis. 1999;6:231–244. doi: 10.1006/nbdi.1999.0243. [DOI] [PubMed] [Google Scholar]
- 24.Nikolic WV. Hou H. Town T. Zhu Y. Giunta B. Sanberg CD. Zeng J. Luo D. Ehrhart J, et al. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Alloza M. Robbins EM. Zhang-Nunes SX. Purcell SM. Betensky RA. Raju S. Prada C. Greenberg SM. Bacskai BJ. Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Jankowsky JL. Slunt HH. Ratovitski T. Jenkins NA. Copeland NG. Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 27.Arendash GW. King DL. Gordon MN. Morgan D. Hatcher JM. Hope CE. Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 28.Alamed J. Wilcock DM. Diamond DM. Gordon MN. Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 29.Tan J. Town T. Crawford F. Mori T. DelleDonne A. Crescentini R. Obregon D. Flavell RA. Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 30.Rezai-Zadeh K. Shytle D. Sun N. Mori T. Hou H. Jeanniton D. Ehrhart J. Townsend K. Zeng J, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh DM. Tseng BP. Rydel RE. Podlisny MB. Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 32.Lesné S. Koh MT. Kotilinek L. Kayed R. Glabe CG. Yang A. Gallagher M. Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ. Lee JK. Lee H. Carter JE. Chang JW. Oh W. Yang YS. Suh JG. Lee BH. Jin HK. Bae JS. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Koyama A. Okereke OI. Yang T. Blacker D. Selkoe DJ. Grodstein F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benzing WC. Wujek JR. Ward EK. Shaffer D. Ashe KH. Younkin SG. Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 36.Bornemann KD. Staufenbiel M. Transgenic mouse models of Alzheimer's disease. Ann N Y Acad Sci. 2000;908:260–266. doi: 10.1111/j.1749-6632.2000.tb06653.x. [DOI] [PubMed] [Google Scholar]
- 37.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 38.DeMattos RB. Bales KR. Cummins DJ. Paul SM. Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 39.Malinin NL. Wright S. Seubert P. Schenk D. Griswold-Prenner I. Amyloid-beta neurotoxicity is mediated by FISH adapter protein and ADAM12 metalloprotease activity. Proc Natl Acad Sci U S A. 2005;102:3058–3063. doi: 10.1073/pnas.0408237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christie R. Yamada M. Moskowitz M. Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158:1065–1071. doi: 10.1016/S0002-9440(10)64053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eikelenboom P. van Gool WA. Neuroinflammatory perspectives on the two faces of Alzheimer's disease. J Neural Transm. 2004;111:281–294. doi: 10.1007/s00702-003-0055-1. [DOI] [PubMed] [Google Scholar]
- 42.Rozemuller AJ. van Gool WA. Eikelenboom P. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer's disease: therapeutic implications. Curr Drug Targets CNS Neurol Disord. 2005;4:223–233. doi: 10.2174/1568007054038229. [DOI] [PubMed] [Google Scholar]
- 43.Bates KA. Verdile G. Li QX. Ames D. Hudson P. Masters CL. Martins RN. Clearance mechanisms of Alzheimer's amyloid-beta peptide: implications for therapeutic design and diagnostic tests. Mol Psychiatry. 2009;14:469–486. doi: 10.1038/mp.2008.96. [DOI] [PubMed] [Google Scholar]
- 44.Townsend KP. Town T. Mori T. Lue LF. Shytle D. Sanberg PR. Morgan D. Fernandez F. Flavell RA. Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- 45.Lixian J. Saporta S. Chen N. Sanberg CD. Sanberg PR. Willing A. The effects of human umbilical cord blood cells on survival and cytokine production by post-ischemic astrocytes in vitro. Stem Cell Rev. 2010;6:523–531. doi: 10.1007/s12015-010-9174-x. [DOI] [PubMed] [Google Scholar]
- 46.Lue LF. Walker DG. Modeling Alzheimer's disease immune therapy mechanisms: interactions of human postmortem microglia with antibody-opsonized amyloid beta peptide. J Neurosci Res. 2002;70:599–610. doi: 10.1002/jnr.10422. [DOI] [PubMed] [Google Scholar]
- 47.Suo Z. Tan J. Placzek A. Crawford F. Fang C. Mullan M. Alzheimer's beta-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- 48.Naert G. Rivest S. The role of microglial cell subsets in Alzheimer's disease. Curr Alzheimer Res. 2011;8:151–155. doi: 10.2174/156720511795256035. [DOI] [PubMed] [Google Scholar]
- 49.Tan J. Town T. Mullan M. CD40-CD40L interaction in Alzheimer's disease. Curr Opin Pharmacol. 2002;2:445–451. doi: 10.1016/s1471-4892(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 50.Giunta B. Figueroa KP. Town T. Tan J. Soluble CD40 ligand in dementia. Drugs Future. 2009;34:333–340. doi: 10.1358/dof.2009.034.04.1358595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giunta B. Rezai-Zadeh K. Tan J. Impact of the CD40-CD40L dyad in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:149–155. doi: 10.2174/187152710791012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Town T. Tan J. Mullan M. CD40 signaling and Alzheimer's disease pathogenesis. Neurochem Int. 2001;39:371–380. doi: 10.1016/s0197-0186(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 53.Calingasan NY. Erdely HA. Altar CA. Identification of CD40 ligand in Alzheimer's disease and in animal models of Alzheimer's disease and brain injury. Neurobiol Aging. 2002;23:31–39. doi: 10.1016/s0197-4580(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 54.Togo T. Akiyama H. Kondo H. Ikeda K. Kato M. Iseki E. Kosaka K. Expression of CD40 in the brain of Alzheimer's disease and other neurological diseases. Brain Res. 2000;885:117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- 55.Riordan NH. Chan K. Marleau AM. Ichim TE. Cord blood in regenerative medicine: do we need immune suppression? J Transl Med. 2007;5:8. doi: 10.1186/1479-5876-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez-Ramos JR. Song S. Kamath SG. Zigova T. Willing A. Cardozo-Pelaez F. Stedeford T. Chopp M. Sanberg PR. Expression of neural markers in human umbilical cord blood. Exp Neurol. 2001;171:109–115. doi: 10.1006/exnr.2001.7748. [DOI] [PubMed] [Google Scholar]
- 57.Rainsford E. Reen DJ. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol. 2002;116:702–709. doi: 10.1046/j.0007-1048.2001.03321.x. [DOI] [PubMed] [Google Scholar]
- 58.Paresce DM. Ghosh RN. Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 59.Borlongan CV. Hadman M. Sanberg CD. Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 60.Paresce DM. Chung H. Maxfield FR. Slow degradation of aggregates of the Alzheimer's disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- 61.Chung H. Brazil MI. Soe TT. Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- 62.Imrich H. Harzer K. On the role of peripheral macrophages during active experimental allergic encephalomyelitis (EAE) J Neural Transm. 2001;108:379–395. doi: 10.1007/s007020170060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Priller J. Flugel A. Wehner T. Boentert M. Haas CA. Prinz M. Fernandez-Klett F. Prass K. Bechmann I, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 64.Town T. Vendrame M. Patel A. Poetter D. DelleDonne A. Mori T. Smeed R. Crawford F. Klein T. Tan J. Mullan M. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer's beta-amyloid(1–42) J Neuroimmunol. 2002;132:49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 65.Town T. Nikolic V. Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Town T. Tan J. Flavell RA. Mullan M. T-cells in Alzheimer's disease. Neuromolecular Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]