Abstract
Stem cells hold great potential for therapeutic angiogenesis due to their ability to directly contribute to new vessel formation or secrete paracrine signals. Adipose-derived stem cells (ADSCs) are a particularly attractive autologous cell source for therapeutic angiogenesis due to their ease of isolation and relative abundance. Gene therapy may be used to further enhance the therapeutic efficacy of ADSCs by overexpressing desired therapeutic factors. Here, we developed vascular endothelial growth factor (VEGF)–overexpressing ADSCs utilizing poly(β-amino esters) (PBAEs), a hydrolytically biodegradable polymer, and examined the effects of paracrine release from nonviral modified ADSCs on the angiogenic potential of human umbilical vein endothelial cells (HUVECs) in vitro. PBAE polymeric vectors delivered DNA into ADSCs with high efficiency and low cytotoxicity, leading to an over 3-fold increase in VEGF production by ADSCs compared with Lipofectamine 2000. Paracrine release from PBAE/VEGF-transfected ADSCs enhanced HUVEC viability and decreased HUVEC apoptosis under hypoxia. Further, paracrine release from PBAE/VEGF-transfected ADSCs significantly enhanced HUVEC migration and tube formation, two critical cellular processes for effective angiogenesis. Our results demonstrate that genetically engineered ADSCs using biodegradable polymeric nanoparticles may provide a promising autologous cell source for therapeutic angiogenesis in treating cardiovascular diseases.
Introduction
Therapeutic angiogenesis is a promising treatment approach for patients suffering from ischemic cardiovascular disease. This approach involves methods of regenerative medicine to augment the host angiogenic response. Angiogenesis is the physiological process of blood vessel growth from pre-existing vessels and is the body's natural mechanism for restoring blood perfusion to sites of ischemia. Stem cell-based therapies hold great promise for therapeutic angiogenesis by either acting as cellular building blocks for new blood vessels or by secreting paracrine signals to promote blood vessel growth [1,2]. The most well-studied cell sources for therapeutic angiogenesis include endothelial progenitor cells (EPCs) and bone marrow-derived mesenchymal stem cells (BMMSCs) [3]. Despite the therapeutic efficacy of these cell sources [3–7], their broad clinical application is largely limited. This is because their use requires an invasive cell isolation procedure that has a low cell yield, and needs to be expanded ex vivo prior to transplanting back into the patient.
Adipose-derived stem cells (ADSCs) represent a promising alternative cell source due to their relative abundance, ease of isolation, and demonstrated potential for therapeutic angiogenesis [8,9]. Indeed, when directly compared with BMMSCs, ADSCs were reported to possess higher angiogenic potency, demonstrating improved blood perfusion and a reduction in infarct size 2 weeks posttransplantation in a mouse model of hindlimb ischemia [10]. Among the mechanisms by which stem cells contribute to angiogenesis, paracrine signaling is regarded as being a key contributor [5,11]. In support of this view, it was shown that direct injection of conditioned medium (CM) from BMMSC versus BMMSC transplantation into a rat model of myocardial ischemia led to similar reductions in infarct size and improvements in ventricular function by 72 h [12]. A broad spectrum of angiogenic cytokines were found to be present in the CM of BMMSCs, including vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), interleukin-6, and monocyte chemotactic protein-1 (MCP-1) [6]. Similarly, ADSCs were shown to release a number of angiogenic and antiapoptotic paracrine factors [13], which led to enhanced new blood vessel formation in vivo [13–18]. Despite the promise of stem-cell-based therapies for therapeutic angiogenesis, the efficacy of using stem cells alone remains limited due to the insufficient production of therapeutic factors in vivo, as well as low cell engraftment and survival in ischemic tissues.
Gene therapy has the potential to improve stem cell-mediated therapeutic angiogenesis by increasing the paracrine release of specific angiogenic factors. In fact, it was shown that transplantation of virally transduced BMMSCs overexpressing VEGF into rat models of myocardial ischemia led to enhanced blood vessel density and heart function in comparison to MSCs alone [19]. Further, it was demonstrated that overexpressing VEGF reduced the number of EPCs needed by 30-fold to achieve a similar therapeutic effect as unmodified EPCs in promoting angiogenesis and limb salvage [20]. However, most studies so far have relied on viral vectors for efficient gene transfer, which are limited by potential risks of mutagenesis, carcinogenesis, and immunogenicity. In the pursuit of a safer alternative approach, strategies utilizing nonviral vectors for therapeutic angiogenesis are beginning to emerge [21]. Conventional nonviral delivery systems involve using positively charged polymers that can condense negatively charged DNA into nanoparticles for cellular uptake. Polyethylenimine (PEI) and Lipofectamine 2000 (Lipo) are the most widely used polymer-based transfection reagents for gene delivery, but their clinical application is limited due to nondegradability, poor transfection efficiency, and high toxicity [21]. Poly(β-amino esters) (PBAEs) are a family of hydrolytically biodegradable polymers, which can deliver DNA into human stem cells with high efficiency and low toxicity [11,22]. We previously reported that MSCs modified using PBAE/VEGF nanoparticles led to substantially enhanced angiogenesis and limb salvage in a mouse hindlimb ischemia model [22]. However, the efficacy of nonviral engineered ADSCs for therapeutic angiogenesis remains largely unknown.
In this study, we assess the effects of paracrine release from ADSCs modified utilizing PBAE/VEGF nanoparticles on human umbilical vein endothelial cell (HUVEC) behavior in vitro. We first developed biodegradable PBAE/VEGF nanoparticles that can transfect ADSCs with high efficiency for VEGF overexpression. CM from transfected ADSCs was then applied to HUVECs to examine the effects of such paracrine release on HUVEC viability and apoptosis under low oxygen (1% O2), as well as on HUVEC migration and tube formation in vitro.
Materials and Methods
Cell isolation and culture
Fat tissue was obtained from the abdominal fat of a female patient who had undergone a free flap breast reconstruction surgery at Stanford University. All procedures were approved and guided under the Stanford Institutional Review Board protocol. The fat tissue was washed 2–3 times with phosphate-buffered saline (PBS) and digested at 37°C for 30 min with 0.5 U/mL Blendzyme 3 (Roche Diagnostics, Indianapolis, IN). Enzyme activity was neutralized with Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA), containing 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (P/S; Invitrogen). Cells were then filtered through a 100 μm nylon mesh to remove cellular debris before seeding. Following the initial 48 h of incubation at 37°C and 5% CO2, cells were washed with PBS and maintained in growth medium containing DMEM, 10% FBS, 1% P/S, and 10 ng/mL of FGF-2 (PeproTech, Rocky Hill, NJ). Once 85%–95% confluent, the cells were passaged by trypsin (0.05%) digestion and plated at a density of 5000–6000 cells/cm2 for further expansion. HUVECs were obtained from Lonza (Basal, Switzerland) and expanded utilizing endothelial growth medium-2 (EGM-2) (Lonza). Passage 4–5 HUVECs were utilized for all experiments.
Transfection
Human ADSCs were transfected with DNA plasmids encoding VEGF165 (pVEGF) (Aldevron, Fargo, ND) or enhanced green fluorescence protein (pEGFP) (ELIM Biopharm, Hayward, CA). Passage 4 ADSCs were seeded at 65,000 cells per well in clear 24-well plates or 10,400 cells per well in clear 96-well plates and incubated overnight prior to transfection. Cells were transfected utilizing PBAE C32-122 as previously reported [11]. Briefly, polyplexes were formed by mixing pDNA and PBAE polymers in 25 mM sodium acetate. Polyplexes were added to the cell culture after 10 min of complexation. Cells were then incubated with polyplexes for 4 h, at which time cell medium was replaced. Transfection parameters were optimized by varying pDNA loading dose (1 μg, 2 μg, 3 μg, 4.5 μg, and 6 μg). Lipofectamine 2000 (Invitrogen) was used as a positive control and nontransfected cells were included as a negative control. Lipo transfection conditions were based on the manufacturer's protocol (1 μg DNA, 1:2.5 DNA/Lipo wt/wt ratio). GFP expression by transfected ADSCs was measured at 48 h posttransfection utilizing fluorescence-activated cell sorting on a BD FACScalibur (BD Biosciences, San Jose, CA). Dead cells were excluded from the analysis by staining with propidium iodide and 10,000 live cells per sample were included for the analysis. The percentage of GFP-positive cells was quantified using Flowjo 7.6 software (Tree Star, Inc., Ashland, OR). Cell viability was determined 48 h posttransfection using Cell Titer 96 Aqueous One Solution Cell Proliferation Assay 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) (Promega, Madison, WI) and results were normalized using nontreated cells as a control.
Preparation of conditioned medium
CM was prepared by culturing ADSCs in 24-well plates (65,000/well) for 48 h in serum-free endothelial basal medium (EBM) (Lonza). Three groups were examined: (1) PBAE/VEGF-transfected ADSCs, (2) Lipo/VEGF-transfected ADSCs, and (3) nontransfected ADSCs. To examine the effects of hypoxia on VEGF release, ADSCs were cultured in either low oxygen (1% O2) or normoxia (20% O2). CM from both groups was collected at 48 h posttransfection and stored at −80°C. CM from ADSCs cultured under normoxia was also collected on days 4, 6, and 8 to quantify VEGF-release kinetics. New medium was added and cultured until collection at next time point. VEGF in the supernatant was determined by VEGF165 enzyme-linked immunosorbent assay (ELISA) (PeproTech). Accumulated VEGF release was calculated by adding up the total amount of VEGF released in the CM from all previous time points. To evaluate the functional responses of HUVECs to the paracrine signals from ADSCs, CM from ADSCs cultured under normoxia was added to the HUVEC culture and outcomes were analyzed by HUVEC apoptosis, migration, and tube formation assays. Given that VEGF concentration is largely dependent upon the number of cells plated and the volume of the medium, results were reported as the total amount of VEGF produced and normalized by the cell number. Specifically, the total amount of VEGF in the CM (VEGF concentration measured by ELISA×volume of CM) was divided by the initial cell seeding number per well, and results were reported as pg per 106 cells (pg/106 cells).
HUVEC survival under hypoxia
Passage 4 HUVECs were plated in either 96-well plates (11,200 cells/well) for MTS assay or in gelatin-coated 8-well chamber slides (28,000 cells/well) for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. HUVECs were cultured in serum-free EBM for 24 h prior to switching to CM, when they were placed in a low-oxygen chamber (1% O2) for 48 h. HUVEC viability was determined by MTS assay. HUVEC viability was normalized to EGM-2 and results were presented as a percentage of HUVEC viability cultured under EGM-2. HUVEC apoptosis was determined utilizing the DeadEnd Fluorometric TUNEL System (Promega), and mounted with Vectashield Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) for counterstain. Fluorescent microscopic images were analyzed utilizing ImageJ software (NIH). HUVEC apoptosis was reported as the ratio of TUNEL-positive cells to DAPI-positive cells.
HUVEC migration
HUVEC migration was assessed in a modified Boyden chamber (E&K Scientific, Santa Clara, CA). The upper wells were pretreated with 50 μL of 10 μg/mL fibronectin (Sigma-Aldrich, St. Louis, MO) and incubated at 4°C for 24 h prior to HUVEC plating. HUVECs were seeded onto the upper chamber (20,000 cells/well) and EBM was added to the bottom chamber. Cells were allowed to settle for 24 h at 37°C. EBM in the bottom wells was then replaced with CM in quadruplicate and cells were allowed to migrate for 6 h. The cells were fixed and stained with Hemacolor (Merck, Darmstadt, Germany) and cell number was quantified by counting the number of cells per high-powered field under a light microscope. Results were reported as the ratio of migrated cells over total number of cells.
Tube formation assay
Tube formation was assessed by culturing HUVECs on BD Matrigel (BD Biosciences). Prior to HUVEC seeding, 96-well microtiter plates were coated with 80 μL of Matrigel per well and incubated for 1 h. Passage 4 HUVECs were suspended in CM or serum-free EBM and plated in triplicate at 12,000 cells per well. The formation of tube-like structures was examined at each hour up to 16 h. Representative images were taken and analyzed utilizing ImageJ software (NIH). Tube formation was analyzed by quantifying the number of branches per high-powered field.
Statistical analysis
All experiments were performed in triplicate and repeated for consistency. The results were reported as mean±standard error of the mean. Statistical analysis was performed using the one-way analysis of variance with Dunnett's multiple comparison test as the posttest to compare all the experimental groups. For transfection experiments, Lipo-transfected cells were used as positive controls and nontransfected cells as negative controls. For HUVEC assays, EGM-2-cultured HUVECs were used as positive controls and EBM-cultured HUVECs were used as negative controls.
Results
Gene delivery efficiency into ADSCs using PBAE nanoparticles
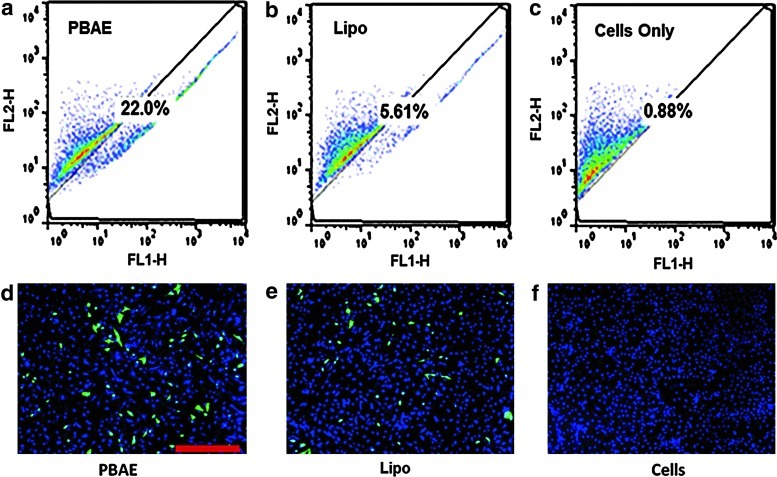
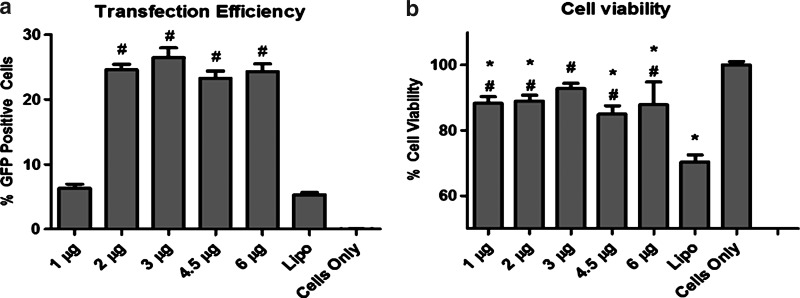
Transfection efficiency of PBAEs was first examined in human ADSCs using a leading PBAE polymer C32-122 [11] and a reporter DNA encoding pEGFP. Fluorescence microscopy and flow cytometry confirmed gene delivery into ADSCs 48 h posttransfection (Fig. 1). Increasing DNA doses from 1 μg to 2 μg significantly increased the transfection efficiency from 6% to 26%, and further increase in DNA doses up to 6 μg did not significantly change the transfection efficiency. The PBAE-mediated transfection also showed 4-fold higher efficiency compared with the positive control transfected using Lipo (5.27%±0.7%) (Fig. 2a). Meanwhile, all groups transfected with PBAEs demonstrated much higher cell viability compared with the Lipo-transfected group (88.6%±6% vs. 70.4%±4%, p<0.05) (Fig. 2b). Varying DNA dose did not lead to a statistically significant difference in cell viability among PBAE-transfected groups.
FIG. 1.
Efficiency of gene delivery to human adipose-derived stem cells (ADSCs) utilizing poly(β-amino ester) (PBAE) nanoparticles. Under leading transfection conditions, PBAE (a) delivered enhanced green fluorescence protein (EGFP) DNA into ADSCs with 4 times the efficiency of Lipofectamine 2000 (Lipo) (b). Fluorescence microscopy showed markedly increased GFP signals in ADSCs transfected using PBAE nanoparticles (d) compared with cells transfected using Lipo (e). No GFP expression was observed in non-transfected cells (c, f). Scale bar: 500 μm. Color images available online at www.liebertpub.com/scd
FIG. 2.
Effects of nanoparticle dose on transfection efficiency and cell viability. (a) PBAEs led to significantly increased transfection efficiency in ADSCs using doses as low as 2 μg/well compared with Lipo (#p<0.001 compared with Lipo). (b) Cells transfected using PBAEs at all doses demonstrated high cell viability, which were about 1-fold higher compared with the cells transfected using Lipo (#p<0.01 vs. Lipo; *p<0.05 vs. cells only).
VEGF release from PBAE/VEGF-transfected ADSCs
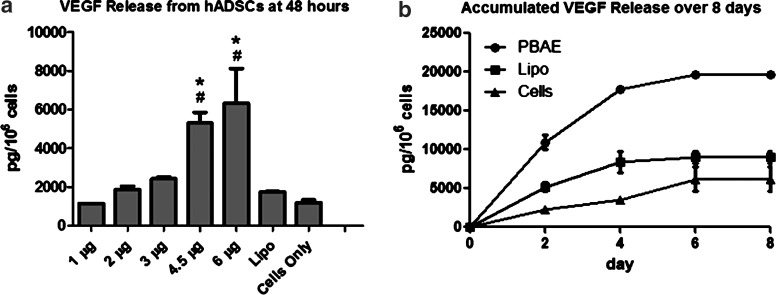
The effect of DNA dose on VEGF release from PBAE/VEGF-transfected ADSCs was examined by VEGF165 ELISA 48 h posttransfection (Fig. 3). ADSCs transfected using PBAE with low DNA doses (1–3 μg DNA) or Lipo did not lead to a significant increase in VEGF production compared with nontransfected ADSC controls. Increasing PBAE/DNA dose to 4.5 μg led to significant increases in VEGF production (5290±563 pg/106 cells) and further increase was observed when DNA dose increased to 6 μg (6308±1810 pg/106 cells), which was over 3-fold higher than Lipo control (1726.25±46.25 pg/106 cells, p<0.05) and nontransfected ADSC control (1188±133.75 pg/106 cells, p<0.05) (Fig. 3a). Accumulated VEGF release over time showed that the optimized PBAE-transfected group produced over 3-fold higher amount of VEGF than released from nontransfected ADSC controls, while Lipo transfection only led to a slight increase in VEGF production.
FIG. 3.
Vascular endothelial growth factor (VEGF) release from ADSCs after transfection using polymeric nanoparticles. (a) VEGF release from PBAE-modified ADSCs increased with DNA dose and reached a maximum at DNA dose of 6 μg, which was significantly higher than groups transfected using Lipo (#p<0.05) and cells alone (*p<0.05). (b) Accumulated VEGF release showed continuously enhanced VEGF release from cells transfected using PBAE/VEGF nanoparticles compared with Lipo control.
Effect of hypoxia on VEGF release and ADSC proliferation
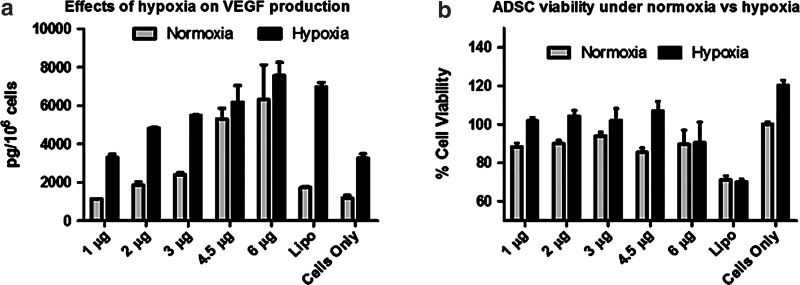
To assess the effects of ischemia on ADSC fate, ADSCs were cultured in a hypoxia chamber (1% O2) for 48 h to mimic ischemic conditions in vivo. Outcomes were analyzed for VEGF release and ADSC viability (Fig. 4). Hypoxia treatment enhanced VEGF release in nontransfected ADSC controls by over 200% when compared with culture under normoxia. PBAE/VEGF transfection led to further increase in VEGF production, with the highest VEGF concentrations observed for the 4.5 μg and the 6 μg DNA doses (Fig. 4a). Groups transfected using PBAE demonstrated high viability (85%–95%) overall. The lowest cell number was observed in the Lipo-transfected group (∼70%). Hypoxia treatment led to further increases in cell number in all PBAE-transfected groups in comparison to the normoxia-cultured, nontransfected ADSC controls (Fig. 4b), except the 6 μg dose. This increase is likely a combination of improved cell survival and increased cell proliferation.
FIG. 4.
Effects of hypoxia on VEGF production and cell viability of nonviral engineered ADSCs. (a) Hypoxia (1% O2 for 48 h) enhanced VEGF release from nonviral engineered ADSCs in a dose-dependent manner. (b) Hypoxia treatment led to an increase in ADSC viability in most PBAE-transfected groups, except in the groups transfected with 6 μg PBAE/DNA or Lipo.
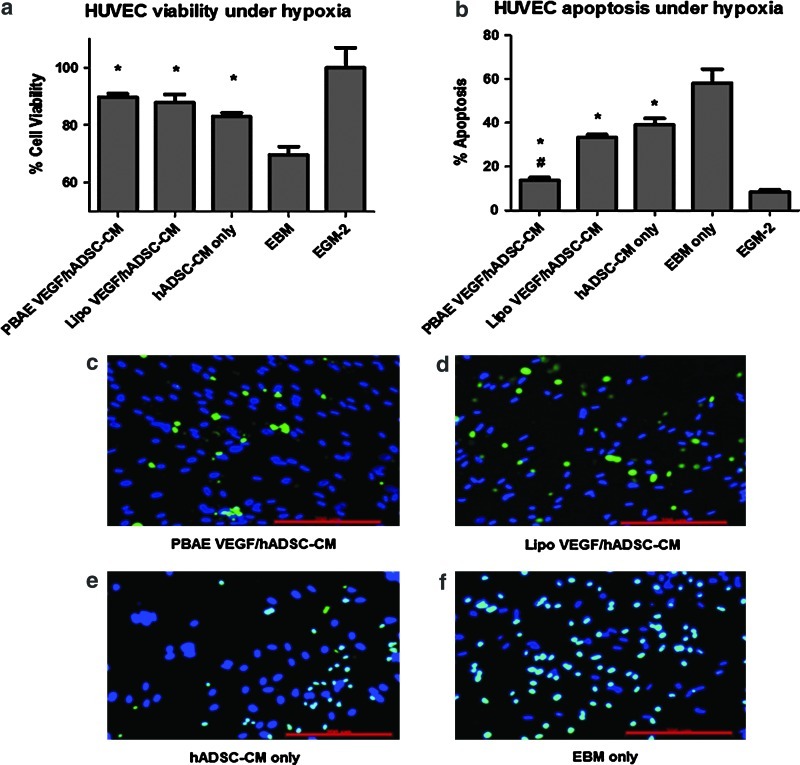
Effects of paracrine release on HUVEC viability and apoptosis under hypoxia
CM from ADSCs (transfected or control) was collected and added to HUVECs cultured under hypoxia to evaluate the effects of paracrine signals from ADSCs on HUVEC viability and apoptosis (Fig. 5). The 4.5 μg DNA dose was the chosen PBAE-transfected group for application to HUVECs. CM from all ADSC groups led to an increase in HUVEC viability (87.3%±3.9%) compared with EBM (69.6%±5.1%, p<0.05) (Fig. 5a). However, no significant difference in HUVEC viability was observed between groups treated with CM from VEGF-overexpressing ADSCs or untransfected ADSCs. The effects of paracrine release from ADSCs on HUVEC apoptosis were analyzed using TUNEL assay. CM from PBAE-transfected ADSCs led to a significant decrease in HUVEC apoptosis (13.7%±2.6%) compared with Lipo (33.2%±2.4%, p<0.05) and nontransfected controls (39%±5.3%, p<0.05) (Fig. 5b).
FIG. 5.
Effects of paracrine signals from nonviral engineered ADSCs on endothelial cell (EC) viability and apoptosis. (a) Paracrine release from ADSCs significantly improved human umbilical vein endothelial cell (HUVEC) viability under hypoxia [*p<0.05 vs. endothelial basal medium (EBM)], with no significant difference in cell viability due to VEGF overexpression. (b) Paracrine release from ADSCs markedly decreased HUVEC apoptosis under hypoxia, and PBAE/VEGF-transfected group led to the greatest decrease in cell apoptosis [*p<0.05 vs. EBM; #p<0.05 vs. Lipo VEGF/ADSC–conditioned medium (CM)]. (c–f) Representative images of TUNEL-stained HUVECs undergoing apoptosis. Scale bars=200 μm. Color images available online at www.liebertpub.com/scd
Effects of paracrine release on HUVEC migration and tube formation
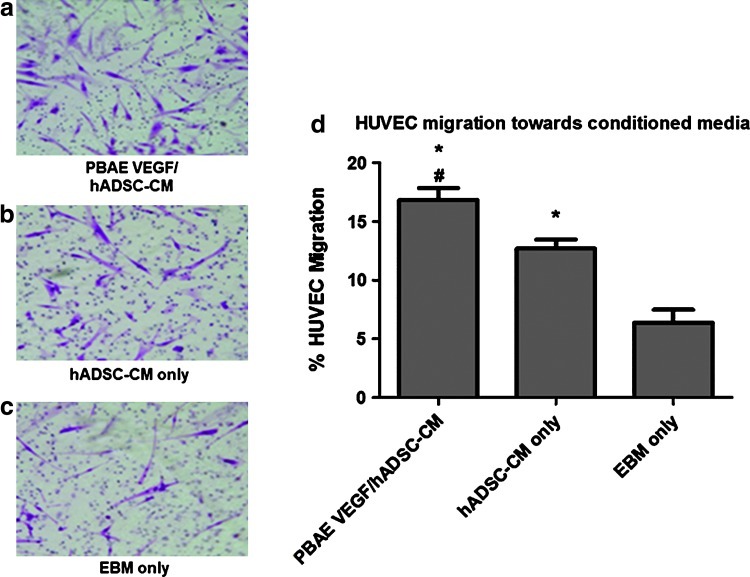
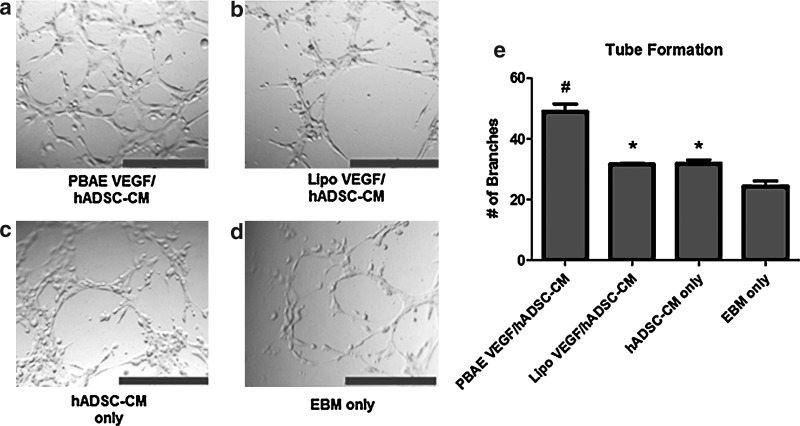
We lastly assessed the effects of VEGF overexpression on EC migration and tube formation, two key steps involved in angiogenesis. HUVEC migration toward ADSC paracrine release was assessed in a modified Boyden chamber (Fig. 6). CM from nontransfected ADSCs increased HUVEC migration (12.7%±1%, p<0.05) compared with EBM (6.4%±2%, p<0.05), and CM from PBAE/VEGF-transfected ADSCs led to further statistically significant increases in HUVEC migration (16.8%±1%, p<0.05). Similarly, HUVECs formed homogeneous and interconnected tubular structures when exposed to CM from PBAE/VEGF-transfected ADSCs (49±4 branches per high-powered field) (Fig. 7), whereas significantly fewer tubular structures were observed in HUVECs exposed to CM from Lipo/VEGF-transfected ADSCs (31.7±1 branches per high-powered field, p<0.05) or ADSCs alone (32.0±2 branches per high-powered field, p<0.05).
FIG. 6.
Paracrine release from PBAE/VEGF-engineered ADSCs enhanced EC migration. (a–c) Representative images of migrated HUVECs in response to CM from ADSCs or EBM (negative control). (d) Percentage of migrated HUVECs toward CM (*p<0.05 vs. EBM only; #p<0.05 vs. ADSC-CM only). Color images available online at www.liebertpub.com/scd
FIG. 7.
Paracrine release from PBAE/VEGF-engineered human ADSCs enhanced EC tube formation. (a–d) CM from PBAE/VEGF-transfected ADSCs led to formation of interconnected tubular structures by HUVECs (scale bars=500 μm). (e) The number of branch points formed by HUVECs in response to CM from all groups (*p<0.05 vs. EBM; #p<0.05 compared with Lipo).
Discussion
Here, we demonstrated that PBAEs are effective nonviral, polymeric vectors for gene delivery to ADSCs. VEGF-overexpressing ADSCs utilizing biodegradable nanoparticles enhanced angiogenesis-related cellular behavior, including EC survival under hypoxia, migration, and tube formation. Although a previous study has shown beneficial effects of VEGF-overexpressing ADSCs on angiogenesis using a viral-based approach [23], the broad clinical translation of viral-mediated gene delivery remains limited by safety concerns. Nonviral-based gene delivery is potentially safer, but often suffers from low transfection efficiency and high toxicity. Our results showed that PBAE nanoparticles are promising nonviral polymeric vectors that could transfect ADSCs with 4–5 times higher transfection efficiency than Lipofectamine 2000, a commercially available transfection reagent, and with higher cell viability (Fig. 2). PEI [24], nucleofection, and electroporation [25,26] are other commonly used nonviral gene delivery methods, which may achieve higher transfection efficiency than Lipo, but these suffer from high toxicity. PBAE nanoparticles are able to achieve high transfection at little expense to cell viability, which makes them a more attractive option for overexpressing desired therapeutic genes. PBAE-mediated VEGF overexpression is transient (Fig. 3), which may limit any negative effects due to constitutive release of VEGF, such as excessive and aberrant vascularity. This demonstrates that PBAE nanoparticles could be utilized to strategically overexpress known therapeutic factors, thereby manipulating stem cells to augment their natural angiogenic responses.
Given that ADSCs would be exposed to hypoxia when transplanted into ischemic tissues, it is important to understand ADSC behavior under hypoxia to determine their response posttransplantation. ADSCs are known to release several paracrine factors with angiogenic effects [13,27]. Hypoxia has been shown to enhance paracrine release of angiogenic factors from ADSCs or BMMSCs, which may induce therapeutic benefit both in vitro and in vivo [27–30]. Paracrine release from ADSCs cultured under hypoxia has previously shown the ability to improve EC survival, where such effects were attenuated by the addition of anti-VEGF blocking antibodies [30]. We examined the effects of hypoxia on ADSC VEGF release and cell viability, as well as how such cellular responses changed upon upregulating VEGF by PBAE. Consistent with previous reports, our results demonstrated that hypoxia led to an increase in both ADSC VEGF release and cell proliferation compared with normoxia culture. VEGF overexpression further improved VEGF release from ADSCs cultured under hypoxia conditions, demonstrating that gene therapy and hypoxia can act synergistically. We also found that hypoxia enhanced ADSC viability, which has been demonstrated in several prior reports utilizing different assays [13,27,30]. Further, endogenous ADSCs have been found to proliferate in situ due to ischemia at a distant site, suggesting that ADSCs respond favorably to low oxygen tension [27]. These findings support ADSCs as an excellent cell source for treating ischemic disease, due to their ability to survive under hypoxia and enhance paracrine release of angiogenic factors. Though most previous work has focused on the use of BMMSCs for angiogenesis [6,7,12,22,31], ADSCs represent an attractive alternative cell source due to their relative abundance and ease of isolation. Both cell sources have been reported to improve angiogenesis in vivo, but their paracrine release profiles are different. While both cell types secrete VEGF, BMMSCs secrete more basic FGF [6] and ADSCs more prominently secrete hepatocyte growth factor and transforming growth factor-β [13].
Angiogenesis is the formation of new blood vessels from pre-existing vessels, which involves EC proliferation, migration, and new tube formation. To determine the effects of paracrine release from VEGF-overexpressing ADSCs on angiogenesis, we applied CM from PBAE/VEGF-transfected ADSCs to HUVECs and examined HUVEC cellular processes related to angiogenesis. Prior studies assessed EC survival under low-serum (2%–5%) containing medium [4,7,28]. In our study, ECs were cultured in serum-free EBM, which represents a more challenging culture condition for ECs. Our results confirmed that hypoxia exposure had detrimental effects on HUVECs, causing decreased cell viability and markedly increased cell apoptosis (Fig. 5). Consistent with previous reports, we found that paracrine release from ADSCs alone enhanced HUVEC viability [13,16], HUVEC migration [6,30], and HUVEC tube formation [28,30]. PBAE/VEGF overexpression led to a marked decrease in HUVEC apoptosis, with improvements in HUVEC migration and more homogeneous tube formation. These results confirmed the beneficial effects of VEGF overexpression on HUVEC behavior. Addition of anti-VEGF blocking antibodies was previously reported to reduce EC migration [30], again suggesting the functional importance of VEGF. While previous studies have also demonstrated protective effects on ECs using VEGF supplementation, the dose of VEGF applied was two orders of magnitude higher than that secreted by cells alone (4–10 ng/mL vs. 0.08–0.1 ng/mL VEGF from cells alone) [6], which may lead to undesirable excessive vascular formation. It is also worth noting that Lipo/VEGF transfection did not improve tube formation or HUVEC apoptosis compared with ADSCs alone, which is likely the result of the low transfection efficiency using Lipofectamine compared with PBAE nanoparticles (Fig. 1). Overall, these results show that PBAE-mediated nonviral gene delivery provides an effective strategy for overexpressing therapeutic factors to induce HUVEC cellular processes related to angiogenesis.
Conclusion
In conclusion, we have shown that nonvirally modified ADSCs using biodegradable polymeric vectors have beneficial effects on HUVEC behavior in vitro. ADSCs are promising autologous cell sources that can serve as delivery vehicles for therapeutic paracrine signals. Biodegradable PBAE nanoparticles can deliver DNA into ADSCs with high efficiency and low cytotoxicity. Paracrine release from VEGF-overexpressing ADSCs improved HUVEC survival, migration, and tube formation, and markedly reduced HUVEC apoptosis. The results of this study suggest that nonvirally engineered ADSCs may be a promising autologous cell source for therapeutic angiogenesis in cardiovascular disease.
Acknowledgments
The authors would like to thank American Heart Association National Scientist Development Grant (10SDG2600001), Stanford Bio-X Interdisciplinary Initiative Program, Donald E. and Delia B. Baxter Foundation, and Stanford Medical Scholars Research Program for funding.
Disclosure Statement
The authors have no conflicts to disclose.
References
- 1.Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 2.Potente M. Gerhardt H. Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 4.Gruber R. Kandler B. Holzmann P. Vogele-Kadletz M. Losert U. Fischer MB. Watzek G. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896–903. doi: 10.1089/ten.2005.11.896. [DOI] [PubMed] [Google Scholar]
- 5.Kamihata H. Matsubara H. Nishiue T. Fujiyama S. Tsutsumi Y. Ozono R. Masaki H. Mori Y. Iba O, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 6.Kinnaird T. Stabile E. Burnett MS. Lee CW. Barr S. Fuchs S. Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 7.Potapova IA. Gaudette GR. Brink PR. Robinson RB. Rosen MR. Cohen IS. Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- 8.Schäffler A. Büchler C. Concise review: Adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ. Kim HK. Cho HH. Bae YC. Suh KT. Jung JS. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20:867–876. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 11.Yang F. Green JJ. Dinio T. Keung L. Cho SW. Park H. Langer R. Anderson DG. Gene delivery to human adult and embryonic cell-derived stem cells using biodegradable nanoparticulate polymeric vectors. Gene Ther. 2009;16:533–546. doi: 10.1038/gt.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi M. He H. Liang OD. Melo LG. Morello F. Mu H. Noiseux N. Zhang L. Pratt RE. Ingwall JS. Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 13.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove CJ. Bovenkerk JE. Pell CL. Johnstone BH. Considine RV. March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 15.Kondo K. Shintani S. Shibata R. Murakami H. Murakami R. Imaizumi M. Kitagawa Y. Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 16.Miranville A. Heeschen C. Sengenes C. Curat CA. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 17.Moon MH. Kim SY. Kim YJ. Kim SJ. Lee JB. Bae YC. Sung SM. Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 18.Rubina K. Kalinina N. Efimenko A. Lopatina T. Melikhova V. Tsokolaeva Z. Sysoeva V. Tkachuk V. Parfyonova Y. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15:2039–2050. doi: 10.1089/ten.tea.2008.0359. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto R. Omura T. Yoshiyama M. Hayashi T. Inamoto S. Koh KR. Ohta K. Izumi Y. Nakamura Y, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 20.Iwaguro H. Yamaguchi J. Kalka C. Murasawa S. Masuda H. Hayashi S. Silver M. Li T. Isner JM. Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 21.Park H-J. Yang F. Cho S-W. Nonviral delivery of genetic medicine for therapeutic angiogenesis. Adv Drug Deliv Rev. 2012;64:40–52. doi: 10.1016/j.addr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang F. Cho S-W. Son SM. Bogatyrev SR. Singh D. Green JJ. Mei Y. Park S. Bhang SH, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabbarzadeh E. Starnes T. Khan YM. Jiang T. Wirtel AJ. Deng M. Lv Q. Nair LS. Doty SB. Laurencin CT. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci U S A. 2008;105:11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn HH. Lee JH. Kim KS. Lee JY. Kim MS. Khang G. Lee IW. Lee HB. Polyethyleneimine-mediated gene delivery into human adipose derived stem cells. Biomaterials. 2008;29:2415–2422. doi: 10.1016/j.biomaterials.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Lopatina T. Kalinina N. Parfyonova E. Nonviral transfection of adipose tissue stromal cells: an experimental study. Bull Exp Biol Med. 2009;147:509–512. doi: 10.1007/s10517-009-0546-7. [DOI] [PubMed] [Google Scholar]
- 26.Zaragosi L-E. Billon N. Ailhaud G. Dani C. Nucleofection is a valuable transfection method for transient and stable transgene expression in adipose tissue-derived stem cells. Stem Cells. 2007;25:790–797. doi: 10.1634/stemcells.2006-0235. [DOI] [PubMed] [Google Scholar]
- 27.Thangarajah H. Vial IN. Chang E. El-Ftesi S. Januszyk M. Chang EI. Paterno J. Neofytou E. Longaker MT. Gurtner GC. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 28.Hung S-C. Pochampally RR. Chen S-C. Hsu S-C. Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 29.Kinnaird T. Stabile E. Burnett MS. Shou M. Lee CW. Barr S. Fuchs S. Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 30.Nakagami H. Maeda K. Morishita R. Iguchi S. Nishikawa T. Takami Y. Kikuchi Y. Saito Y. Tamai K. Ogihara T. Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue–derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 31.Burdon TJ. Paul A. Noiseux N. Prakash S. Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326. doi: 10.1155/2011/207326. [DOI] [PMC free article] [PubMed] [Google Scholar]