Abstract
Background
Autopsy studies show that smoking contributes to airway wall hyperplasia and narrowing of the airway lumen. Studies of smoking and airway measures on computed tomography (CT) scan are limited to case-control studies of measures that combine airway lumen and wall thickness.
Objectives
We hypothesized that cumulative cigarette smoking would be associated with increased airway wall thickness in a large, population-based cohort.
Methods
The Multi-Ethnic Study of Atherosclerosis enrolled participants age 45-84 years from the general population. Smoking history was assessed via standardized questionnaire items; current smoking was confirmed in half the cohort with cotinine. Airway lumen and wall thickness were measured in two dimensions in posterior basal segmental bronchi on cardiac-gated CT scans. Analyses were adjusted for age, gender, genetic ancestry, education, height, weight, asthma history, particulate matter, scanner type, and scanner current.
Results
Half of the 7,898 participants had smoked and 14% were current smokers. Pack-years of smoking were associated with thicker airway walls (mean increase 0.002 mm per ten pack-years [95% CI: 0.00002, 0.004] p=0.03). Current smoking was associated with narrower airway lumens (mean decrease −0.11 mm [95% CI: −0.2, −0.02] p=0.02). There was no evidence that either association was modified by genetic ancestry, and findings persisted among participants without clinical disease.
Conclusions
Long-term cigarette smoking was associated with subclinical increases in wall thickness of sub-segmental airways whereas current smoking was associated with narrower airway lumen diameters. Smoking may contribute to airway wall thickening prior to the development of overt chronic obstructive pulmonary disease.
Keywords: smoking, airway remodeling, Pi10, wall thickness, lumen, chronic obstructive pulmonary disease
INTRODUCTION
Chronic lower respiratory disease is the third leading cause of death in the United States, primarily due to deaths from chronic obstructive pulmonary disease (COPD) (1). COPD is defined as airflow limitation that is not fully reversible (2). Airflow limitation in COPD results from structural changes in the airway wall, bronchoconstriction and lack of tethering due to emphysema (3-7).
Smoking is the major risk factor for COPD (8-11), and chronic smoking is associated with pigmented macrophages, edema, fibrosis and epithelial hyperplasia in first and second order respiratory bronchioles in autopsy specimens from healthy smokers (12). Chronic smoking is associated with inflammatory infiltrates, fibrosis and squamous cell metaplasia in lung biopsy specimens (13).
Two prior case-control studies examined the relationship between chronic smoking and airway dimensions on computed tomography (CT) scan (14, 15). Both studies, however, used measures of airway dimensions that are a function of both wall thickness and lumen diameter. The first measure, airway wall area percent (WA%), is usually defined as the total airway area minus the lumen area divided by the total airway area (7). The second, Pi10, regresses the wall thickness on lumen diameter from all visualized airways to calculate the hypothetical airway wall thickness (AWT) of an airway at an internal perimeter of 10 mm (16). An increased WA% or Pi10 are usually interpreted as evidence of thicker airway walls (17-19) but higher values of these measures could result mathematically from thicker walls, narrower lumens or a combination of the two. Hence, if acute and chronic cigarette smoke exposure differentially affect airway lumen diameter and AWT, the use of a combined measure such as Pi10 or WA% may not detect these differences.
In addition, the two existing case-control studies may be limited by selection bias due to differential selection of cases compared to controls, reverse-causality in which aspects of clinical COPD affect airway dimensions, or limited generalizability.
We therefore examined AWT and airway lumen diameter on CT scan in a large, population-based cohort study hypothesizing that cumulative smoking would be associated specifically with thicker airway walls in segmental bronchi. Given prior paradoxical findings of the association of emphysema on CT scan with current smoking (20), we also examined the association of current smoking with AWT and airway lumen diameter.
METHODS
Multi-Ethnic Study of Atherosclerosis
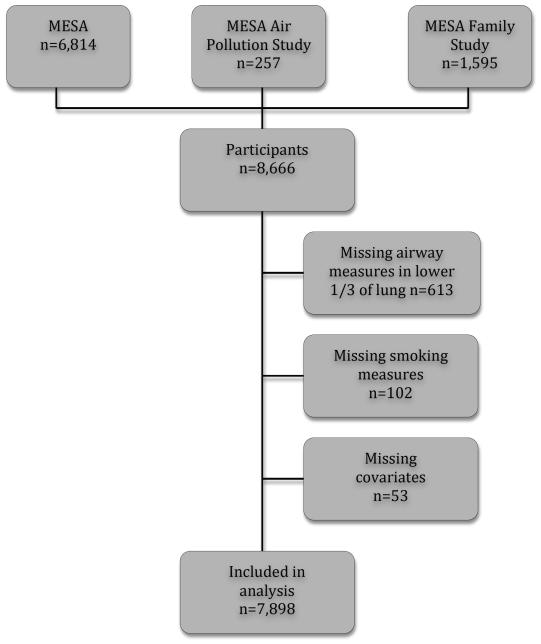
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter cohort study of subclinical cardiovascular disease in whites, African Americans, Hispanics, and Asians (21). Between 2000 and 2002, MESA recruited 6,814 men and women 45 to 84 years of age from Forsyth County, North Carolina; New York City; Baltimore; St. Paul, Minnesota; Chicago; and Los Angeles. Exclusion criteria were clinical cardiovascular disease, weight exceeding 136 kg (300 lb.), pregnancy, and impediment to long-term participation.
The MESA Family Study recruited 1,595 African American and Hispanic participants, generally siblings of MESA participants, using the same inclusion and exclusion criteria as MESA except that clinical cardiovascular disease was permitted (Figure 1) (22).
Figure 1.
Participant in the Multi-Ethnic Study of Atherosclerosis (MESA) studies included in the analysis.
The MESA Air Pollution Study recruited an additional 257 participants from Los Angeles and Riverside County, CA, and Rockland County, NY, using the same criteria as MESA except that participants were ages 50 to 89 who lived in the area ≥ 50% of the year and had no plans to move in the next five years(23).
MESA, MESA Family, and MESA Air Pollution studies used the same study protocol for the measures presented here.
The protocols were approved by the institutional review boards of all collaborating institutions and by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
Smoking status and pack years
Smoking status and pack years were assessed via standard American Thoracic Society questionnaire items: (24)
“Have you smoked at least 100 cigarettes in your lifetime?”
“How old were you when you first started smoking cigarettes?”
“Have you smoked during the last 30 days?”
“How old were you when you quit smoking cigarettes?
“On average, about how many cigarettes a day do/did you smoke?”
Participants who reported smoking fewer than 100 cigarettes in their lifetimes were classified as never smokers. Among participants who reported smoking greater than 100 cigarettes in their lifetime, those who reported smoking during the last 30 days were classified as current smokers and those who did not were classified as former smokers. Pack-years of cigarette smoking were calculated from age of starting to quitting (or current age among current smokers) × (cigarettes per day/20). Urinary cotinine levels were measured via immunoassay in a subset of 3,674 MESA participants (Immulite 2000 Nicotine Metabolite Assay; Diagnostic Products Corp., Los Angeles, CA) (25).
CT Measures
Lung structure was assessed on the lung fields of cardiac CT scans, which included approximately 70% of the lung volume from the carina to the lung bases. Cardiac CT scans were obtained at full inspiration on multidetector-row (MDCT) and electron-beam (EBT) CT scanners according to a standardized protocol (26). The scans were cardiac-gated, thereby reducing cardiac-related motion artifact in the left lower lobe. Two scans were obtained for each participant within the same session. The scan with the greater volume of lung air was used for analyses, except in cases of discordant scan quality, when the higher-quality scan was analyzed (27).
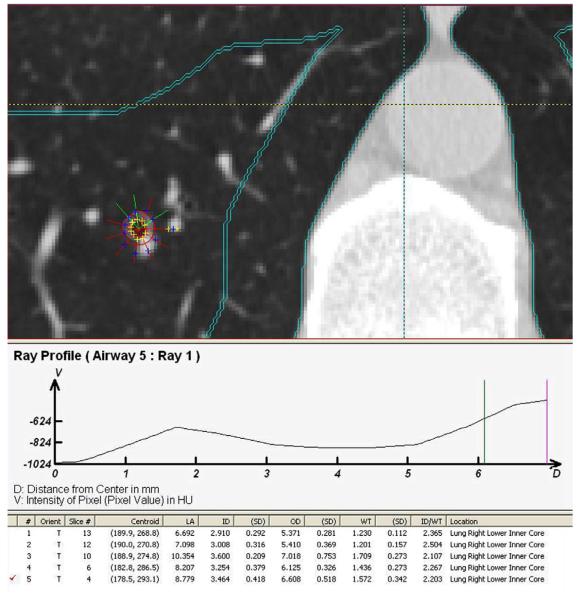
The CT scans were sent to a single center, the Iowa Comprehensive Lung Imaging Center, where the scans were read by nine radiology analysts using a modified version of the Pulmonary Analysis Software Suite (PASS) (28, 29) with semi-automated airway analysis (30). PASS is a software package that enables the manipulation, display and analysis of multidimensional digital imaging data and was used in the NHLBI-funded National Emphysema Treatment Trial (31).The airway tree was identified and labeled, paths identified and straightened so as to provide luminal and wall dimensions measured as a function of the distance along the path and perpendicular to the local long axis (Figure 2). All airways that were sampled by CT imaging approximately perpendicular to the airway long axis (i.e., the airway lumen was approximately circular) were measured in two dimensions using a modified full-width-half-maximum principal to identify the outer and inner airway wall borders (30, 32-34). Earlier full-width-half-maximum methods gave inaccurate results for small thin-walled structures like airways. To address this problem, PASS assessed the point-spread function of the particular scanner/slice selection/reconstruction algorithm of interest and then used a model-based deconvolution to account for the scanning process. This approach was more accurate than earlier methods for thin-walled structures (29, 35, 36). Upon locating an airway perpendicular to the plane, a centroid was placed in the center of the airway, from which the PASS system generated rays and inner and outer airway diameters. If a ray extended into adjacent tissue the analyst would manually exclude it and the PASS system would regenerate the inner and outer diameter to better conform to the shape of the airway. The remaining rays were averaged to calculate airway wall thickness and lumen diameter (Figure 2). Readers underwent standardized training and were certified based upon achievement of less than 5% inter-reader and intra-reader variability on a set of training scans.
Figure 2. Airway measurements on CT scan.
Upon locating an airway perpendicular to the plane, a centroid was placed in the center of the airway, from which the Pulmonary Analysis Software Suite generated rays and inner and outer airway diameters. If a ray extended into adjacent tissue the analyst would manually exclude it and the PASS system would regenerate the inner and outer diameter to better conform to the shape of the airway. The remaining rays were averaged to calculate airway wall thickness and lumen diameter.
AWT and airway lumen diameter were measured on the same slice in the airway with the largest luminal diameter in the basilar 1/3 of the scan, the posterior basal segmental bronchi (LB10 or RB10). We validated this approach among 31 MESA participants who underwent concurrent full-lung CT scans and found that 95% of airways selected using this algorithm were either LB10 or RB10.
A total of nine readers read the 7,898 scans. Four of these readers read 85% of the scans (n=6,727; mean 1,682 scans per reader). The mean difference in airway wall thickness (AWT) between readers was 0.09 mm. The intra-scan intraclass correlation coefficients (ICCs) were excellent for airway lumen and airway wall area percent (0.94 and 0.92, respectively) and good/very good for AWT (0.68) among 266 randomly selected scans read by the same reader twice (with an intervening 6-month interval). The intra-scan, inter-reader ICCs were slightly lower at 0.87, 0.82 and 0.55, respectively, among 330 randomly selected scans read by different readers. The intra-scan ICCs for lumen diameter and wall thickness did not vary appreciably by smoking category: 0.88 and 0.58 for never smokers, 0.91 and 0.53 for former smokers, and 0.84 and 0.62 for current smokers. This quantitative approach to the assessment of airway analysis was more reproducible than physician assessment of airway dimensions (37).
To facilitate comparisons with the literature, we also report AW% and Pi10. AW% in segmental airways was defined as:
Pi10 in the lower lobes was calculated following the standard method. Individual regression plots were created for each participant, plotting the square root of the wall area against the corresponding internal perimeter for each measured airway belonging to that participant. As is standard, airways with an internal perimeter ≤ 6 mm were excluded given the technical limitations of the CT scanners. The resulting regression line was used to calculate the standardized measure of airway wall thickness for a hypothetical airway with an internal perimeter of 10mm (Pi10) for each participant (16, 17).
Covariates
Age, gender, educational attainment, occupational exposure to dust and physician-diagnosed asthma were self-reported. Anthropometry was assessed as previously described(38). Genetic ancestry was defined by principal components derived from approximately 1 million, genome-wide SNPs (Affymetrix 6.0) in the current cohort (39). Average level of ambient particulate matter smaller than 2.5 μm (PM2.5) in the year prior to the CT scan was estimated from a temporal-spacial model as previously described (23).
Statistical Analysis
The cohort was stratified by smoking status for descriptive purposes. Linear regression was employed to test the relationship between smoking and airway dimensions with generalized estimating equations to account for the correlation among related family members (40). Initial models were adjusted for age, sex and the first three principle components of ancestry. We then additionally adjusted for the following potential confounders of education, height, weight, asthma history and PM2.5, in addition to CT scanner type and tube current as precision variables. An additional analysis for pack years additionally adjusted for cigarettes smoked per day among current smokers. Interactions were tested in the full model with the −2 log likelihood test. The linearity of association of packyears and AWT was evaluated using plots generated with fully adjusted generalized additive models. The tests of the primary hypothesis, 95% confidence intervals, and P-values were estimated from linear regression models. Statistical significance was defined as two-tailed P-values <0.05. Analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
The mean age of the 7,898 participants at the time of the CT scan was 62 ± 10 years, 46% were male, and the self-reported race/ethnic distribution was 33% white, 33% African-American, 25% Hispanic and 9% Chinese-American.
Fifty-one percent were never smokers, 35% were past smokers (median pack years, 14, inter-quartile range 5-31) and 14% were current smokers (median pack years, 24, IQR 12-40). Current smokers were younger and more likely to be male and African-American, whereas former smokers were older and more likely to be male and white (Table 1). The airway dimensions listed in Table 1 are crude and not adjusted for important covariates related to body size such as age, gender, and race/ethnicity.
Table 1.
Characteristics of the study sample stratified by smoking status.
| N=7,898 | Never Smokers N=3,999 |
Former Smokers N=2,796 |
Current Smokers N=1,103 |
|---|---|---|---|
| Age, mean (SD), years | 62 (10) | 63 (10) | 58 (9) |
| Female gender – no. (%) | 2,521 (63) | 1,228 (44) | 513 (47) |
| Race/ethnicity – no. (%) | |||
| White | 1,148 (29) | 1,141 (41) | 296 (27) |
| African American | 1,212 (30) | 887 (32) | 490 (44) |
| Hispanic | 1,078 (27) | 622 (22) | 276 (25) |
| Chinese | 561 (14) | 146 (5) | 41 (4) |
| Principal components of ancestry (PC) – median (IQR) |
|||
| PC1 | 0.09 (0.03-0.61) |
0.06 (0.02-0.58) |
0.10 (0.02-0.77) |
| PC2 | 0.23 (0.04-0.35) |
0.17 (0.03-0.27) |
0.22 (0.04-0.28) |
| PC3 | 0.23 (0.17-0.26) |
0.2 (0.17-0.26) |
0.24 (0.18-0.27) |
| Pack years, median (IQR) | 0 (0) | 14 (4.63-31) | 24 (11.7-40) |
| Education, years – no. (%) | |||
| < 12 | 813 (20) | 425 (15) | 209 (19) |
| 12 | 715 (18) | 475 (17) | 224 (20) |
| > 12 | 2,471 (62) | 1,896 (68) | 670 (61) |
| Asthma self report – no. (%) | 393 (10) | 295 (11) | 100 (9) |
| Height, mean (SD), cm | 164 (10) | 168 (9) | 169 (10) |
| Weight, mean (SD), lbs. | 171 (38) | 182 (38) | 177 (39) |
| Occupational exposure to dust – no. (%)b | 613 (32) | 522 (39) | 215 (47) |
| PM2.5 , mean (SD) | 12 (8.4) | 12 (7.7) | 11 (8.2) |
| CT type – no. (%) | |||
| Imatron | 2,124 (53) | 1,308 (47) | 474 (43) |
| Volume Zoom | 907 (23) | 780 (28) | 312 (28) |
| LightSpeed Plus | 379 (9) | 335 (12) | 125 (11) |
| Sensation Cardiac 64 | 168 (4) | 107 (4) | 30 (3) |
| LightSpeed Pro 16 | 142 (4) | 77 (3) | 44 (4) |
| Aquilion | 120 (3) | 59 (2) | 51 (5) |
| Sensation 16 | 89 (2) | 60 (2) | 45 (4) |
| LightSpeed QX/i | 70 (2) | 70 (3) | 22 (2) |
| Airway luminal diameter, mm, mean (SD) | 3.32 (1.17) | 3.43 (1.21) | 3.21 (1.18) |
| Airway wall thickness, mm, mean (SD) | 1.43 (0.19) | 1.44 (0.19) | 1.43 (0.19) |
| Airway wall area percent, %, mean (SD) | 71.9 (8.03) | 71.3 (8.15) | 72.9 (8.12) |
| Airway Pi10 mm, mean (SD) | 4.59 (0.54) | 4.59 (0.34) | 4.6 (0.55) |
MESA = Multiethnic Study of Atherosclerosis, SD = standard deviation, IQR= inter-quartile range, PC = principal components, PM2.5 = ambient particulate matter smaller than 2.5 μm.
Among n=3,701 participants for whom data were available.
Cumulative Smoking
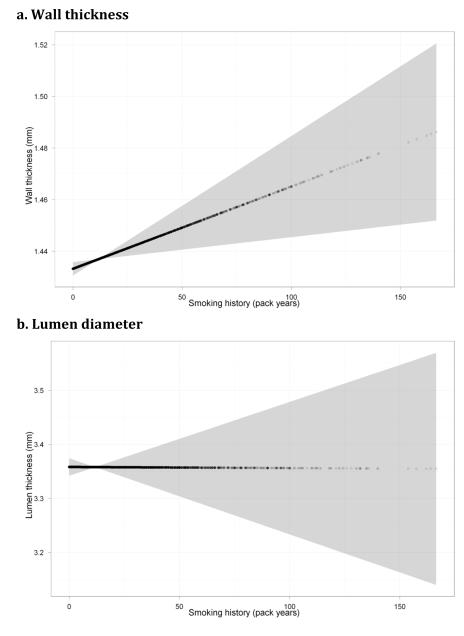
Pack years of smoking were associated with thicker segmental airway walls in both minimal and fully adjusted models (Table 2 and Figure 3). There was no evidence that the magnitude of this association with AWT varied by race/ethnicity (p-interaction=0.40), gender (p-interaction=0.11) or scanner type (p-interaction=0.43). In contrast, there was no evidence for an association of pack years with segmental airway lumen diameter (Table 2). Findings were similar after the exclusion of participants with clinically diagnosed emphysema (0.002 mm [−0.0001 to −0.004]; p = 0.06).
Table 2.
Mean differences in airway dimensions per ten pack years of cigarette smoking.
| Per Ten Pack Years | ||
|---|---|---|
| N=7,898 | Mean difference (95% CI) |
P-value |
| Airway wall thickness, mm | ||
| Mean Difference Model 1a | 0.004 (0.002 to 0.006) |
0.0003 |
| Mean Difference Model 2b | 0.003 (0.0007 to 0.004) |
0.008 |
| Mean Difference Model 3c | 0.002 (0.00002 to 0.004) |
0.03 |
| Airway lumen diameter, mm | ||
| Mean Difference Model 1a | −0.01 (−0.02 to 0.008) |
0.4 |
| Mean Difference Model 2b | −0.01 (−0.02 to 0.002) |
0.1 |
| Mean Difference Model 3c | 0 (−0.02 to 0.01) |
0.7 |
Model 1: age, gender, principal components of ancestry
Model 2: Model 1 + education, height, weight, asthma, scanner type, CT voltage and PM2.5
Model 3: Model 2 + cigarettes per day among current smokers
Never-smokers were included in these analyses and coded as having zero pack years.
Figure 3. Continuous relationships of cigarette pack years to airway measures.
a. Wall thickness
b. Lumen diameter
The plots show the smoothed association for airway dimensions per ten pack years for mean levels of other covariates from a multivariable generalized additive model using repeated measures assuming compound symmetric covariance within MESA family units. Never-smokers were coded as having zero pack years.
In secondary analyses, pack years were also associated with increased Pi10 (0.005 mm per 10 pack-years of smoking [95%CI 0.001, 0.009]; p=0.008) and a consistent but non-significant increase in WA% (0.063% [95%CI −0.04, 0.162]; p=0.2).
Current Smoking
Current smoking was associated with narrower airway lumens in both minimal and fully adjusted models (Table 3) and there was no evidence that the magnitude of this association varied by race/ethnicity (p-interaction=0.49), gender (p-interaction=0.68) or scanner type (p-interaction=0.83). In contrast, current smoking was not associated with segmental AWT (Table 2). Findings were similar after the exclusion of participants with clinically diagnosed emphysema (−0.11 mm [−0.20 to −0.02]; p = 0.02).
Table 3.
Mean differences in airway dimensions among 1,103 current smokers compared to never smokers.
| Mean difference (95% CI) |
P-value | |
|---|---|---|
| Airway wall thickness, mm | ||
| Mean Difference Model 1a | 0.008 (−0.01 to 0.02) |
0.3 |
| Mean Difference Model 2b | 0.005 (−0.01 to 0.02) |
0.5 |
| Airway lumen diameter, mm | 3.21 (1.18) | |
| Mean Difference Model 1a | −0.17 (−0.2 to −0.09) |
<0.0001 |
| Mean Difference Model 2b | −0.11 (−0.2 to −0.02) |
0.02 |
Model 1: age, gender, principal components of ancestry
Model 2: Model 1 + education, height, weight, asthma, scanner type, CT voltage, PM2.5 and pack years.
The reference group is comprised of non-smokers. Findings for former smokers are reported in the text.
In secondary analyses, current smoking was associated with increased airway WA% (1.0% [95% CI 0.3, 1.6]; p=0.002) but not increased Pi10 (0.02 mm [−0.02, 0.05]; p=0.4) in fully adjusted models. Defining current smoking as cotinine level > 500 ng/ml in the subset with cotinine measures (467 of 3,674 participants) yielded qualitatively similar results: an association of current smoking with narrower airway lumens (−0.13 mm [95%CI −0.25, 0.00018]; p=0.05) and increased WA% (1.3 % [95% CI 0.44, 2.11]; p=0.003).
Sensitivity Analyses
Associations were similar after additionally controlling for occupational exposure to dust among the 3,701 participants with available measures: pack years were associated with thicker airway walls (0.002 mm [95%CI 0, 0.004]; p=0.03) and current smoking with narrower lumens (−0.12mm [95%CI −0.24, 0.011]; p=0.07). Results were similar when analyses were restricted to the four readers who read the majority (n=6,727) of the CT scans: in these analyses, pack years were associated with thicker airway walls (0.002 mm [95%CI 0.0001, 0.004]; p=0.04) and current smoking with narrower lumens (−0.13mm [95%CI −0.22, −0.03]: p = 0.009).
Findings were similar when the (n=84) participants with other lung diseases such as bronchiectasis, lung cancer, sarcoidosis or tuberculosis were excluded: pack years of smoking were associated with thicker airway walls (mean increase 0.002 per ten pack years [0.0002 to 0.0042]; p = 0.03) and current smoking was associated with narrower airway lumens (mean decrease −0.10 mm [−0.2 to −0.01]; p = 0.03) in fully adjusted models.
Findings also were qualitatively similar when analyses were stratified by scanner type. Every 10 pack years was associated with a 0.003 mm increase in AWT on MDCT scanners (95% CI 0.00029, 0.005; p=0.03) and a 0.002 mm increase in AWT on EBT scanners (95% CI 0.000, 0.005; p=0.3). Current smoking was associated with narrower lumens on MDCT scanners (−0.08 mm [95% CI −0.18, 0.027]; p=0.1) and EBT scanners (−0.16 mm [95% CI −0.27, −0.05]; p=0.005). The exclusion results from the Toshiba scanners yielded identical results for pack years and increased the significance of those for current smoking (−0.12 mm [95% CI −0.21, −0.03]; p=0.009).
In order to assess for potential selection bias due to the exclusion for clinical cardiovascular disease, analyses were restricted to participants age less than 65 years. These analyses also yielded similar, significant results for pack years with AWT (0.003 mm [95% CI 0.00008, 0.006]; p=0.01) and current smoking with airway lumens (−0.13 mm [95% CI −0.23, −0.03]; p=0.01).
DISCUSSION
Cumulative smoking was associated with thicker airway walls in this large, population-based cohort study. In addition, current smoking was associated with narrowed airway lumens. This is by far the largest study of which we are aware to assess these measures and is unique in doing so in a general population sample.
Two prior case-control studies reported associations between smoking and Pi10. Patel et al. studied airway dimensions on CT scan in 519 smokers with COPD and 640 smokers (siblings of the cases), and reported an association between Pi10 and pack years (r = 0.26), although that study did not control for current smoking (15). Grydeland et al. studied 463 smokers with COPD and 431 smokers, and reported an association between pack years and Pi10, but only among controls (14). The present study replicated these prior results for Pi10 in a much larger, multi-ethnic, population-based cohort, which is less subject to selection bias.
Increases in the Pi10, however, may reflect an increase in AWT or a decrement in airway wall lumen and it is unclear which aspect contributed to the findings in the prior case-control studies. The present study addressed this gap using a novel approach to examine AWT separately from lumen diameter and demonstrated definitively that cumulative smoking is associated with increased AWT.
Changes in dimensions of segmental airways on CT scan are correlated with dimensions of the small conducting airways measured histologically (16) and likely reflect airway remodeling, goblet cell hyperplasia or both. Increased mucus in the airway lumen is an alternative explanation as mucus is indistinguishable from wall on quantitative CT scanning; however, this is an unlikely explanation for the finding of increased AWT as cumulative smoking was not associated with reduced lumen diameter, as would be expected with mucus.
The mechanism of smoking-induced small airway remodeling in humans remains an open question. Chronic smoke exposure causes increased airway wall collagen in mouse (41-43) and guinea pig models (44). Guinea pigs exposed to cigarette smoke for six months had increased AWT and increased thick collagen fibers in airway walls, which were associated with reduced peak expiratory flow and FEV1/FVC, and increased airway resistance (45). Acute smoke exposure leads to upregulation of type I pro-collagen and transforming growth factor-β (TGF-β) gene expression in mice, (42) and upregulation of collagen and TGF-β in rats (46). In guinea pigs exposed to cigarette smoke, small airway remodeling was blocked by an inhibitor of matrix matalloproteinases 9 and 12 (44).
Alternatively, higher levels of exposure to cigarette smoke were associated with more severe pathophysiologic changes in the small airways, including goblet cell metaplasia, and the severity of these changes was associated with decreased lung function in smokers undergoing resection for pulmonary tumors (47). Several investigators have observed increased numbers of goblet cells in the small airways of smokers (7, 48). Smoking-related increases in the number, size or function of goblet cells in the small airways might reduce lung function either via mucus plugs that completely occlude the airway lumen, or by altering airway surface tension (47, 49).
We also observed that current smoking was associated with smaller airway lumens. Mucus may be one explanation for this finding, and greater WA% on CT scan previously has been associated with chronic bronchitis (50); however, current smoking was not associated with increased AWT, as would be expected with goblet cell hyperplasia and mucus secretion. Alternative explanations include broncho-constricting effects of acute smoke exposure (51) or of acute smoking-related inflammation (52).
The present study has a number of limitations. The magnitude of the increase in wall thickness observed in this study was modest. However, the study participants were recruited from the general population, so the increase related to smoking is expected to be smaller than that previously observed in clinical populations of patients with COPD. In addition, measurement error in AWT may have led to an underestimate of the true association, given that the measurement error was not related to smoking status and hence likely biased associations toward the null. Airflow in the small peripheral airways can be modeled using Poiseuille’s Law, in which airflow is proportional to airway radius to the fourth power (49). Thus, to the extent that airway dimensions in segmental airways predict dimensions in small peripheral airways, even the comparatively small differences in airway dimensions associated with smoking in this study may have important implications for airflow limitation. This is consistent with the findings of Hogg and colleagues, who observed relatively greater airway wall thickening in patients with early stage COPD and greater destruction of the small airways in participants with more advanced disease (53).
There is potential for misclassification of smoking history given its retrospective ascertainment. Current smoking may have been misrepresented; however, analysis based upon cotinine-confirmed smoking status in half the cohort yielded similar results. Furthermore, discrepancies of self-reported vs. cotinine-confirmed smoking were rare and non-differential by airway wall thickness, which suggests results for current smoking and possibly pack years are conservative.
Cross-sectional studies may be limited by selection bias, but such bias is unlikely to explain the results since the study was population-based, participants were not selected based on airway dimensions, and findings persisted when analyses were restricted to participants less than 65 years of age, a group relatively free of exclusions for cardiovascular disease.
Lung structure was measured on the lung regions of cardiac-gated CT scans. Two dimensional measures of airway wall thickness and lumen diameter were assessed using methods similar to those used by Aysola et. al, whose CT airway measures correlated with airway epithelial thickness on endobronchial biopsy samples (54). The cardiac scans did not allow assessment of upper-lobe airways; however it is unclear if lower or upper airway measures are preferred and they are correlated (54). The resolution of the scans was sufficient to measure segmental airways but not smaller airways that are arguably the more relevant site for investigation of smoking-related pathophysiology. Other investigators have shown that segmental airway thickening is correlated with thickening in the more distal small airways (16). The scans did not allow for three-dimensional reconstruction, which may affect external validity, but would not affect internal validity. Cardiac-gating reduced the motion artifact typical in the left lower lobe of full lung scans, allowing more precise measures in both lower lobes. Variations in slice thickness and scanner resolution by scanner type may have affected airway measurement accuracy. However, this variability existed across all subgroups, scanner type was accounted for within the statistical models, and findings were similar when analyses were stratified by scanner type. Finally, observational studies are potentially subject to confounding; however, smoking is the dominant risk factor for COPD and we adjusted for multiple potential confounders.
In conclusion, our findings suggest that chronic cigarette smoking, as measured by pack years, is associated with thicker airway walls, and that current smoking is associated with narrower airway lumens. Smoking likely contributes to changes in airway wall structure prior to the development of clinical disease.
Comments.
This is the first large, population-based study of the relationship between smoking and airway dimensions on CT scan. We used a novel approach to assess separately the contributions of cumulative and current smoking on airway wall thickness and lumen diameter among an n= 7,898 participant multi-ethnic cohort. We found that cumulative cigarette smoking was associated with subclinical increases in sub-segmental airway wall thickness, whereas current cigarette smoking was associated with reduced lumen diameter. Smoking may contribute to chronic obstructive pulmonary disease chronically via airway wall thickening, and acutely via narrower lumens.
ACKNOWLEDGEMENT
The authors wish to thank David McAllister, MD for helpful comments. This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.MESA-nhlbi.org. Dr. Donohue had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) through N01 HC-95159 through N01-HC-95169 and RR-024156. MESA Air is conducted and supported by the United States Environmental Protection Agency through RD831697. MESA Family is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) through R01HL071051, R01HL071205, R01HL071250 through R01HL0712502, R01HL071251, R01HL071252, R01HL071258, and R01HL071259. The MESA Lung Study is supported by R01HL077612 and RC1HL100543.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: Dr. Donohue performed the statistical analysis and drafted the manuscript. Drs. Hoffman, Guo, Budoff, Austin, and Ms. Baumhauer provided data collection and critical revisions. Drs. Kalhan, Kawut and Tracy provided critical revisions. Dr. Barr provided funding, data collection and critical revisions.
REFERENCES
- 1.Minino AM, Xu J. National Vital Statistics Reports: Deaths: Preliminary Data for 20082010. 2010 Dec 16; Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. [PubMed]
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American journal of respiratory and critical care medicine. 2007;176(6):532–55. doi: 10.1164/rccm.200703-456SO. Epub 2007/05/18. [DOI] [PubMed] [Google Scholar]
- 3.Aziz ZA, Wells AU, Desai SR, Ellis SM, Walker AE, MacDonald S, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005;185(6):1509–15. doi: 10.2214/AJR.04.1578. Epub 2005/11/24. [DOI] [PubMed] [Google Scholar]
- 4.Timmins SC, Diba C, Farrow CE, Schoeffel RE, Berend N, Salome CM, et al. The Relationship between Airflow Obstruction, Emphysema Extent and Small Airways Function in Copd. Chest. 2012 doi: 10.1378/chest.11-2169. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa H, Fujimoto K, Fukushima Y, Kaji Y. Thin-section CT imaging that correlates with pulmonary function tests in obstructive airway disease. Eur J Radiol. 2011;80(2):e157–63. doi: 10.1016/j.ejrad.2010.06.010. Epub 2010/07/14. [DOI] [PubMed] [Google Scholar]
- 6.Achenbach T, Weinheimer O, Biedermann A, Schmitt S, Freudenstein D, Goutham E, et al. MDCT assessment of airway wall thickness in COPD patients using a new method: correlations with pulmonary function tests. Eur Radiol. 2008;18(12):2731–8. doi: 10.1007/s00330-008-1089-4. Epub 2008/07/22. [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. American journal of respiratory and critical care medicine. 2000;162(3 Pt 1):1102–8. doi: 10.1164/ajrccm.162.3.9907120. Epub 2000/09/16. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–8. doi: 10.1136/bmj.1.6077.1645. Epub 1977/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dockery DW, Speizer FE, Ferris BG, Jr., Ware JH, Louis TA, Spiro A., 3rd Cumulative and reversible effects of lifetime smoking on simple tests of lung function in adults. Am Rev Respir Dis. 1988;137(2):286–92. doi: 10.1164/ajrccm/137.2.286. Epub 1988/02/01. [DOI] [PubMed] [Google Scholar]
- 10.Peat JK, Woolcock AJ, Cullen K. Decline of lung function and development of chronic airflow limitation: a longitudinal study of non-smokers and smokers in Busselton, Western Australia. Thorax. 1990;45(1):32–7. doi: 10.1136/thx.45.1.32. Epub 1990/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115(2):195–205. doi: 10.1164/arrd.1977.115.2.195. Epub 1977/02/01. [DOI] [PubMed] [Google Scholar]
- 12.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291(15):755–8. doi: 10.1056/NEJM197410102911503. Epub 1974/10/10. [DOI] [PubMed] [Google Scholar]
- 13.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298(23):1277–81. doi: 10.1056/NEJM197806082982303. Epub 1978/06/08. [DOI] [PubMed] [Google Scholar]
- 14.Grydeland TB, Dirksen A, Coxson HO, Pillai SG, Sharma S, Eide GE, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;34(4):858–65. doi: 10.1183/09031936.00167908. Epub 2009/03/28. [DOI] [PubMed] [Google Scholar]
- 15.Patel BD, Coxson HO, Pillai SG, Agusti AG, Calverley PM, Donner CF, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2008;178(5):500–5. doi: 10.1164/rccm.200801-059OC. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 16.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. American journal of respiratory and critical care medicine. 2005;171(2):142–6. doi: 10.1164/rccm.200407-874OC. Epub 2004/11/02. [DOI] [PubMed] [Google Scholar]
- 17.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. American journal of respiratory and critical care medicine. 2010;181(4):353–9. doi: 10.1164/rccm.200907-1008OC. Epub 2009/11/21. [DOI] [PubMed] [Google Scholar]
- 18.Rutten EP, Grydeland TB, Pillai SG, Wagers S, Dirksen A, Coxson HO, et al. Quantitative CT: Associations between Emphysema, Airway Wall Thickness and Body Composition in COPD. Pulm Med. 2011 Aug 06;2011:419328. doi: 10.1155/2011/419328. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grydeland TB, Thorsen E, Dirksen A, Jensen R, Coxson HO, Pillai SG, et al. Quantitative CT measures of emphysema and airway wall thickness are related to D(L)CO. Respiratory medicine. 2011;105(3):343–51. doi: 10.1016/j.rmed.2010.10.018. Epub 2010/11/16. [DOI] [PubMed] [Google Scholar]
- 20.Ashraf H, Lo P, Shaker SB, de Bruijne M, Dirksen A, Tonnesen P, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66(1):55–60. doi: 10.1136/thx.2009.132688. Epub 2010/10/28. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. Epub 2002/10/25. [DOI] [PubMed] [Google Scholar]
- 22.Manichaikul A, Chen WM, Williams K, Wong Q, Sale MM, Pankow JS, et al. Analysis of family- and population-based samples in cohort genome-wide association studies. Hum Genet. 2011 doi: 10.1007/s00439-011-1071-0. Epub 2011/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen MA, Adar SD, Allen RW, Avol E, Curl CL, Gould T, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43(13):4687–93. doi: 10.1021/es8030837. Epub 2009/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. Epub 1978/12/01. [PubMed] [Google Scholar]
- 25.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201–10. doi: 10.1059/0003-4819-152-4-201002160-00004. Epub 2010/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr., et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. Epub 2004/12/25. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–99. doi: 10.1016/j.acra.2008.12.024. Epub 2009/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo JRJ, Kitaoka H, Zhang L, Sonka M, McLennon G, Hoffman EA. Integrated system for CT-based assessment of parenchymal lung disease. IEEE International Symposium on Biomedical Imaging. 2002:871–4. [Google Scholar]
- 29.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):519–32. doi: 10.1513/pats.200603-086MS. Epub 2006/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Souza N. RJaHE. ASAP: Interactive Quantification of 2D Airway Geometry. SPIE Medical Imaging. 1996;2709:197–208. [Google Scholar]
- 31.Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. The National Emphysema Treatment Trial Research Group. Chest. 1999;116(6):1750–61. doi: 10.1378/chest.116.6.1750. Epub 1999/12/14. [DOI] [PubMed] [Google Scholar]
- 32.Amirav I, Kramer SS, Grunstein MM, Hoffman EA. Assessment of methacholine-induced airway constriction by ultrafast high-resolution computed tomography. J Appl Physiol. 1993;75(5):2239–50. doi: 10.1152/jappl.1993.75.5.2239. Epub 1993/11/01. [DOI] [PubMed] [Google Scholar]
- 33.Reinhardt JMDSN, Hoffman EA. Accurate Measurement of Intra-thoracic Airways. IEEE Transactions on Medical Imaging. 1998;16(6):820–7. doi: 10.1109/42.650878. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman EA, Reinhardt JM, Sonka M, Simon BA, Guo J, Saba O, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of computed tomography images of lung structure and function. Acad Radiol. 2003;10(10):1104–18. doi: 10.1016/s1076-6332(03)00330-1. Epub 2003/11/01. [DOI] [PubMed] [Google Scholar]
- 35.Reinhardt J, Raab S, D’Souza N, et al., editors. Proceedings of SPIE. Newport Beach, CA: 1997. Intrathoracic airway measurement: ex-vivo validation. [Google Scholar]
- 36.Saba OI, Hoffman EA, Reinhardt JM. Maximizing quantitative accuracy of lung airway lumen and wall measures obtained from X-ray CT imaging. J Appl Physiol. 2003;95(3):1063–75. doi: 10.1152/japplphysiol.00962.2002. Epub 2003/05/20. [DOI] [PubMed] [Google Scholar]
- 37.Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. Copd. 2012;9(2):151–9. doi: 10.3109/15412555.2012.654923. Epub 2012/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field center and laboratory procedures. University of Washington; Seattle: [cited 2011 February 16]. 2008. MESA Manual of Operations. Available from: http://www.mesa-nhlbi.org/manuals.aspx. [Google Scholar]
- 39.Divers J. Principal component analysis of the MESA-SHARE data. Wake Forest University; 2010. p. 5. [Google Scholar]
- 40.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. Epub 1986/03/01. [PubMed] [Google Scholar]
- 41.Bracke KR, D’Hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177(7):4350–9. doi: 10.4049/jimmunol.177.7.4350. Epub 2006/09/20. [DOI] [PubMed] [Google Scholar]
- 42.Churg A, Tai H, Coulthard T, Wang R, Wright JL. Cigarette smoke drives small airway remodeling by induction of growth factors in the airway wall. American journal of respiratory and critical care medicine. 2006;174(12):1327–34. doi: 10.1164/rccm.200605-585OC. Epub 2006/09/30. [DOI] [PubMed] [Google Scholar]
- 43.Bracke KR, D’Hulst AI, Maes T, Demedts IK, Moerloose KB, Kuziel WA, et al. Cigarette smoke-induced pulmonary inflammation, but not airway remodelling, is attenuated in chemokine receptor 5-deficient mice. Clin Exp Allergy. 2007;37(10):1467–79. doi: 10.1111/j.1365-2222.2007.02808.x. Epub 2007/09/22. [DOI] [PubMed] [Google Scholar]
- 44.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62(8):706–13. doi: 10.1136/thx.2006.068353. Epub 2007/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright JL, Postma DS, Kerstjens HA, Timens W, Whittaker P, Churg A. Airway remodeling in the smoke exposed guinea pig model. Inhal Toxicol. 2007;19(11):915–23. doi: 10.1080/08958370701515563. Epub 2007/09/13. [DOI] [PubMed] [Google Scholar]
- 46.Wang RD, Wright JL, Churg A. Transforming growth factor-beta1 drives airway remodeling in cigarette smoke-exposed tracheal explants. Am J Respir Cell Mol Biol. 2005;33(4):387–93. doi: 10.1165/rcmb.2005-0203OC. Epub 2005/07/05. [DOI] [PubMed] [Google Scholar]
- 47.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–82. doi: 10.1148/radiol.11110173. Epub 2011/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacNee W, Wiggs B, Belzberg AS, Hogg JC. The effect of cigarette smoking on neutrophil kinetics in human lungs. N Engl J Med. 1989;321(14):924–8. doi: 10.1056/NEJM198910053211402. Epub 1989/10/05. [DOI] [PubMed] [Google Scholar]
- 49.Lambert RK, Wilson TA, Hyatt RE, Rodarte JR. A computational model for expiratory flow. J Appl Physiol. 1982;52(1):44–56. doi: 10.1152/jappl.1982.52.1.44. Epub 1982/01/01. [DOI] [PubMed] [Google Scholar]
- 50.Drost EM, Selby C, Bridgeman MM, MacNee W. Decreased leukocyte deformability after acute cigarette smoking in humans. Am Rev Respir Dis. 1993;148(5):1277–83. doi: 10.1164/ajrccm/148.5.1277. Epub 1993/11/01. [DOI] [PubMed] [Google Scholar]
- 51.Da Silva AM, Hamosh P. Effect of smoking a single cigarette on the “small airways”. Journal of applied physiology. 1973;34(3):361–5. doi: 10.1152/jappl.1973.34.3.361. Epub 1973/03/01. [DOI] [PubMed] [Google Scholar]
- 52.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. American journal of respiratory and critical care medicine. 1999;159(2):473–9. doi: 10.1164/ajrccm.159.2.9804080. Epub 1999/02/02. [DOI] [PubMed] [Google Scholar]
- 53.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–75. doi: 10.1056/NEJMoa1106955. Epub 2011/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008;134(6):1183–91. doi: 10.1378/chest.07-2779. Epub 2008/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]