Abstract
Depression has been associated with reduced expression of brain-derived neurotrophic factor (BDNF) in the hippocampus. Genetic association studies of the BDNF Val66Met polymorphism (rs6265) in geriatric depression have produced inconsistent results. A meta-analysis of studies was conducted to compare the frequency of the BDNF Val66Met variant between cases with geriatric depression and age-matched controls. A total of five studies involving 523 cases with geriatric depression and 1,220 psychiatrically healthy controls was included. Met allele carriers had an increased risk for geriatric depression when compared to Val/Val homozygotes (P =0.004, OR =1.48, 95% CI =1.13–1.93). Our findings suggest the BDNF Met allele may confer increased risk for depression as individual age.
Keywords: BDNF, Val66Met, geriatric, depression, meta-analysis
INTRODUCTION
Depression among the elderly (geriatric depression) is a common psychiatric condition, with a prevalence of 10–20% [Borson et al., 1986; Beekman et al., 1995; Copeland et al., 1999]. Geriatric depression is associated with personal suffering as well as elevated risk of mortality due to suicide and medical illness; it is also associated with increased disability associated with medical and cognitive disorders [Lebowitz et al., 1997; Alexopoulos and Kelly, 2009].
The causes of geriatric depression are likely diverse and complex, and heritable factors contribute to the disorder [Alexopoulos and Kelly, 2009]. Studies to date have moved the field of mood disorders research beyond the monoamine hypothesis of depression. Neurotrophic factors in limbic brain regions play an important role in the regulation of mood and cognition. Brain-derived neurotrophic factor (BDNF) is widely distributed in the central nervous system (CNS), including the hippocampus, neocortex, amygdala, cerebellum, and hypothalamus, all of which are key regions in the regulation of mood. BDNF is necessary for dendritic growth, synaptic plasticity, and long-term potentiation [Maisonpierre et al., 1990]. Additionally, it modulates the activity of neurotransmitter systems in the CNS involved in many mood disorders including serotonin, dopamine, and glutamate [Mossner et al., 2000; Guillin et al., 2001; Carvalho et al., 2008]. Hippocampal BDNF levels decrease in depression and increase following anti-depressant treatment [Kuroda and McEwen, 1998; Madsen et al., 2000; Chen et al., 2001; Dranovsky and Hen, 2006; Duman and Monteggia, 2006]. Similarly, serum BDNF levels decrease in adult patients with major depression disorder and increase in patients treated with antidepressants [Karege et al., 2002; Castren et al., 2007; Post, 2007; Yoshimura et al., 2007; Piccinni et al., 2008; Sen et al., 2008; Hashimoto, 2010]. One study [Diniz et al., 2010] also found that serum BDNF level is reduced in antidepressant-free patients with late-life depression.
Basic and clinical studies have examined the association between BDNF polymorphisms and depression, including geriatric depression. To date, most published studies have focused on a functional valine-to-methionine polymorphism at codon 66 (Val66Met also known as G196A or rs6265) that results from a guanine to adenine substitution at base 196 [Schumacher et al., 2005]. Many studies have shown that the BDNF valine-to-methonine substitution at codon 66 produces functional differences [Hariri et al., 2003; Egan et al., 2003a,b; Rybakowski et al., 2003, 2006; Chen et al., 2005, 2006; Dempster et al., 2005; Tan et al., 2005]. In cultured hippocampal neurons, the Met allele was associated with differing activity-dependent secretion of the BDNF protein and failure of the protein to localize to secretory granules or synapses [Egan et al., 2003a,b; Chen et al., 2004]. These trafficking abnormalities are likely to reflect impaired binding of the Met allele to sortilin, a Vps10p domain protein that influences sorting of BDNF into regulated secretory pathways [Chen et al., 2005].
Association studies examining the role of the BDNF Val66Met polymorphism and geriatric depression have been inconsistent, potentially due to low power in individual studies, etiological heterogeneity, or because of random error in the absence of a true effect [Glatt et al., 2003; Lau and Eley, 2010]. In this study, we carefully reviewed and selected qualified studies, using a meta-analysis to investigate the role of the BDNF Val66Met polymorphism in geriatric depression. Meta-analysis provides the most accurate estimate of the nature and magnitude of an effect by combining the results of multiple independent studies, to reduce the potential influence of types I and II errors that occur within individual studies [Gu et al., 2001].
MATERIALS AND METHODS
Search Strategy
All published studies examining the association of the BDNF Val66Met polymorphism with geriatric depression were carefully selected by three independent authors (Y.J. Wang, Y. Pei, and W.G. Pan. Data were collected from the electronic databases PubMed and Ovid. These databases were searched from the first date available up to September 30, 2011. The keywords “BDNF”, “brain-derived neurotrophic factor,” “Val66Met,” “rs6265,” “G196A,” “196G/ A,” “mood,” “affective,” “depress,*” “unipolar,” “geriatric,” “old,*” “elder,*” “aged,” and “late onset” were used for searching. Meanwhile, we also searched the reference lists of included studies to identify other potentially eligible studies. We only included data from full-length papers, not abstracts from conference proceedings.
Inclusion Criteria
All case–control studies reporting genotype or allele frequencies of the Val66Met polymorphisms in geriatric depression and in psychiatrically healthy controls were eligible for inclusion in this meta-analysis. Case status was defined as having a current or 1-year diagnosis of geriatric depression (60 years or older) assessed by established psychiatric interviews. The diagnosis of depression was based on either DSM or ICD, including DSM-III, DSM-III-R, DSM-IV, DSM-IV-R, and ICD-10, or if participants had clinically significant depressive symptoms according to a validated depression rating scale (Table I). We included studies involving samples with any ethnic background. Case-only studies, family-based designs, and population-based studies of healthy subjects were not included. In addition, we did not include studies on post-stroke depression or Alzheimer’s disease (AD)-related depression to limit phenotypic heterogeneity.
TABLE I.
Characteristics of the Studies Included in the Meta-Analysis
| Geriatric disorder Refs. | Ethnic | Depression measure | Case
|
Control
|
||
|---|---|---|---|---|---|---|
| Met carriers | Val/Val | Met carriers | Val/Val | |||
| Hwang et al. [2006] | Asian | DSM-IV | 83 | 27 | 106 | 65 |
| Taylor et al. [2007] | Caucasian | DDES | 95 | 150 | 23 | 71 |
| Kim et al. [2007] | Asian | GMS | 75 | 26 | 473 | 158 |
| Kanellopoulos et al. [2011] | Caucasian | DSM-IV | 16 | 17 | 11 | 12 |
| Czira et al. [2011] | Caucasian | CES-D | 14 | 20 | 87 | 214 |
| Total | 355 | 344 | 721 | 587 | ||
Met, methionine; Val, valine; DDES, Duke Depression Evaluation Schedule; GMS, Geriatric Mental State diagnostic schedule; CES-D, Center for Epidemiologic Studies-Depression.
Data Extraction
Three authors (C.L. Tie, W.G. Pan, and Y. Pei) independently extracted data to avoid potential mistakes. Discrepancies were resolved by discussions within the research team. From each study, we extracted the first author’s name, year of publication, source of publication, ethnicity of samples, depression measure (or diagnostic system), number of cases and controls, and the available genotype and allele frequency information of the BDNF Val66Met polymorphism. Ethnicity was coded as Caucasian or Asian as determined by the published report. No other ethnicities were involved in the studies included in our analysis.
Meta-Analysis Methods
We examined the relationship between the frequency of the Met allele and diagnosis of geriatric depression. The odds ratio (OR) and its 95% confidence interval (95% CI) were estimated for each study. We performed a chi-squared-based Q-statistic test to assess between-study heterogeneity [Lau et al., 1997]. In this analysis, P <0.1 indicates the presence of significant heterogeneity. The inconsistency index I2 was also calculated to evaluate heterogeneity. We also measured the effect of heterogeneity by another measure, I2 =100% × (Q − df)/Q [Higgins and Thompson, 2002]. Depending on the results of the heterogeneity tests among individual studies, we used a fixed-effects model (if P >0.1) [Mantel and Haenszel, 1959] or a random-effects model (if P <0.1) [DerSimonian and Laird, 1986] to summarize the pooled OR. We used a Z-test with a significance threshold of P <0.05 to evaluate the OR.
Evaluation of Publication Bias
Publication bias, that is, the preferential publication of a study with positive findings, was evaluated with a funnel plot such that asymmetry of the funnel plot suggests bias. Both Begg’s and Egger’s tests were used to statistically assess publication bias (P <0.05). Sensitivity analysis was conducted by sequential deletion of a single study in an attempt to assess the contribution of each individual dataset to the pooled OR obtained.
All analyses were performed using the software Stata (version 10.1, Stata Corp LP, College Station, TX).
RESULTS
Description of Studies Identified in Meta-Analysis
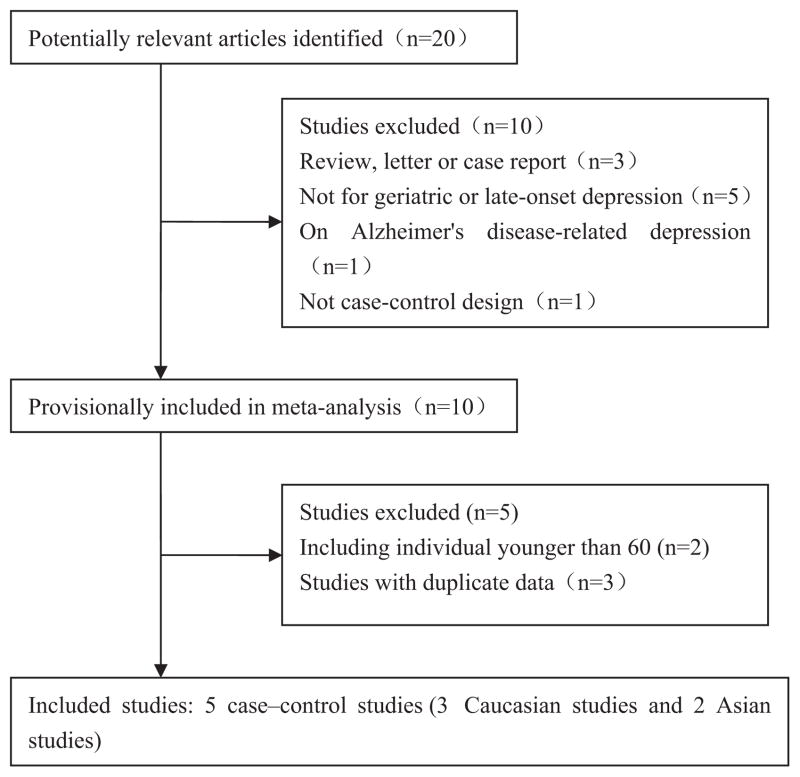
We identified 20 potentially relevant research papers using our search strategies, but 15 did not meet the inclusion criteria (Fig. 1) after reviewing the abstracts or papers. The excluded papers included five studies that were not focusing on geriatric depression [von Bohlen und Halbach et al., 2006; Hayden et al., 2010; Hedner et al., 2010; Erickson et al., 2011], three review articles [Arantes-Goncalves and Coelho, 2006; Frodl et al., 2008; Savitz and Drevets, 2009], and one that was not a case–control design [Taylor et al., 2011]. We also excluded one study on AD-related depression [Borroni et al., 2009] and two other studies that contained cases or controls younger than 60-year old [Surtees et al., 2007; You et al., 2010], were also excluded. Studies with overlapping patient samples were discarded and the most complete dataset was selected for the meta-analysis [Kim et al., 2008; Lin et al., 2009; Benjamin et al., 2010].
FIG. 1.
Flow diagram of the study selection process. Studies included in this meta-analysis were limited to primary reports of non-overlapping individuals >60-year old with depression. Depression that occurred following a stroke in cohorts with Alzheimer’s disease were excluded to limit phenotypic heterogeneity.
Five case–control datasets were included in this meta-analysis (Table I) [Hwang et al., 2006; Kim et al., 2007; Taylor et al., 2007; Czira et al., 2011; Kanellopoulos et al., 2011]. Overall, the studies included 523 cases and 1,220 psychiatrically healthy controls (3 Caucasians and 2 Asians cohorts).
Effect of BDNF Val66Met on Geriatric Depression
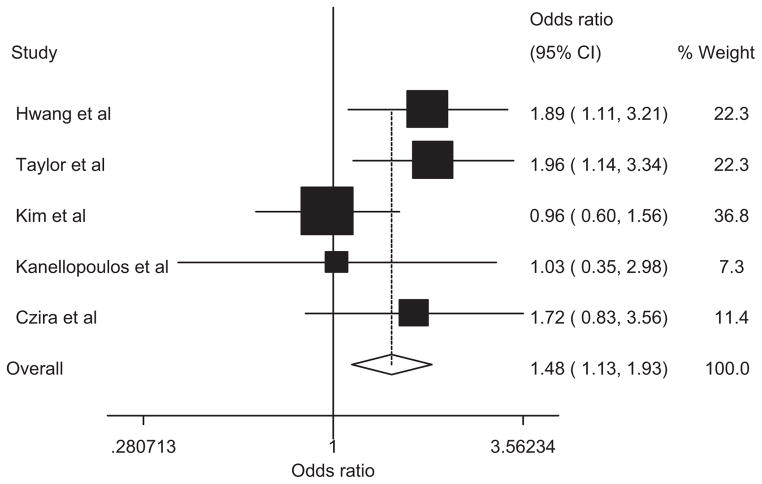
We tested the association between geriatric depression and the BDNF Val66Met polymorphism by estimating the OR of Met carriers (Met/Met and Val/Met) versus the Val/Val genotype. The pooled analysis was carried out with a fixed-effects model, as no evidence of heterogeneity was found (I2 =27.3%, P >0.1). The Met allele was significantly associated with geriatric depression (P =0.004, OR =1.48, 95% CI =1.13–1.93). The summary of the meta-analysis for the BDNF Val66Met polymorphisms (Met carriers vs. the Val/Val) and geriatric depression is shown in Figure 2.
FIG. 2.
Forest plots for meta-analyses. The weight of each study in the overall analysis is reflected by the size of squares. Whiskers represent 95% confidence intervals. The pooled OR for Met carries versus Val/Val is based on the fixed-effect model.
Publication Bias and Sensitivity Analysis
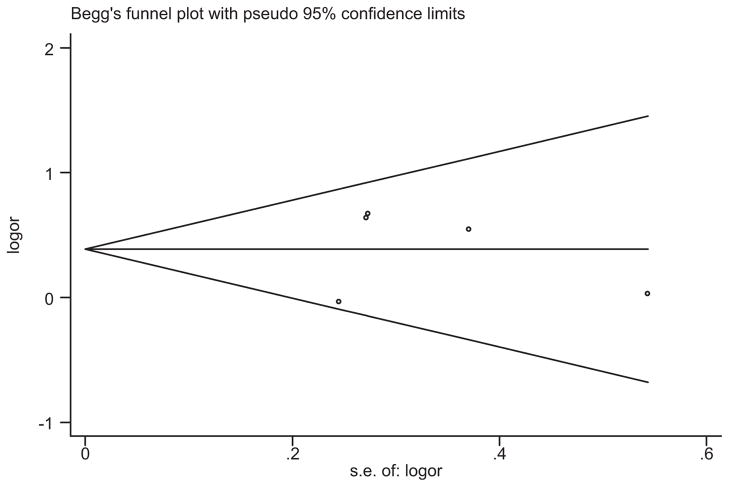
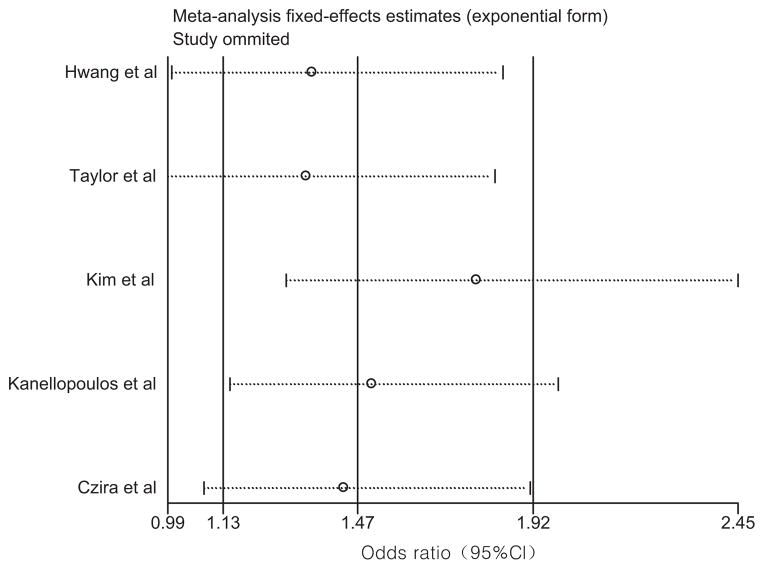
A funnel plot was generated to explore publication bias, but no evidence asymmetry was observed (Begg’s test; Z =0; P =1; Fig. 3). Egger’s test was also performed to investigate the symmetry of the funnel plot, and the results were consistent (t = −0.04, P =0.97). Sensitivity analyses were conducted to assess the degree to which each individual study influenced the results of the overall analysis (Fig. 4). If the study with the largest effect size [Taylor et al., 2007] was excluded, the association of the Met allele with geriatric depression would be reduced (P =0.06) in a meta-analysis of the four remaining studies. However, this is consistent with loss of power, and removal of any other study did not significantly impact the results of the overall analysis.
FIG. 3.
Funnel plot analysis to detect publication bias. Each dot represents one study. Location outside the delineated triangle (pseudo 95% confidence intervals) suggests a publication bias.
FIG. 4.
Sensitivity of individual studies used in the meta-analysis. The circles represent odds ratio of the overall analyses if the individual study is not included. The lines represent the confidence intervals for the individual study.
DISCUSSION
To our knowledge, this is the first comprehensive meta-analysis of studies examining the association of the BDNF Val66Met polymorphism with geriatric depression. Previously meta-analyses of this polymorphism in adults suggest little, if any, association of this polymorphism with major depressive disorder (MDD) [Chen et al., 2008; Verhagen et al., 2010] or mood disorders [Gratacos et al., 2007].
Our analysis suggests that Met carriers are at increased risk for geriatric depression. The Met allele is associated with decreased activity of the BDNF system [Rybakowski, 2008]. Mice with the BDNF Met/Met genotype showed almost a 30% reduction in regulated release of BDNF [Chen et al., 2006]. Further, BDNFMet/Met mice have decreased N-methyl-D-aspartic acid (NMDA) receptor neurotransmission in the CA1 pyramidal neurons as well as decreased NMDA receptor-dependent long-term depression compared to wild-type (BDNFVal/Val) mice [Ninan et al., 2010]. BDNFMet/Met mice also exhibit increased anxiety-related behaviors [Chen et al., 2006] and reduced extinction of fear learning compared with BDNFVal/Val mice [Heldt et al., 2007; Soliman et al., 2010].
Decreased activity of the BDNF system resulting from the Met allele may have a greater effect on the elderly. Neuropsychological, neuroimaging, and neuropathological studies support the multi-factorial nature of geriatric depression and delineate it from depression in younger adults [Beekman, 2011]. Studies of geriatric depression show deficits consistent with normal aging as well as cerebrovascular and neurodegenerative disease processes [Smith et al., 2007]. In healthy humans, Met allele carriers have poorer episodic memory performance and reduced hippocampal physiologic engagement during functional magnetic resonance imaging (fMRI) studies; they also have reduced prefrontal and hippocampal gray matter volume [Egan et al., 2003a; Pezawas et al., 2004; Szeszko et al., 2005; Bueller et al., 2006; Yu et al., 2009]. Met carriers also showed a greater age-related decline in hippocampal activation during both encoding and retrieval tasks relative to Val/Val individuals [Sambataro et al., 2010]. Reduced hippocampal volume is also reported in older people with depression, those with both early-onset and late-onset disorders [Hickie et al., 2005]. Patients with late-onset depression also have more white matter hyperintensity than younger adults [Lesser et al., 1996]. In individuals with late-onset depression, the Met66 allele is associated with greater white matter hyperintensity volumes prompting the need to determine how this might be associated with other clinically relevant findings [Taylor et al., 2008]. Above all, we hypothesize that the Met allele might play a more important role in geriatric depression.
A few limitations of this meta-analysis should be acknowledged. First, we did not perform gender-specific analyses because power was limited to detect associations in stratified datasets. Second, we only focused on geriatric depression and excluded studies in which the age of onset was less than or equal to 60 years as well as studies in older adults with AD. Because depression can be a prodromal symptom of AD [Ahmed, 2001], studies in this meta-analysis ruled out possible dementia using the Mini Mental State Examination (MMSE). The relationship between BDNF polymorphisms, AD and geriatric depression needs further examination in light of the association between the BDNF Met allele and AD [Fukumoto et al., 2010]. Third, studies in this analysis were limited to Caucasians and Asians. As studies in other ethnic groups are conducted, they should be examined in a meta-analytical framework to evaluate the generalizability of these results.
In summary, our analysis suggests that the BDNF Met allele is a risk factor for geriatric depression. The differential role of age of onset in the association should be examined in future studies.
Acknowledgments
Grant sponsor: National Natural Science Foundation of China; Grant number: 81071102; Grant sponsor: National Institute of Mental Health; Grant number: MH085806.
This study was supported by the grants of National Natural Science Foundation of China (No. 81071102 to X.M.) and the National Institute of Mental Health (MH085806 to A.K.S.). The authors would like to thank Yilang Tang, M.D., Ph.D., Department of Psychiatry and Behavioral Sciences, Emory University and Yang Li, Ph.D., Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen for his constructive comments on an earlier version of this manuscript.
Footnotes
The authors declared that they have no conflicts of interest.
References
- Ahmed MB. Alzheimer’s disease: Recent advances in etiology, diagnosis, and management. Tex Med. 2001;97:50–58. [PubMed] [Google Scholar]
- Alexopoulos GS, Kelly RE., Jr Research advances in geriatric depression. World Psychiatry. 2009;8:140–149. doi: 10.1002/j.2051-5545.2009.tb00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Goncalves F, Coelho R. Depression and treatment. Apoptosis, neuroplasticity and antidepressants. Acta Med Port. 2006;19:9–20. [PubMed] [Google Scholar]
- Beekman AT. Neuropathological correlates of late-life depression. Expert Rev Neurother. 2011;11:947–949. doi: 10.1586/ern.11.88. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, van Tilburg T, Smit JH, Hooijer C, van Tilburg W. Major and minor depression in later life: A study of prevalence and risk factors. J Affect Disord. 1995;36:65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Benjamin S, McQuoid DR, Potter GG, Payne ME, MacFall JR, Steffens DC, Taylor WD. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. Am J Geriatr Psychiatry. 2010;18:323–331. doi: 10.1097/JGP.0b013e3181cabd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Archetti S, Costanzi C, Grassi M, Ferrari M, Radeghieri A, Caimi L, Caltagirone C, Di Luca M, Padovani A. Role of BDNF Val66Met functional polymorphism in Alzheimer’s disease-related depression. Neurobiol Aging. 2009;30:1406–1412. doi: 10.1016/j.neurobiolaging.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Borson S, Barnes RA, Kukull WA, Okimoto JT, Veith RC, Inui TS, Carter W, Raskind MA. Symptomatic depression in elderly medical outpatients. I. Prevalence, demography, and health service utilization. J Am Geriatr Soc. 1986;34:341–347. doi: 10.1111/j.1532-5415.1986.tb04316.x. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl 1):S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lawlor DA, Lewis SJ, Yuan W, Abdollahi MR, Timpson NJ, Day IN, Ebrahim S, Smith GD, Shugart YY. Genetic association study of BDNF in depression: Finding from two cohort studies and a meta-analysis. Am J Med Genet Part B. 2008;147B:814–821. doi: 10.1002/ajmg.b.30686. [DOI] [PubMed] [Google Scholar]
- Copeland JR, Beekman AT, Dewey ME, Hooijer C, Jordan A, Lawlor BA, Lobo A, Magnusson H, Mann AH, Meller I, et al. Depression in Europe. Geographical distribution among older people. Br J Psychiatry. 1999;174:312–321. doi: 10.1192/bjp.174.4.312. [DOI] [PubMed] [Google Scholar]
- Czira ME, Wersching H, Baune BT, Berger K. Brain-derived neurotrophic factor gene polymorphisms, neurotransmitter levels, and depressive symptoms in an elderly population. Age (Dordr) 2011 doi: 10.1007/s11357-011-9313-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet Part B. 2005;134B:73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Talib LL, Mendonca VA, Gattaz WF, Forlenza OV. Serum brain-derived neurotrophic factor level is reduced in antidepressant-free patients with late-life depression. World J Biol Psychiatry. 2010;11:550–555. doi: 10.3109/15622970903544620. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003a;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Egan MF, Weinberger DR, Lu B. Schizophrenia III: Brain-derived neurotropic factor and genetic risk. Am J Psychiatry. 2003b;160:1242. doi: 10.1176/appi.ajp.160.7.1242. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist. 2011;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Moller HJ, Meisenzahl E. Neuroimaging genetics: New perspectives in research on major depression? Acta Psychiatr Scand. 2008;118:363–3372. doi: 10.1111/j.1600-0447.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- Fukumoto N, Fujii T, Combarros O, Kamboh MI, Tsai SJ, Matsushita S, Nacmias B, Comings DE, Arboleda H, Ingelsson M, et al. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer’s disease: New data and meta-analysis. Am J Med Genet Part B. 2010;153B:235–242. doi: 10.1002/ajmg.b.30986. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT. Meta-analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiatry. 2003;8:911–915. doi: 10.1038/sj.mp.4001321. [DOI] [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: Meta-analysis of case–control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61:911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Gu C, Province MA, Rao DC. Meta-analysis for model-free methods. Adv Genet. 2001;42:255–272. doi: 10.1016/s0065-2660(01)42027-x. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Dyson MW, Durbin CE, Sheikh HI, Singh SM. The role of brain-derived neurotrophic factor genotype, parental depression, and relationship discord in predicting early-emerging negative emotionality. Psychol Sci. 2010;21:1678–1685. doi: 10.1177/0956797610385357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner M, Nilsson LG, Olofsson JK, Bergman O, Eriksson E, Nyberg L, Larsson M. Age-related olfactory decline is associated with the BDNF Val66met polymorphism: Evidence from a population-based study. Front Aging Neurosci. 2010;2:24. doi: 10.3389/fnagi.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos D, Gunning FM, Morimoto SS, Hoptman MJ, Murphy CF, Kelly RE, Glatt C, Lim KO, Alexopoulos GS. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. Am J Geriatr Psychiatry. 2011;19:13–22. doi: 10.1097/jgp.0b013e3181f61d62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. BDNF genotype potentially modifying the association between incident stroke and depression. Neurobiol Aging. 2008;29:789–792. doi: 10.1016/j.neurobiolaging.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- Lau JY, Eley TC. The genetics of mood disorders. Annu Rev Clin Psychol. 2010;6:313–337. doi: 10.1146/annurev.clinpsy.121208.131308. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- Lebowitz BD, Pearson JL, Schneider LS, Reynolds CF, III, Alexopoulos GS, Bruce ML, Conwell Y, Katz IR, Meyers BS, Morrison MF, et al. Diagnosis and treatment of depression in late life. Consensus statement update. JAMA. 1997;278:1186–1190. [PubMed] [Google Scholar]
- Lesser IM, Boone KB, Mehringer CM, Wohl MA, Miller BL, Berman NG. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- Lin E, Hong CJ, Hwang JP, Liou YJ, Yang CH, Cheng D, Tsai SJ. Gene–gene interactions of the brain-derived neurotrophic-factor and neurotrophic tyrosine kinase receptor 2 genes in geriatric depression. Rejuvenation Res. 2009;12:387–393. doi: 10.1089/rej.2009.0871. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- Mossner R, Daniel S, Albert D, Heils A, Okladnova O, Schmitt A, Lesch KP. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF) Neurochem Int. 2000;36:197–202. doi: 10.1016/s0197-0186(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008;105:279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: Clinical and theoretical implications. J Psychiatr Res. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK. BDNF gene: Functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 2008;9:1589–1593. doi: 10.2217/14622416.9.11.1589. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Skibinska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disord. 2003;5:468–472. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Szczepankiewicz A, Kapelski P, Leszczynska-Rodziewicz A, Czerski PM, Hauser J. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin Neurosci. 2006;60:70–76. doi: 10.1111/j.1440-1819.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Lemaitre HS, Reed JD, Das S, Goldberg TE, Callicott JH, Weinberger DR, Mattay VS. BDNF modulates normal human hippocampal ageing [corrected] Mol Psychiatry. 2010;15:116–118. doi: 10.1038/mp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: Genetic correlates. Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Schulze TG, Deschner M, Schmal C, Hofels S, et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Gunning-Dixon FM, Lotrich FE, Taylor WD, Evans JD. Translational research in late-life mood disorders: Implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32:1857–1875. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Sandhu MS, Luben R, Day NE, Flint J. No association between the BDNF Val66Met polymorphism and mood status in a non-clinical community sample of 7389 older adults. J Psychiatr Res. 2007;41:404–409. doi: 10.1016/j.jpsychires.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Tan YL, Zhou DF, Cao LY, Zou YZ, Wu GY, Zhang XY. Effect of the BDNF Val66Met genotype on episodic memory in schizophrenia. Schizophr Res. 2005;77:355–356. doi: 10.1016/j.schres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Zuchner S, McQuoid DR, Steffens DC, Speer MC, Krishnan KR. Allelic differences in the brain-derived neurotrophic factor Val66Met polymorphism in late-life depression. Am J Geriatr Psychiatry. 2007;15:850–857. doi: 10.1097/JGP.0b013e318050c9d5. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Zuchner S, McQuoid DR, Payne ME, MacFall JR, Steffens DC, Speer MC, Krishnan KR. The brain-derived neurotrophic factor VAL66MET polymorphism and cerebral white matter hyperintensities in late-life depression. Am J Geriatr Psychiatry. 2008;16:263–271. doi: 10.1097/JGP.0b013e3181591c30. [DOI] [PubMed] [Google Scholar]
- Taylor WD, McQuoid DR, Ashley-Koch A, MacFall JR, Bridgers J, Krishnan RR, Steffens DC. BDNF Val66Met genotype and 6-month remission rates in late-life depression. Pharmacogenomics J. 2011;11:146–154. doi: 10.1038/tpj.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: Effects of gender and ethnicity. Mol Psychiatry. 2010;15:260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Krause S, Medina D, Sciarretta C, Minichiello L, Unsicker K. Regional- and age-dependent reduction in trkB receptor expression in the hippocampus is associated with altered spine morphologies. Biol Psychiatry. 2006;59:793–800. doi: 10.1016/j.biopsych.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, Ueda N, Nakamura J. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1034–1037. doi: 10.1016/j.pnpbp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- You J, Yuan Y, Zhang Z, Zhang X, Li H, Qian Y. A preliminary association study between brain-derived neurotrophic factor (BDNF) haplotype and late-onset depression in mainland Chinese. J Affect Disord. 2010;120:165–169. doi: 10.1016/j.jad.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS, Chen ZY. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]