Abstract
Recent work has emphasized the role that orbitofrontal cortex (OFC) plays in value-based decision-making. However, it is also clear that a number of discrepancies have arisen when comparing the findings from animal models to those from humans. In this paper, we examine several possibilities that might explain these discrepancies including anatomical difference between species, the behavioral tasks used to probe decision-making and the methodologies used to assess neural function. Understanding how these differences affect the interpretation of experimental results will help us better integrate future results from animal models. This will enable us to fully realize the benefits of using multiple approaches to understand OFC function.
Introduction
In 1998, at the Forum of European Neuroscience in Berlin, there was a symposium entitled “The Mysterious Orbitofrontal Cortex” 1. The feeling was that within the frontal lobe, an area with a long history of frustrating researchers, the function of orbitofrontal cortex (OFC) was particularly baffling. Despite that pessimism, the intervening 13 years have seen remarkable progress in our understanding. Much of this progress was driven by two factors. First, there has been theoretical convergence: researchers from a variety of fields determined that OFC plays a fundamental role in value-based decision-making. Second, researchers have employed increasingly sophisticated behavioral methods drawn from economics and psychology in order to measure decision-making. This period has been satisfying, as researchers from disparate fields have formed links between their research, and the mystery of OFC has looked increasingly solvable. However, it is perhaps time to assess how deeply this theoretical convergence extends. It is becoming clear that discrepancies exist between results from different methodologies. The goal of this review is to highlight these discrepancies and examine whether they can be explained by species differences in the function and anatomy of OFC.
Psychologists distinguish between two conceptually distinct types of decision-making. Perceptual decision-making refers to the process by which a subject makes a judgment about sensory input 2. The baggage screener examines an x-ray trying to decide whether the bag contains a gun or a hair dryer. On the other hand, value-based decision-making resembles the folk definition of decision-making: for example, deciding whether to have bacon or cereal for breakfast 3. Unlike perceptual decision-making, value-based decision-making is inherently subjective. You could make a best guess as to what I will choose based on your past experience of my choices (I usually choose bacon) and your knowledge of my current goals (I recently went on a diet), but without knowing my precise internal state it remains a guess. It is the process of valuing alternatives to determine the best choice that is thought to be a core OFC function.
Early studies of the effects of frontal lobe damage in humans emphasized the importance of OFC and the adjacent medial frontal cortex for ‘everyday’ decision-making 4. Laboratory tests sought to mimic this process with gambling tasks where money could be won or lost probabilistically 5. The flavor was certainly of value-based decision-making even though the terminology had yet to be agreed on. Later studies explicitly tested patients with OFC damage on perceptual and value-based decision-making tasks and showed impairments only on the latter 6. Furthermore, if damage was restricted to dorsolateral prefrontal areas, decision-making usually (although not always 7) remained intact 6, 8, 9. Neuroimaging studies are in broad agreement with these findings: value-based decision-making typically activates orbital and medial frontal regions rather than dorsolateral frontal areas 10–20.
Studies in monkeys have also shown that OFC damage impairs various aspects of value-based decision-making, including the ability to assign 21 and update 22 stimulus values. Early neurophysiological studies showed that OFC neurons encoded a subject’s relative preferences between different rewards 23, and that reward information was encoded more quickly in OFC than dorsolateral prefrontal cortex 24 and anterior cingulate cortex 25. Later studies, employing sophisticated methods from economics, showed that OFC neuronal activity matched the animal’s subjective valuation of the reward 26. OFC neurons in both rats and monkeys also encode a wide range of other variables necessary for decision-making, including positive and negative expected outcomes 27–29, hypothetical as well as actual outcomes 30, the amount of time 31, 32 and effort 33 necessary to acquire an outcome, confidence in the decision 34 and the probability that one’s choice will be fruitful 33.
In summary, an impressive body of evidence from an array of methods has implicated OFC in value-based decision-making. Although it is not surprising that the field has tended to emphasize the remarkable agreement in the findings 3, 35–37, discrepancies do exist. However, before discussing them, we will first review the anatomy of OFC across species.
Anatomy of OFC
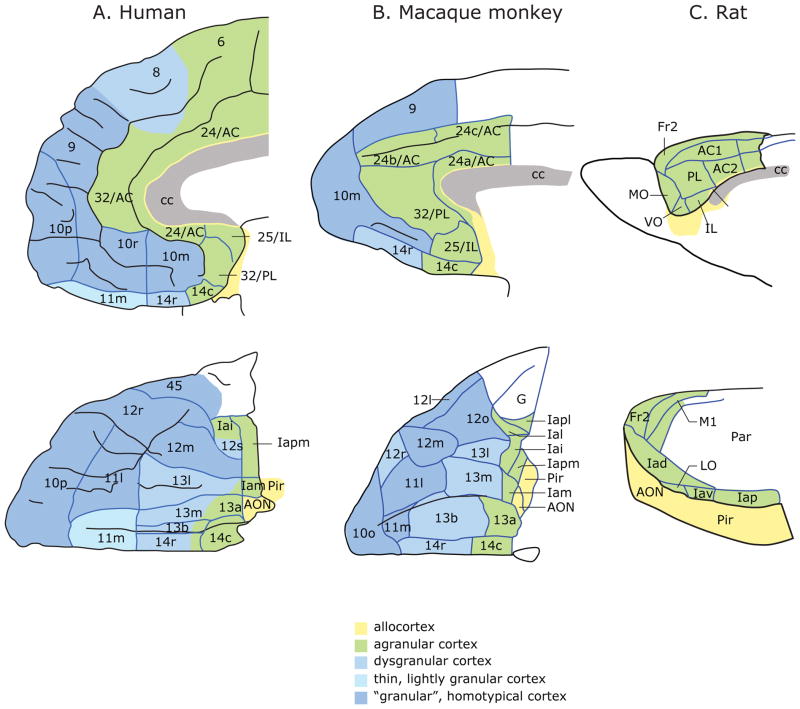
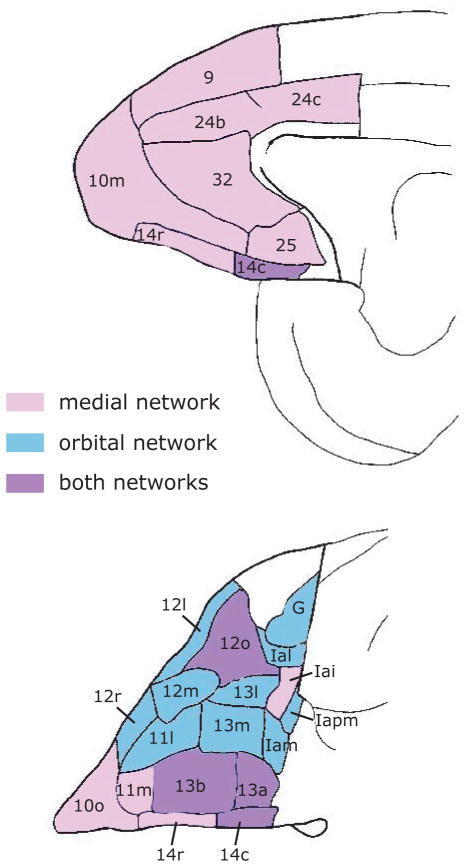
In primates, OFC lies on the ventral surface of the frontal lobe. It consists of three major cytoarchitectonic regions: area 11 anteriorly, area 13 posteriorly and area 14 medially, but also includes the ventral parts of area 10 and area 47/12 38, 39 (Fig. 1). Posterior to OFC lie the more rostral areas of insular cortex. The OFC is positioned at the intersection of multimodal sensory networks and circuits mediating emotion and memory. It has strong connections with the limbic system, including the hypothalamus, amygdala, hippocampus, nucleus accumbens and cingulate cortex 40–42, and is unique within the frontal lobe in receiving information from all sensory modalities 43–45. However, although OFC has extensive sensory connections, it only weakly connects with the motor system 44. The organization of intrinsic connections within OFC and neighboring medial prefrontal cortex suggests the presence of two functional networks 46. One network consists of areas in central OFC and the other network consists largely of areas in medial OFC and medial frontal cortex (Fig. 2). Areas within a network are strongly interconnected and show few connections with areas in the other network. A few areas are members of both networks, most obviously areas 13a and 12o, but to a lesser extent also 13b and 14c.
Figure 1.
Architectonic maps of the medial (top) and orbital (bottom) surfaces of the frontal lobe in A) humans 89 and B) monkeys 39. C) Medial (top) and lateral (bottom) frontal cortex in rats 90. Agranular cortex lacks layer IV. Dysgranular cortex contains a rudimentary layer IV. Granular cortex has a well-developed layer IV. Layer IV neurons are described as granular because their cell bodies are small and round and changes in this layer are clearly visible as one transitions from agranular to granular cortex. Abbreviations: AON, anterior olfactory nucleus; Fr2, second frontal area; I, insula; LO, lateral orbital area; M1, primary motor area; Par, parietal cortex; Pir, Piriform cortex; AC, anterior cingulate area; cc, corpus callosum; IL, infralimbic cortex; MO, medial orbital area; PL, prelimbic cortex; VO, ventral orbital area; l, lateral; m, medial; o, orbital; r, rostral; c, caudal; i, inferior; p, posterior; s, sulcal; v, ventral. Numbers indicate cortical fields, except that after certain areas, such as Fr2 and AC1, they indicate subdivisions of cortical fields. a has two meanings: in Ia, it means agranular; in 13a, it distinguishes that area from area 13b. Figures reproduced with permission 91.
Figure 2.
The medial (top) and orbital (bottom) surfaces of the macaque frontal lobe, color-coded according to the areas with which they interconnect 46. Areas in pink connect strongly with pink and purple areas, but weakly or not at all with blue areas. Areas in blue connect strongly with blue and purple areas, but weakly or not at all with pink areas. Abbreviations as in Figure 1.
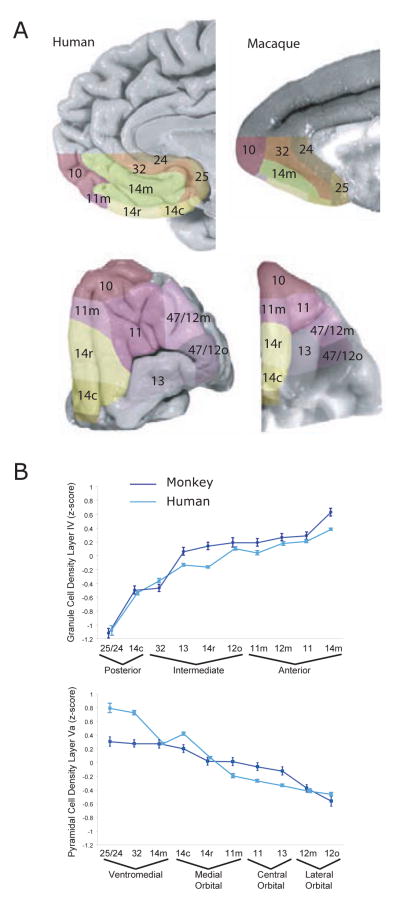
Monkey and human OFC share similar organization (Fig. 1). However, studies of value-based decision-making frequently concentrate on different regions of OFC in the two species. Studies in monkeys usually focus on areas 11 and 13 located between the lateral and medial orbital sulci 23, 26, 33, whereas human studies tended to focus on the ventral part of medial prefrontal cortex (vmPFC) 12–17, 47, 48. In early cytoarchitectonic studies, the homology between human and monkey vmPFC was not straightforward 39. The maps identified vmPFC as area 10 (Fig. 1) and it was noticeably bigger with more subdivisions in humans than monkeys. This implied that there had been anatomical reorganization of vmPFC in humans relative to other primates. However, older studies relied on the anatomist’s subjective opinion as to where one cytoarchitectonic area began and another ended. Recent studies have used quantifiable image processing methods 49. These methods have supported the close parallels in the organization of OFC in humans and monkeys (Fig. 3), showing that vmPFC consists largely of area 14 in both species. Studies that have compared patterns of connectivity across species using diffusion tensor imaging have also supported the close similarity in monkey and human OFC organization 50. This does not mean that there are not differences between monkey and human OFC. For example, the spine density of prefrontal pyramidal neurons is about 70% greater in humans compared to monkeys 51. Nevertheless, the homology of OFC areas in the two species is clear.
Figure 3.
a) Architectonic parcellation of the human and macaque monkey orbital and ventromedial surface 92. b) Mean density of layer IV and layer Va between comparable architectonic areas in the monkey (dark blue) and the human (light blue) brains. Error bars indicate standard deviation. Figures reproduced with permission 92.
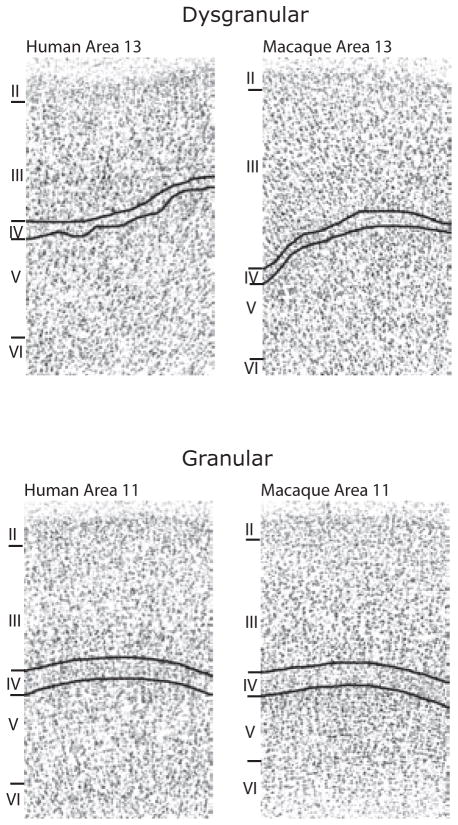
Connectivity studies suggest that rat OFC may have a similar organization to primates, with two distinct networks: a medial network, comprising medial OFC and the medial wall of the frontal cortex, and an orbital network, comprising more lateral OFC areas 52. In other respects, the homology between rodents and primates is less clear (Fig. 1). One example relates to the presence or absence of Layer IV, which contains small granular neurons (Fig. 4). Within prefrontal cortex, there is a progressive posterior to anterior gradient that begins with agranular cortex, lacking layer IV, to dysgranular cortex with a rudimentary layer IV, through to granular cortex that has a well-developed layer IV. While all three stages are evident in primates, rat OFC consists solely of agranular cortex (Fig. 1).
Figure 4.
Photomicrographs of cortical architecture in dysgranular and granular regions of OFC. Cortical layers are identified by Roman numerals. Layer IV consists of granule cells, neurons with small, round cell bodies. Figures reproduced with permission 92.
Having reviewed OFC anatomy, we are now in a position to examine whether species differences in OFC anatomy may or may not account for functional differences between species. We will begin by examining discrepancies in studies examining decision-making in humans and monkeys and then move on to examine how this work relates to rodents.
Discrepancies between studies in humans and monkeys
In humans, although some neuroimaging studies report activation of central OFC (areas 11 and 13) during value-based decision-making tasks 10, 11, 20 these tend to be the exception rather than the rule. More common are activations of vmPFC (area 14) 12–18. In contrast, neurophysiological studies in monkeys usually record from central OFC 23, 26, 33. This raises the concern that neurophysiologists are perhaps recording from the wrong area and would see many more value-related neurons if they focused on vmPFC. While this is an obvious possibility, my laboratory and that of Padoa-Schioppa (personal communication) have recorded pilot data from vmPFC and seen few value-related responses, at least in comparison to other OFC areas. The lack of published reports on value coding in vmPFC does not mean that neurophysiologists have ignored this area, only that they have not seen enough to motivate a formal report. Results from vmPFC seem to be lying in the neurophysiologist’s ‘bottom-drawer’.
Many reviews of decision-making ignore this discrepancy in anatomical localization and discuss results from central OFC in monkeys and vmPFC in humans as though the two areas constitute a single functional unit 3, 36, 53. Yet, as the above discussion of anatomy made clear, central OFC in monkeys is homologous to central OFC in humans, and vmPFC in monkeys is homologous to vmPFC in humans. Furthermore, rather than constituting a functional unit, the two regions are parts of distinct networks (Fig. 2). Thus, we need to explain why value-based decision-making appears to be localized in vmPFC in humans, but central OFC in monkeys. One possible reason is that the tasks which activate vmPFC in humans are not quite matched in terms of cognitive demands to the tasks used to probe OFC in monkeys. Indeed, when efforts are made to precisely match behavioral tasks across species, there can be remarkable similarity in findings. Monkeys 22 (or rats 54) with OFC lesions show impairments in updating the value of a stimulus when the value of its associated outcome changes. In humans, this same process also activates central OFC rather than vmPFC 19, 55. The fact that closely matched tasks involve similar OFC regions in both species is further evidence that anatomical reorganization is unlikely to have occurred. Instead it raises the possibility that vmPFC and central OFC may perform related but distinct functions that are differentially taxed by the behavioral tasks used to test decision-making in different species.
Early neuroimaging studies did indeed suggest functional differences between central OFC and vmPFC. Positive outcomes, such as rewards, tended to activate vmPFC, while more lateral regions of OFC, were associated with negative outcomes, such as punishment 56. Subsequent studies have cast doubt on aspects of this putative functional organization. For example, vmPFC also responds to monetary losses as well as gains 57, costs as well as benefits 14, and the signal in this area correlates with the willingness of a subject to pay in order to avoid eating unpleasant food 58. These findings suggest that the difference between central OFC and vmPFC is more complex than a simple dichotomy based on valence. Furthermore, neurophysiological studies examined the activity of single neurons in OFC to the delivery of rewards (drops of juice in the mouth) or punishments (air puffs to the face). Many OFC neurons responded to positive or negative outcomes but there was no evidence of anatomical organization, with neurons encoding different outcomes intermingled throughout OFC 27. Therefore, functional distinctions between vmPFC and central OFC are likely not based on valence alone.
Recent findings suggest an alternative medial-lateral organization. Central OFC neurons tend to encode the value of outcomes associated with external stimuli while vmPFC neurons encode the value of outcomes associated with internal states, such as the amount of reward one expects for a self-initiated movement 59. Consistent with this idea, vmPFC neurons are also more sensitive to the effects of satiation than central OFC neurons. Meanwhile neuropsychological studies found that central OFC is important for updating 60 and assigning 61 values to sensory stimuli, while vmPFC is necessary for choosing between alternative outcomes 60, 61 and extinguishing responding when reward is omitted 60. The flavor of these results is similar. Central OFC is more concerned with assigning value to external stimuli in the environment, while vmPFC is more concerned with values associated with internal processes, such as might be involved when a monkey deliberates as to which is the better of two alternatives or decides to give up responding. To some extent, this distinction seems to map on to human neuroimaging results. Studies involving updating stimulus-outcome associations activate more lateral regions of OFC 19, 55, whereas studies focusing on evaluating and choosing between different options activate vmPFC 12–17, 47, 48.
Another possibility is that vmPFC may be more important for processing the value of social stimuli. Neuroimaging studies show that a wide variety of social rewards, including cooperation 62, love 63 and trust 64, activate vmPFC. Furthermore, in male monkeys lesions of area 32 65 (the area directly dorsal to area 14) disrupt behavioral responses to socially relevant stimuli, such as other aggressive males or female genitalia 66. It is possible that there could be an additional social component to human decision-making tasks that is not present in monkey tasks, leading to greater activation of vmPFC and its adjacent regions in humans. In many of the tasks used in humans, subjects are trying to maximize the amount of money they win, but the amounts of money are not usually large, and subjects’ motivations might have more to do with impressing the experimenter than winning money per se. However, it is difficult to apply this explanation to tasks that have explicitly tested valuation, for instance, when a subject is simply indicating their preference among options and there is no right or wrong answer 13, 16, 47. Further studies are needed to directly test these hypotheses by contrasting the function of vmPFC and central OFC across species.
Reconciling different measures of neural activity
A final consideration is that differences in the methodologies used to study decision-making in monkeys and humans might account for the differential focus on central OFC and vmPFC. Neuroimaging data may be more sensitive to value signals in vmPFC than OFC. Susceptibility artifacts arise in functional magnetic resonance imaging (fMRI) scans near air-tissue boundaries and the nasal sinuses lie directly underneath OFC making it particularly prone to these kinds of artifacts 67. Supporting this, other imaging methods that are not prone to susceptibility artifacts, such as positron emission tomography, do show activations of central OFC 68, 69. Presenting power maps in fMRI studies, similar to those that have recently been used for neuropsychological studies 9, would help in assessing whether negative results in OFC are genuine. Sensitivity could also be reduced at the group level if OFC responses showed greater inter-individual variability than vmPFC responses. Presentation of single-subject data would be helpful to determine whether this is the case 16.
A second possibility is that different results could arise because fMRI and single-unit neurophysiology are sensitive to different physiological parameters. The blood oxygen level dependent (BOLD) response, measured by fMRI, correlates with the local field potential (LFP) which measures the summation of somatodendritic potentials over 0.5–3 mm of tissue rather than the action potentials of individual neurons 70. This has sometimes been interpreted to mean that the BOLD response reflects the inputs of an area, while single-unit neurophysiology reflects the outputs, but the reality is more complex. For example, an increase in activity in inhibitory interneurons can increase energy consumption and the BOLD response 71, even though the functional consequence may be deactivation of the area. In addition, neuromodulatory systems can affect large numbers of cells and potentially induce greater changes in the fMRI signal than changes in the spiking rate of a small set of function-specific neurons 72. Similarly, top-down feedback signals can induce a larger BOLD response in sensory cortex than bottom-up signals related to the processing of the stimulus 73, 74. The interaction of these factors could considerably complicate the interpretation of the fMRI signal in vmPFC.
Finally, the functional organization within an area may affect how difficult it is to detect signals with fMRI. Sensorimotor areas frequently show a topographic mapping of the sensorimotor parameter space. In such cases, averaging across large populations of neighboring neurons, as the BOLD response does, could still extract the parameter. However, there is little evidence of such topography in OFC 27, 33, with neurons recorded on the same electrode showing selectivity to very different decision parameters. Furthermore, OFC neurons show a diametrically opposed encoding scheme: approximately half of value encoding neurons increase their firing rate as value increases while half increase their firing rate as value decreases 25–27, 75, 76. These two populations could potentially have opposing effects on the BOLD signal, canceling one another out.
Given these problems in comparing the results from neurophysiology and neuroimaging, what can be done to reconcile the literatures? One possibility is to analyze the fMRI data using more sophisticated methods, such as multivariate decoding techniques 77, 78. However, studies that have applied this approach to reward processing have broadly reached the same conclusion as univariate methods. Significant reward information could be decoded from vmPFC rather than areas 11 and 13 17. Thus, it remains an open question as to whether these methods will prove more sensitive than univariate methods at quantifying value information in OFC. A second possibility is for neurophysiologists to analyze LFPs, particularly in vmPFC, since LFPs may better correlate with the fMRI response. LFPs in rat OFC do contain decision related information such as the magnitude 79 and probability 80 of expected rewards. In addition, there is evidence that the LFP may be one mechanism by which functional ensembles of neurons can be coordinated and communicate with one another 81. For example, in an odor discrimination task, spikes from movement-related OFC neurons phase-locked to the gamma band of the LFP while spikes from odor-related OFC neurons phase-locked to the theta band 82. The LFP may play a crucial role in coordinating functional ensembles of OFC neurons responsible for implementing distinct cognitive processes that may underlie decision-making.
Discrepancies between studies in monkeys and rats
The results from studies investigating rodent OFC are broadly similar to those from studies of primates: damage impairs the ability to learn stimulus values 83 and make adaptive decisions 84 and neurons encode decision-related information 29, 32, 34, 79, 80. However, there are some discrepancies that have prompted speculation that OFC is not directly comparable between rodents and primates 27, 85.
A notable feature of OFC neurons in monkeys is that, although they encode the value of expected outcomes, they often do not encode anything about the motor response necessary to obtain the outcome 23–26, 33. In contrast, rodent OFC neurons show coding of responses leading to outcomes 85, 86. However, there are clear differences in the way rats and monkeys are tested. With rats, different outcomes are typically associated with different responses (e.g. go left or go right in a t-maze), whereas with monkeys, the different outcomes are typically associated with different stimuli and the response simply serves to indicate which stimulus the monkey wishes to choose. Indeed, when monkeys are trained on a task where outcomes are associated with different responses rather than stimuli, OFC neurons do encode response information 87 although, unlike the rat, this information is encoded at the time of feedback rather than at the time of making the response.
Another potential difference between rats and monkeys relates to what information OFC encodes about the outcome. Neurophysiological studies in monkeys consistently report that, while some OFC neurons encode specific information about an outcome 26, 88, many neurons integrate outcome information to derive an abstract value signal 26, 27, 33. Thus, the firing rate of many OFC neurons is a function of multiple decision parameters (e.g. a reward’s taste as well as its magnitude 26) that can be used to predict the animal’s choice behavior. In contrast, in rats, OFC neurons do not appear to integrate such information 32. Furthermore, lesion results suggest that rat OFC is important for encoding specific information about the outcome rather than its general affective value 83. However, it is again possible that differences in testing procedures between rats and monkeys could be responsible for this apparent functional difference. Rat neurophysiological studies have typically manipulated a single decision variable at a time 32, 79, 80, for example, testing the effects of reward magnitude and delay costs in separate blocks of trials 32. This could reduce the likelihood of seeing neuronal responses that integrate across parameters. Future rodent neurophysiology studies could clarify this by using paradigms that require the simultaneous consideration of multiple decision parameters.
Finally, it is worth noting that not all areas of rat and monkey OFC have been studied to an equal degree. Neurophysiological studies in primates typically focus on anterior rather than posterior OFC, while studies in rats typically focus on lateral OFC rather than medial OFC. Furthermore, these studies rarely acknowledge that the data has been collected from a restricted part of OFC. Thus, before concluding that there are functional differences between rat and monkey OFC, it is important to ensure that the data giving rise to the putative functional difference has been collected from homologous areas in the two species.
Conclusion
Despite the broad agreement that OFC is critical for value-based decision-making, there are discrepancies in the literature between different species and methodologies. In this review, I have contrasted findings from humans, monkeys and rats. The similarity of OFC anatomy in monkeys and humans makes it unlikely that anatomical differences will account for differences in findings between the two species. In addition, although recent studies have highlighted the functional heterogeneity of OFC, it is difficult to see how these results could account for species differences. The most likely explanation resides in the techniques used to assess OFC function and the difficulty of translating between methodologies. Most notably, the correspondence between findings from neuroimaging and neurophysiology currently remains murky. Regarding rats, although there are marked differences in OFC anatomy relative to primates, there are also marked differences in testing procedures. Until those differences in testing procedures are controlled for, it is perhaps premature to conclude that rat and primate OFC are functionally different.
Although this review has focused on cross-species differences, it is worth emphasizing that substantial homologies do exist between the OFC of different species. Indeed, within the frontal cortex, OFC exhibits some of the clearest homologies. This is of great benefit for those of us interested in understanding OFC mechanisms, since each species opens up opportunities unavailable in others. Although our ultimate goal is to understand human OFC, monkey neurophysiology affords better spatial and temporal resolution than the imaging techniques currently available to study humans, while the rat affords an array of molecular tools that will allow for precise manipulation of OFC mechanisms. However, in order to capitalize on these methods, it is important to keep in mind the limitations of each method and be precise in comparing results across species. For example, given the anatomy, we ought not to be treating vmPFC in humans and central OFC in monkeys as homologous. There are also a number of experimental directions currently available that would help build bridges between different methods and potentially reconcile some discrepancies in the literature. For instance, if LFP data is reported in addition to single neuron data, we may be able to better link neurophysiological and neuroimaging results. Studies aimed at understanding functional differences between primate posterior and anterior OFC might provide insight into the relationship between primate and rodent OFC. Building these bridges will enable us to better benefit from a multipronged approach to understanding OFC function and increase our chances of seeing as much progress in the next decade as we have in the last.
Acknowledgments
The preparation of this manuscript was supported by NIDA grant R01DA19028 and NINDS grant P01NS04081. The author thanks Roshan Cools, Elisabeth Murray, Camillo Padoa-Schioppa, Erin Rich, Geoffrey Schoenbaum and Steven Wise for their valuable comments on an earlier draft of this manuscript.
References
- 1.Cavada C, Schultz W. The mysterious orbitofrontal cortex. foreword. Cereb Cortex. 2000;10:205. doi: 10.1093/cercor/10.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 3.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damasio AR. Descartes’ error: emotion, reason, and the human brain. Putman; New York: 1994. [Google Scholar]
- 5.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 6.Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cereb Cortex. 2007;17:2669–2674. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- 7.Manes F, et al. Decision-making processes following damage to the prefrontal cortex. Brain: a journal of neurology. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- 8.Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sescousse G, Redoute J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters J, Buchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29:15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DV, et al. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FitzGerald TH, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. J Neurosci. 2009;29:8388–8395. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basten U, Biele G, Heekeren HR, Fiebach CJ. How the brain integrates costs and benefits during decision making. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talmi D, Dayan P, Kiebel SJ, Frith CD, Dolan RJ. How humans integrate the prospects of pain and reward during choice. J Neurosci. 2009;29:14617–14626. doi: 10.1523/JNEUROSCI.2026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahnt T, Heinzle J, Park SQ, Haynes JD. The neural code of reward anticipation in human orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:6010–6015. doi: 10.1073/pnas.0912838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 20.Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 24.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 25.Kennerley SW, Wallis JD. Encoding of reward and space during a working memory task in the orbitofrontal cortex and anterior cingulate sulcus. J Neurophysiol. 2009;102:3352–3364. doi: 10.1152/jn.00273.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 29.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Lee D. Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron. 2011;70:731–741. doi: 10.1016/j.neuron.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roesch MR, Olson CR. Neuronal activity in primate orbitofrontal cortex reflects the value of time. J Neurophysiol. 2005;94:2457–2471. doi: 10.1152/jn.00373.2005. [DOI] [PubMed] [Google Scholar]
- 32.Roesch MR, Taylor AR, Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–520. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kepecs A, Uchida N, Zariwala HA, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455:227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- 35.Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padoa-Schioppa C. Neurobiology of Economic Choice: A Good-Based Model. Annu Rev Neurosci. 2010 doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallis JD. Neuronal mechanisms in prefrontal cortex underlying adaptive choice behavior. Ann N Y Acad Sci. 2007;1121:447–460. doi: 10.1196/annals.1401.009. [DOI] [PubMed] [Google Scholar]
- 38.Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Elsevier; New York: 1994. pp. 17–57. [Google Scholar]
- 39.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 40.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 41.Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 42.Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 45.Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 46.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackey S, Petrides M. Architectonic mapping of the medial region of the human orbitofrontal cortex by density profiles. Neuroscience. 2009;159:1089–1107. doi: 10.1016/j.neuroscience.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Croxson PL, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elston GN. Specialization of the neocortical pyramidal cell during primate evolution. In: Kaas J, Preuss TM, editors. Evolution of Nervous Systems: A Comprehensive Reference. Elsevier; New York: 2007. pp. 191–242. [Google Scholar]
- 52.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 53.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27:4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 57.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 58.Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouret S, Richmond BJ. Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys. J Neurosci. 2010;30:8591–8601. doi: 10.1523/JNEUROSCI.0049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. doi: 10.1523/JNEUROSCI.0091-11.2011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rilling J, et al. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 63.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 64.King-Casas B, et al. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 65.Noonan MP, Sallet J, Rudebeck PH, Buckley MJ, Rushworth MF. Does the medial orbitofrontal cortex have a role in social valuation? Eur J Neurosci. 2010;31:2341–2351. doi: 10.1111/j.1460-9568.2010.07271.x. [DOI] [PubMed] [Google Scholar]
- 66.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 67.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 68.Arana FS, et al. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaudhry AM, Parkinson JA, Hinton EC, Owen AM, Roberts AC. Preference judgements involve a network of structures within frontal, cingulate and insula cortices. The European journal of neuroscience. 2009;29:1047–1055. doi: 10.1111/j.1460-9568.2009.06646.x. [DOI] [PubMed] [Google Scholar]
- 70.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 71.Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 73.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 74.Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29:2061–2073. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. J Neurosci. 2009;29:14004–14014. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat Rev Neurosci. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 78.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 79.van Duuren E, et al. Neural coding of reward magnitude in the orbitofrontal cortex of the rat during a five-odor olfactory discrimination task. Learn Mem. 2007;14:446–456. doi: 10.1101/lm.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Duuren E, et al. Single-cell and population coding of expected reward probability in the orbitofrontal cortex of the rat. J Neurosci. 2009;29:8965–8976. doi: 10.1523/JNEUROSCI.0005-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canolty RT, et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Wingerden M, Vinck M, Lankelma JV, Pennartz CM. Learning-associated gamma-band phase-locking of action-outcome selective neurons in orbitofrontal cortex. J Neurosci. 2010;30:10025–10038. doi: 10.1523/JNEUROSCI.0222-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burke KA, Franz TM, Miller DN, Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–344. doi: 10.1038/nature06993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 85.Furuyashiki T, Holland PC, Gallagher M. Rat orbitofrontal cortex separately encodes response and outcome information during performance of goal-directed behavior. J Neurosci. 2008;28:5127–5138. doi: 10.1523/JNEUROSCI.0319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 87.Tsujimoto S, Genovesio A, Wise SP. Monkey orbitofrontal cortex encodes response choices near feedback time. J Neurosci. 2009;29:2569–2574. doi: 10.1523/JNEUROSCI.5777-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lara AH, Kennerley SW, Wallis JD. Encoding of gustatory working memory by orbitofrontal neurons. J Neurosci. 2009;29:765–774. doi: 10.1523/JNEUROSCI.4637-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 90.Palomero-Gallagher N, Zilles K. Isocortex. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academiuc Press; San Diego, CA: 2004. pp. 729–757. [Google Scholar]
- 91.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mackey S, Petrides M. Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. Eur J Neurosci. 2010;32:1940–1950. doi: 10.1111/j.1460-9568.2010.07465.x. [DOI] [PubMed] [Google Scholar]