Abstract
Bypass surgery for limb salvage in cases of chronic limb ischemia is a well-established treatment modality. Use of an autogenous vein provides the best conduit for infrainguinal arterial bypass procedures, particularly for bypass to the infrapopliteal arteries. In this article, we discuss infrainguinal vein bypass surgery including indications, perioperative care, and long-term follow up. We also discuss the outcomes of the procedure with regard to patient survival and limb salvage. The autogenous vein continues to be the best available conduit with the highest patency rate and the best treatment option. Compared to all other revascularization options for infrainguinal disease, the vein bypass has the best limb salvage and long-term survival in patients appropriately selected for the procedure.
Keywords: Critical limb ischemia, infrainguinal vein bypass, limb salvage, revascularization, outcomes

Introduction
Critical limb ischemia (CLI) is characterized by rest pain and tissue loss in the form of ulceration and/or gangrene. It leads to significant patient morbidity and mortality and consumes considerable health and social resources in both developed and developing countries.1 In cases of CLI, bypass surgery for limb salvage is an established treatment. Autogenous vein use is the more effective conduit for infrainguinal arterial bypass procedures, especially for bypass to the infrapopliteal arteries.2, 3 This article explores infrainguinal vein bypass surgery including indications, perioperative care, long-term follow up, and outcomes regarding patient survival and limb salvage.
Indications
Patients with CLI have a considerably bad prognosis, with 40% of patients progressing to major amputation within 6 months and most patients losing their limbs eventually if no attempt at revascularization is performed.4, 5 For this reason, these patients have to undergo revascularization if their limbs are to be salvaged, either by endovascular means or through bypass surgery with vein or prosthetic conduits. Relatively recently, two decision-making guidelines have been developed for these revascularization options. The first is the TASC guidelines, which were developed in 2000 by the Trans-Atlantic Inter-Society Consensus for the management of Peripheral Arterial Disease (TASC). The TASC guidelines document was authored by a working group of representatives from 14 surgical, vascular, cardiovascular, and radiologic societies.6 An upgraded document (TASC II) was published in 2007. The TASC working group classified patients according to the anatomic patterns of disease involvement — types A through D (Figure 1). Based on their recommendations, TASC type A lesions are best treated with angioplasty and TASC type D lesions with bypass surgery. There was insufficient evidence concerning TASC type B and C lesions to definitively recommend one modality over the other; however, type B lesions are probably best treated with angioplasty while type C lesions may be best treated with surgery.1
Figure 1.
TASC disease classifications. From Norgren L, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007 Jan;45 Suppl S:S5-67.
J Vasc Surg. 2007 Jan;45 Suppl S:S5-67. © 2007, Reprinted with permission from Elsevier.
The second set of treatment guidelines was developed from the United Kingdom-based multicenter Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial. This remains the only randomized controlled trial (RCT) to compare the clinical and cost effectiveness of “bypass surgery first” and “balloon angioplasty first” revascularization strategies for CLI due to infrainguinal disease.7 The treatment recommendations based on the BASIL trial data included the following:8
Patients expected to live more than 2 years based on their other comorbidities should usually be offered bypass surgery first. The strength or the recommendation appears to be the greatest where vein is available as a conduit.
Patients expected to live less than 2 years should usually be offered angioplasty first because they are unlikely to reap the longer-term benefits of bypass and because angioplasty is significantly less expensive and morbid in the short term.
Many patients who cannot undergo a vein bypass may be better served by a first attempt at angioplasty than by prosthetic bypass. Surgeons should make every effort to use vein and view prosthetic material as a last resort.
Preoperative Evaluation
The preoperative evaluation for patients undergoing vein bypass for infrainguinal disease includes evaluation of the patient, the anatomy of the disease, and the conduit. Preoperative patient evaluation and surgical risk assessment are covered in detail in other articles within this issue and will therefore not be discussed here. Similarly, preoperative evaluation of the vascular anatomy of the limb using various invasive and noninvasive procedures and imaging modalities is also discussed at length in this issue by Duran and Bismuth (see page 43). After assessment of the patient and the vascular anatomy, the third preoperative step is evaluation of the conduit. Assessment of vein availability and quality is critical and should be carried out before embarking on the operation. This can be performed using vein mapping with duplex ultrasound.9 The evaluation includes the vein’s size, quality, and length available as a conduit. Veins more than 3 mm in diameter are good conduits.10 Veins between 2 and 3 mm are worthy of exploration.11 The veins should be soft and compressible; small, calcified, and sclerotic veins should be rejected. The ipsilateral great saphenous vein is preferred. If this vein is not available or inadequate, scanning of the ipsilateral short saphenous, contralateral great and short saphenous veins, and arm veins is also performed. The type and quality of the bypass conduit are the most important determinants of infrainguinal bypass success. Efforts to maximize conduit quality will be rewarded.
The Operative Technique
Selection of the Proximal and Distal Anastomotic Sites
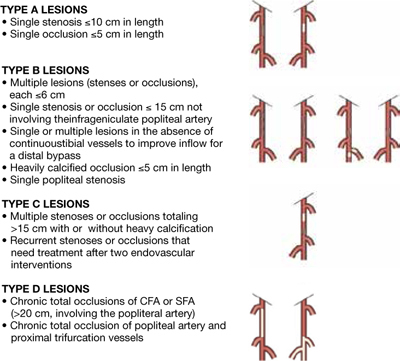
The first step in the operation is to identify the inflow and the runoff targets for the bypass. The principle is to have an inflow target that has no significant disease proximal to it that can interfere with the inflow into the bypass. The inflow vessel is usually the common femoral artery; however, it can be any other vessel of the lower extremity with unrestricted arterial inflow. This can be the profunda femoris artery, the superficial femoral artery, the popliteal artery, and, in some less common instances, one of the tibial vessels. Using another more distal target as an inflow vessel will reduce the length of the bypass, thus maximizing the use of a favorable conduit, especially in cases when the vein conduit is not long enough. There is abundant evidence that originating shorter bypasses distal to the common femoral artery results in patency rates equivalent to those achieved when the common femoral artery serves as the bypass origin. Any significant disease in the inflow vessel should be treated with endarterectomy and patch angioplasty, which is commonly performed in the common femoral artery and origin of the profunda. The patch will then serve as the origin of the proximal anastomosis of the bypass (Figure 2). Correction of significant deep femoral artery disease at the time of infrainguinal bypass is clinically important; should the bypass ever fail, adequate deep femoral artery perfusion may prevent the development of severe, recurrent limb ischemia. In some cases, when there is no available conduit long enough for the bypass, we might have to choose a more distal inflow target even though there is a significant lesion proximal to it. In these cases, the proximal lesion must be dealt with either by open surgery with endarterectomy or endovascularly using angioplasty or remote endarterectomy, creating what is known as a hybrid procedure.
Figure 2.
Vein bypass for infrainguinal disease: endarterectomy and patch angioplasty to treat significant disease in the inflow vessel. The patch serves as the origin of the proximal anastomosis of the bypass.
©2010, Reprinted with permission from Elsevier.30
The selection of the distal anastomosis site requires more judgment than the proximal anastomosis. Generally, the principle is to select the most proximal artery that has unrestricted flow through at least one runoff artery to the foot. This is an easy decision when the popliteal artery is patent with at least one tibial vessel runoff to the foot. The process is more complex when the target is the tibial, peroneal, or pedal vessel. In general, the most proximal segment of the tibial artery that is continuous with the foot is selected. This is particularly important when there is tissue loss, in which case in-line flow to the foot is crucial.12 In some cases, the tibial target requires longer-than-available conduit to ensure in-line flow. In those cases, especially where there is only rest pain and no tissue loss, the peroneal artery can be a satisfactory target and carries a comparable patency rate to the anterior or posterior tibial bypass.13 As stated earlier, in cases of tissue loss, techniques to choose a more distal inflow site are particularly important.
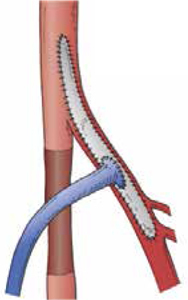
Another important aspect is the angiosome concept of leg and foot perfusion. Tibial bypass is performed only to one tibial target. In cases of tissue loss, the target should be the vessel responsible for in-line perfusion to the area of tissue loss. Based on Taylor and Palmer’s work in 1987, the body is divided into three-dimensional vascular territories supplied by specific source arteries and drained by specific veins (i.e., angiosomes).14 They defined five distinct angiosomes for the lower leg and six for the foot derived from the anterior tibial, posterior tibial, and peroneal arteries (Figure 3). Whenever possible, bypass is performed to the vessel responsible for perfusion of the angiosome containing the tissue loss. It was found that bypass surgery for tissue loss resulted in a limb salvage rate of 91% with the use of direct revascularization of the specific angiosome, whereas the salvage rate was only 62% with indirect revascularization.15
Figure 3.
Angiosomes of the lower leg and foot. ATA: anterior tibial artery; PTA: posterior tibial artery; PA: peroneal artery. From Iida O, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions.
J Vasc Surg. 55(2):363-70. © 2012, Reprinted with permission from Elsevier.
Vein Conduit Harvest and Preparation
If available, the ipsilateral great saphenous vein is the preferred choice for a conduit. If it is not available, the contralateral great saphenous vein is the next choice. There is no merit in saving the contralateral great saphenous vein for future use in the contralateral leg, since only 20–25% of patients will require revascularization of the other leg in the future.16 The only exceptions are if the contralateral leg is already ischemic and the vein is needed for revascularization, or if there is fear of wound healing issues in the contralateral ischemic leg after the vein is harvested. In cases where the great saphenous vein is unavailable or inadequate in length, other vein options are available as conduits.17 The short saphenous vein is suitable, especially if the required bypass is short. Arm veins are also good conduits, and long conduits can be created by harvesting the arm’s basilic, median antecubital, and cephalic vein in one piece as described by Holzenbein and associates.18 In cases where no adequate single piece is available for a conduit, splicing vein pieces together can be used to create longer conduits.
Vein harvesting can be performed through long incisions, skip incisions, or endoscopically. Endoscopic vein harvest is more common in the cardiac surgery world and has not been widely adopted by vascular surgeons, mostly due to equipment cost, lack of reimbursement for the procedure, learning-curve issues, and concerns about damaging vein segments when long conduits are needed. To date, there are no convincing data to support any one specific harvesting technique over another, although some authors suggest that endoscopic vein harvest may have lower long-term patency compared to open harvesting, especially in CLI patients.19 The most important point in vein harvesting is choosing a high quality conduit irrespective of the harvesting technique used.20, 21
The Bypass Technique
The vein bypass can be performed in a reversed, nonreversed, or in situ configuration. The theoretical advantages of the in situ saphenous vein bypass technique (i.e., preserved endothelial function resulting from intact vasa vasora and improved graft hemodynamics) have not been supported by biochemical, morphologic, or hemodynamic data from laboratory studies. Furthermore, superior long-term graft patency rates for in situ vein bypass compared with reversed vein bypass have not been documented in randomized clinical trials.22-24 The technique chosen for vein bypass is primarily dictated by conduit availability, anatomic considerations, and surgeon preferences and experience. However, whenever possible, the proximal and distal anastomoses should be performed in an end-to-side pattern maintaining the continuity of the native arterial system. This preserves any possible endovascular options should the patient need them in the future.
Intraoperative Evaluation
Technical errors in bypass surgery are usually responsible for graft failure in the first 30 days after the procedure.25 This should be an uncommon problem in modern vascular surgery. The most important factor in avoiding early graft failure is to recognize any technical errors during the procedure that may contribute to failure and correct them intraoperatively. This is accomplished using intraoperative monitoring of bypass function, including the inflow vessel, proximal and distal anastomoses, the conduit, and the distal runoff. Technical errors requiring intraoperative correction have been found in 8–12% of bypass cases, signifying the magnitude of the problem.26, 27 There are three methods for performing intraoperative graft monitoring:
Doppler assessment. This is the basic method for evaluating the graft and is most useful for discovering major defects in the bypass procedure. Doppler assessment is performed by evaluating distal pulse using digital examination and continuous Doppler signals in the runoff artery distally, with and without graft compression, which shows significant augmentation of the signals with the bypass open compared to manual occlusion of the graft. Although this is the most commonly used method of intraoperative evaluation, it fails to identify significant technical lesions that may contribute to graft failure, especially compared to using a more detailed examination with angiography or duplex ultrasound.28
Completion arteriogram. Intraoperative arteriogram is the simplest method for a completion study. The availability of X-ray-compatible operating room tables and capable C-arms have made the procedure very easy to perform and very efficient in obtaining the required information. Using completion arteriograms, Mills and associates were able to identify significant technical problems in 8% of infrainguinal bypass grafts that required revision.26 A completion arteriogram can evaluate the bypass, including the inflow and runoff; however, it is less likely to evaluate problems with retained valve cusps in cases of nonreversed and in situ vein bypass.29
Intraoperative duplex examination. Duplex ultrasound is a very effective and accurate method for evaluating bypass grafts intraoperatively. In addition to being as effective as angiography, duplex examination has the advantage of evaluating for retained valve cusps. Duplex examination can also evaluate flow dynamics, even in the absence of identifiable technical defects, in which case the grafts can be extended more distally or at least the physician can be guided towards putting the patient on postoperative anticoagulation to assist in graft patency.30 One drawback is that it requires the availability of both an ultrasound machine and a vascular lab technician in the operating room, which makes duplex examination a more cumbersome modality.
Postoperative Management
In addition to routine post-operative care in vascular surgery procedures, two important points deserve mentioning regarding the long-term postoperative care after infrainguinal vein bypass procedure.
Pharmacologic therapy
Vascular surgeons agree that all patients should be on antiplatelet therapy before and definitely following vein bypass procedures as part of the pharmacologic therapy for peripheral arterial occlusive disease. This is routinely done by using low-dose aspirin (81 or 325 mg daily). Aspirin is well known for its beneficial cardiac and cerebral protective effects and may improve graft patency. Clopidogrel can also be used for this purpose, although it is more expensive and has no evidence of being superior to aspirin in its effects.
What remains controversial is the use of anticoagulants after infrainguinal bypass surgery. The landmark Dutch Bypass Oral Anticoagulants or Aspirin trial found that oral anticoagulation improved vein bypass graft patency compared to aspirin alone.31 On the other hand, a Veterans Affairs cooperative trial randomizing patients to coumadin and aspirin or aspirin alone concluded that vein bypass graft patency was not increased by the addition of coumadin to aspirin, but there was a significant increase in hemorrhagic complications in these patients.32 Many surgeons, however, use anticoagulation selectively in patients after vein bypass in high-risk grafts, which are defined as poor conduit, poor arterial runoff, and reoperative cases.33
Graft surveillance
Over time, nearly one-third of vein grafts will develop significant lesions that threaten their patency.34 Most of these lesions develop within the first 2 years after the operation and are mainly due to neointimal hyperplasia. In their prospective randomized trial, Lundell and associates reported that duplex ultrasound graft surveillance of vein grafts improved the long-term patency by about 15% compared to no surveillance.35 Graft surveillance is performed in the noninvasive vascular lab using duplex ultrasound examination coupled with ankle brachial index measurement. Interference is warranted if there is a significant stenosis (defined as greater than 50%), low flow velocities in the graft that signify failure, or a drop of 0.15 in ankle brachial index.36 Graft surveillance is performed every 3 months for the first year, every 6 months for 2 additional years, and annually thereafter.
Outcomes
Infrainguinal vein bypass procedures for patients with CLI have excellent outcomes. Discussing the results of lower extremity vein bypasses requires an examination of some hard objective endpoints including patency, limb salvage, and patient survival and examination of other endpoints such as functional outcome, quality of life, and cost-benefit analysis. These outcomes should be compared with all other lower extremity revascularization procedures, including endovascular interventions and prosthetic bypass surgery, so we can define the role of lower extremity vein bypass for CLI.
With respect to patency, infrainguinal vein bypasses have the highest patency rates across the board compared to all other conduits, including human umbilical vein, homografts, and prosthetic grafts. Tables 1–3 show pooled meta-analysis data from the literature regarding bypass patency and limb salvage of different conduits and bypass levels.30 Randomized controlled trials have shown no difference in patency between in situ bypass and reversed vein.37, 38 The difference in patency becomes more accentuated the more distal the bypass is. For infragenicular bypass, meta-analysis of a pooled series in the literature shows that prosthetic bypass secondary patency is around 20% in 4 years compared to greater than 75% for vein bypasses (Table 3). In above-the-knee bypass, the vein and prosthetic graft bypasses carry the same patency rates (Table 1) yet have different patterns of failure. Prosthetic graft failure is generally acute with acute limb ischemia and carries a high risk of distal embolization into the outflow tract, in which case the price of graft occlusion is the risk of limb loss. In comparison, vein bypass failure is more chronic and much less likely to result in limb loss.
Table 1.
Patency of above-knee femoropopliteal grafts. all series published since 1981.30
| Graft Type | Primary Patency (%) | |||||
| 1-Mo | 6-Mo | 1-Yr | 2-Yr | 3-Yr | 4-Yr | |
| Reversed Saphenous Vein | 99 | 91 | 84 | 82 | 73 | 69 |
| Arm Vein | 99 | 82 | 65 | 60 | 60 | |
| Polytetrafluoroethylene | 89 | 79 | 74 | 66 | 60 | |
Table 3.
Patency of infrapopliteal grafts. all series published since 1981.30
| Graft and Patency Type | Primary Patency (%) | |||||
| 1-Mo | 6-Mo | 1-Yr | 2-Yr | 3-Yr | 4-Yr | |
| Primary Patency | ||||||
| Reversed saphenous vein | 92 | 81 | 77 | 70 | 66 | 62 |
| In situ vein bypass | 94 | 84 | 82 | 76 | 74 | 68 |
| Secondary Patency | ||||||
| Reversed saphenous vein | 93 | 89 | 84 | 80 | 78 | 76 |
| In situ vein bypass | 95 | 90 | 89 | 87 | 84 | 81 |
| Arm vein | 94 | 73 | 62 | 58 | ||
| Human umbilical vein | 80 | 65 | 52 | 46 | 40 | 37 |
| Polytetrafluoroethylene | 89 | 58 | 46 | 32 | 21 | |
Table 2.
Patency of below-knee femoropopliteal grafts. all series published since 1981.30
| Graft and Patency Type | Primary Patency (%) | |||||
| 1-Mo | 6-Mo | 1-Yr | 2-Yr | 3-Yr | 4-Yr | |
| Primary Patency | ||||||
| Reversed Saphenous Vein | 98 | 90 | 84 | 79 | 78 | 77 |
| In Situ vein bypass | 95 | 87 | 80 | 76 | 73 | 68 |
| Secondary Patency | ||||||
| In situ vein bypass | 97 | 96 | 96 | 89 | 86 | 81 |
| Arm Vein | 97 | 83 | 83 | 73 | 70 | |
| Polytetrafluoroethylene | 96 | 80 | 68 | 61 | 44 | 40 |
| Human umbilical vein | 88 | 82 | 77 | 70 | 61 | 60 |
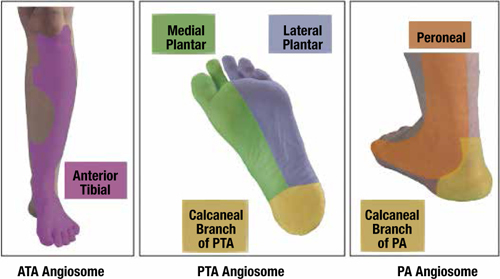
Regarding limb salvage and overall survival, the BASIL trial is the best available evidence comparing vein bypass, prosthetic bypass, and balloon angioplasty for infrainguinal revascularization in CLI.8 The study showed that, compared to prosthetic material, bypass using a vein offers the best long-term amputation-free survival and overall survival (Figure 4).
Figure 4.
A) Amputation-free survival, and B) overall survival for patients undergoing bypass surgery according to type of bypass conduit. From Bradbury AW, et al. Bypass versus angioplasty in severe ischemia of the leg (BASIL) trial: analysis of amputation free and overall survival by treatment received.
J Vasc Surg. 51(Suppl 5):18S-31S. © 2010, Reprinted with permission from Elsevier.
Conclusion
Vein bypass surgery for treating CLI of the lower extremities remains an integral part of the therapy for this difficult to treat patient population. The autogenous vein continues to be the best available conduit with the highest patency rate and the best treatment option. Compared to all other revascularization options for infrainguinal disease, the vein bypass has the best limb salvage and long-term survival in patients appropriately selected for the procedure.
Conflict of Interest Disclosure: The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: The author has no funding disclosures.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Klinkert P, Schepers A, Burger DH, van Bockel JH, Breslau PJ. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomized controlled trial. J Vasc Surg. 2003 Jan;37(1):149–55. doi: 10.1067/mva.2002.86. [DOI] [PubMed] [Google Scholar]
- 3.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, Sheahan M, Campbell DR, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003 Feb;37(2):307–15. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SM, Kalbaugh CA, Gray BH, Mackrell PJ, Langan EM, 3rd, Cull DL, et al. The LEGS score: a proposed grading system to direct treatment of chronic lower extremity ischemia. Ann Surg. 2003 Jun;237(6):812. doi: 10.1097/01.SLA.0000071563.19208.50. 8; discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long-term mortality and its predictors in patients with critical leg ischaemia. The I.C.A.I. Group (Gruppo di Studio dell’Ischemia Cronica Critica degli Arti Inferiori). The Study Group of Criticial Chronic Ischemia of the Lower Exremities. Eur J Vasc Endovasc Surg. 1997 Aug;14(2):91–5. [PubMed] [Google Scholar]
- 6.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000 Jan;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- 7.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005 Dec 3;366(9501):1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury AW. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial in perspective. J Vasc Surg. 2010 May;51(5 Suppl):1S–68S. doi: 10.1016/j.jvs.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Seeger JM, Schmidt JH, Flynn TC. Preoperative saphenous and cephalic vein mapping as an adjunct to reconstructive arterial surgery. Ann Surg. 1987 Jun;205(6):733–9. doi: 10.1097/00000658-198706000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wengerter KR, Veith FJ, Gupta SK, Ascer E, Rivers SP. Influence of vein size (diameter) on infrapopliteal reversed vein graft patency. J Vasc Surg. 1990 Apr;11(4):525–31. doi: 10.1067/mva.1990.18327. [DOI] [PubMed] [Google Scholar]
- 11.Slim H, Tiwari A, Ritter JC, Rashid H. Outcome of infrainguinal bypass grafts using vein conduit with less than 3 millimeters diameter in critical leg ischemia. J Vasc Surg. 2011 Feb;53(2):421–5. doi: 10.1016/j.jvs.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Elliott BM, Robison JG, Brothers TE, Cross MA. Limitations of peroneal artery bypass grafting for limb salvage. J Vasc Surg. 1993 Nov;18(5):881–8. doi: 10.1067/mva.1993.49636. [DOI] [PubMed] [Google Scholar]
- 13.Bergamini TM, George SM, Jr., Massey HT, Henke PK, Klamer TW, Lambert GE, Jr., et al. Pedal or peroneal bypass: which is better when both are patent? J Vasc Surg. 1994 Sep;20(3):347. doi: 10.1016/0741-5214(94)90132-5. 55; discussion 55-6. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987 Mar;40(2):113–41. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 15.Neville RF, Attinger CE, Bulan EJ, Ducic I, Thomassen M, Sidawy AN. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg. 2009 May-Jun;23(3):367–73. doi: 10.1016/j.avsg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Chew DK, Owens CD, Belkin M, Donaldson MC, Whittemore AD, Mannick JA, et al. Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein. J Vasc Surg. 2002 Jun;35(6):1085–92. doi: 10.1067/mva.2002.124628. [DOI] [PubMed] [Google Scholar]
- 17.Gentile AT, Lee RW, Moneta GL, Taylor LM, Edwards JM, Porter JM. Results of bypass to the popliteal and tibial arteries with alternative sources of autogenous vein. J Vasc Surg. 1996 Feb;23(2):272. doi: 10.1016/s0741-5214(96)70271-9. 9; discussion 9-80. [DOI] [PubMed] [Google Scholar]
- 18.Holzenbein TJ, Pomposelli FB, Jr., Miller A, Gibbons GW, Campbell DR, Freeman DV, et al. The upper arm basilic-cephalic loop for distal bypass grafting: technical considerations and follow-up. J Vasc Surg. 1995 Apr;21(4):586. doi: 10.1016/s0741-5214(95)70190-7. 92; discussion 92-4. [DOI] [PubMed] [Google Scholar]
- 19.Julliard W, Katzen J, Nabozny M, Young K, Glass C, Singh MJ, et al. Long-term results of endoscopic versus open saphenous vein harvest for lower extremity bypass. Ann Vasc Surg. 2011 Jan;25(1):101–7. doi: 10.1016/j.avsg.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Erdoes LS. Endoscopic vein harvest in peripheral vascular surgery. J Vasc Surg. 2006 Aug;44(2):417–8. doi: 10.1016/j.jvs.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Gazoni LM, Carty R, Skinner J, Cherry KJ, Harthun NL, Kron IL, et al. Endoscopic versus open saphenous vein harvest for femoral to below the knee arterial bypass using saphenous vein graft. J Vasc Surg. 2006 Aug;44(2):282. doi: 10.1016/j.jvs.2006.03.047. 7; discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 22.Back MR, Seeger JM. Reversed and nonreversed transposed autogenous vein grafting for atherosclerotic lower extremity occlusive disease. In: Ernst CB Stanley JC, editors. Current therapy in vascular surgery. St. Louis, Missouri: Mosby, Inc.; 2001. p. 459-62. [Google Scholar]
- 23.Harris PL, How TV, Jones DR. Prospectively randomized clinical trial to compare in situ and reversed saphenous vein grafts for femoropopliteal bypass. Br J Surg. 1987 Apr;74(4):252–5. doi: 10.1002/bjs.1800740409. [DOI] [PubMed] [Google Scholar]
- 24.Watelet J, Cheysson E, Poels D, Menard JF, Papion H, Saour N, et al. In situ versus reversed saphenous vein for femoropopliteal bypass: a prospective randomized study of 100 cases. Ann Vasc Surg. 1987 May;1(4):441–52. doi: 10.1016/S0890-5096(06)60729-2. [DOI] [PubMed] [Google Scholar]
- 25.Shoenfeld NA, O’Donnell TF, Bush HL, Jr., Mackey WC, Callow AD. The management of early in situ saphenous vein bypass occlusions. Arch Surg. 1987 Aug;122(8):871–5. doi: 10.1001/archsurg.1987.01400200021002. [DOI] [PubMed] [Google Scholar]
- 26.Mills JL, Fujitani RM, Taylor SM. Contribution of routine intraoperative completion arteriography to early infrainguinal bypass patency. Am J Surg. 1992 Nov;164(5):506. doi: 10.1016/s0002-9610(05)81190-0. 10; discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 27.Bandyk DF, Mills JL, Gahtan V, Esses GE. Intraoperative duplex scanning of arterial reconstructions: fate of repaired and unrepaired defects. J Vasc Surg. 1994 Sep;20(3):426. doi: 10.1016/0741-5214(94)90142-2. 32; discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 28.Renwick S, Royle JP, Martin P. Operative angiography after femoropopliteal arterial reconstruction--its influence on early failure rate. Br J Surg. 1968 Feb;55(2):134–6. doi: 10.1002/bjs.1800550217. [DOI] [PubMed] [Google Scholar]
- 29.Gilbertson JJ, Walsh DB, Zwolak RM, Waters MA, Musson A, Magnant JG, et al. A blinded comparison of angiography, angioscopy, and duplex scanning in the intraoperative evaluation of in situ saphenous vein bypass grafts. J Vasc Surg. 1992 Jan;15(1):121. doi: 10.1067/mva.1992.32985. 7; discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 30.Mills JL. Infrainguinal Disease: Surgical Treatment. In: Cronenwett JL Johnston KW, editors. Rutherford Vascular Surgery. Philadelphia, PA: Saunders; 2010. p. 1682-703. [Google Scholar]
- 31.Tangelder MJ, Algra A, Lawson JA, Hennekes S, Eikelboom BC. Optimal oral anticoagulant intensity to prevent secondary ischemic and hemorrhagic events in patients after infrainguinal bypass graft surgery. Dutch BOA Study Group. J Vasc Surg. 2001 Mar;33(3):522–7. doi: 10.1067/mva.2001.111986. [DOI] [PubMed] [Google Scholar]
- 32.Johnson WC, Williford WO. Benefits morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg. 2002 Mar;35(3):413–21. doi: 10.1067/mva.2002.121847. [DOI] [PubMed] [Google Scholar]
- 33.Sarac TP, Huber TS, Back MR, Ozaki CK, Carlton LM, Flynn TC, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg. 1998 Sep;28(3):446–57. doi: 10.1016/s0741-5214(98)70130-2. [DOI] [PubMed] [Google Scholar]
- 34.Szilagyi DE, Elliott JP, Hageman JH, Smith RF, Dall’olmo CA. Biologic fate of autogenous vein implants as arterial substitutes: clinical angiographic and histopathologic observations in femoro-popliteal operations for atherosclerosis. Ann Surg. 1973 Sep;178(3):232–46. doi: 10.1097/00000658-197309000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundell A, Lindblad B, Bergqvist D, Hansen F. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: a prospective randomized study. J Vasc Surg. 1995 Jan;21(1):26. doi: 10.1016/s0741-5214(95)70241-5. 33; discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 36.Mills JL, Sr., Wixon CL, James DC, Devine J, Westerband A, Hughes JD. The natural history of intermediate and critical vein graft stenosis: recommendations for continued surveillance or repair. J Vasc Surg. 2001 Feb;33(2):273. doi: 10.1067/mva.2001.112701. 8; discussion 8-80. [DOI] [PubMed] [Google Scholar]
- 37.Harris PL, Veith FJ, Shanik GD, Nott D, Wengerter KR, Moore DJ. Prospective randomized comparison of in situ and reversed infrapopliteal vein grafts. Br J Surg. 1993 Feb;80(2):173–6. doi: 10.1002/bjs.1800800213. [DOI] [PubMed] [Google Scholar]
- 38.Lawson JA, Tangelder MJ, Algra A, Eikelboom BC. The myth of the in situ graft: superiority in infrainguinal bypass surgery? Eur J Vasc Endovasc Surg. 1999 Aug;18(2):149–57. doi: 10.1053/ejvs.1999.0865. [DOI] [PubMed] [Google Scholar]


